Abstract
Optimization of treatment strategies for prostate cancer patients treated with curative radiation therapy (RT) represents one of the major challenges for the radiation oncologist. Dose escalation or combination of RT with systemic therapies is used to improve tumor control in patients with unfavorable prostate cancer, at the risk of increasing rates and severity of treatment-related toxicities. Elevation of temperature to a supra-physiological level has been shown to both increase tumor oxygenation and reduce DNA repair capabilities. Thus, hyperthermia (HT) combined with RT represents a compelling treatment strategy to improve the therapeutic ratio in prostate cancer patients. The aim of the present systematic review is to report on preclinical and clinical evidence supporting the combination of HT and RT for prostate cancer, discussing future applications and developments of this combined treatment.
1. Introduction
Radiation therapy (RT) represents one of the standard treatments for prostate cancer. Despite the curative intent, a variable proportion of patients treated with RT will develop a local relapse (LR) over time, defined as a prostate-specific antigen (PSA) increase in conjunction with positive prostate biopsy and/or positive positron emission tomography (PET) scanner findings [Citation1]. In the definitive setting, this proportion ranges from 3 to 10%, the highest percentages being found in the high-risk disease population [Citation2]. To date, several randomized trials showed both improved local and biochemical control with dose-escalation [Citation3–5], with a dose of 76–78 Gy recommended as the standard dose for conventional fractionation. Still, if 78 Gy protocols could achieve a 95.6% and a 99.5% rate of 15-year local and biochemical control in low-risk disease population, these proportions dropped, respectively to 91.3 and 88.7% in high-risk disease patients [Citation6]. Similarly, in the post-operative setting, the development of LR in the prostate bed represents a challenging situation for salvage RT, characterized by high variability in treatment paradigms and an overall poor outcome [Citation7–9]. Treatment intensification with dose-escalated RT or addition of systemic agents, such as androgen deprivation therapy (ADT) has been explored in both the definitive [Citation10,Citation11] and salvage [Citation12] settings to optimize outcomes of patients with unfavorable characteristics. However, despite improvements in irradiation techniques including routine use of intensity-modulated and image-guided RT (IMRT and IGRT), improvements in tumor control are often associated with increased long-term side effects [Citation13], highlighting the need to find strategies to improve the therapeutic ratio.
The combination of hyperthermia (HT) and RT represents an appealing treatment strategy to improve tumor local control without increasing the risk of toxicities to the surrounding healthy tissues. While several studies suggest a synergistic effect between RT and HT, Kok et al. estimated that the addition of HT provides an equivalent delivered dose of 10 Gy higher than RT alone [Citation14]. From a clinical point of view, the addition of HT has been shown to improve local control in many tumor sites [Citation15]. A 10–20% benefit in local control rates has been reported for cervix [Citation16], rectal [Citation17], and bladder cancer [Citation18], translating also in a substantial survival benefit for cervix cancer (3-year overall survival of 51 vs. 27% with or without additional HT, respectively, p = 0.009) [Citation19,Citation20].
Recent technical advances in HT systems make the combination of HT and RT an innovative strategy to improve tumor control and avoid long-term toxicity in the curative treatment of prostate cancer. The aim of the present systematic review is to report evidence supporting the combination of HT and RT for prostate cancer, including future applications and development of this new treatment strategy.
2. Materials and methods
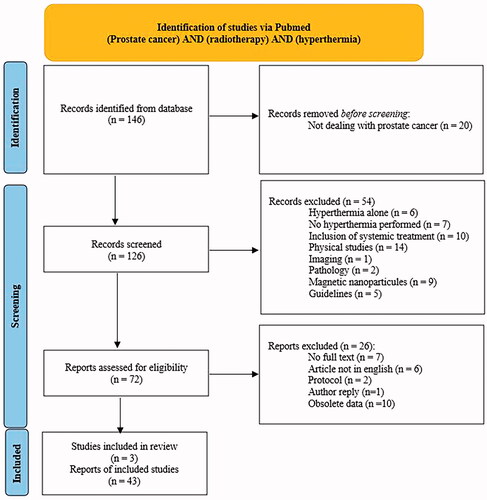
A systematic review was performed using the Pubmed database on 21 July 2021, with the terms (prostate cancer) AND (radiotherapy) AND (hyperthermia). We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement for reporting [Citation21]. After screening of 126 records, 54 were excluded, because they did not directly address the issue of combined RT and HT (). Limitations were placed with respect to publication language (English language was mandatory), year of publication, and full-text availability.
3. Results
3.1. Synergistic mechanisms of HT and RT
From a radiobiological point of view, HT acts as a radiosensitizing agent through distinct mechanisms. The elevation of the temperature (between 41 and 43 °C) to a supraphysiological level generates vascular dilatation and thus an increase in the oxygen supply to tissues, thereby reducing hypoxia and increasing radiosensitivity [Citation22,Citation23]. HT has been proven to enhance the effectiveness of RT by inhibiting the repair processes of DNA damage through inhibition of both base excision repair (BER) [Citation24] and homologous recombination (HR) [Citation25] pathways. The dominant mechanism of HT depends on the temperature level. Enhanced oxygenation is probably the more important mechanism at temperatures around 41 °C, while inhibition of DNA repair may be more significant at temperatures around 43 °C [Citation14]. Combined HT and RT have also demonstrated a direct cell-killing effect, specifically on radioresistant hypoxic tumor cells [Citation26–28], probably related to an accumulation of lactic acid [Citation29]. HT induces the production of heat shock proteins and increases immune cell infiltration, leading to the activation of both innate and adaptive immune cells [Citation30].
3.2. Pre-clinical data
Both in vitro and animal experiments highlight the synergistic tumor-killing effect of HT and RT for prostate cancer.
One of the first in vitro experiments was performed on DU145 prostate cancer cells cultured as spheroids. A higher rate of DNA damage was reported after exposure of cells to an HT session of 43 °C for 90 min, performed with a radiofrequency (RF) capacitive system, followed by a 4 Gy external beam radiation therapy (EBRT) irradiation, as opposed to HT or EBRT only treatments [Citation31]. As a result, a higher percentage of apoptotic cells was observed among cells treated with combined HT and RT (64.48 ± 3.40% vs. 27.70 ± 3.5% without HT), emphasizing the ability of HT to increase sensitivity to RT. Additionally, on the same cell lines, a DNA damage survey demonstrates that combined EBRT (4 Gy) and HT was found to be equally effective as a dose-escalated schedule combining 2 Gy delivered with EBRT with 2.24 Gy brachytherapy (BT) boost [Citation32]. Even in radioresistant cell populations, such as prostate cancer stem cells, the combination of HT and RT has proven its ability in reducing colony survival fractions in comparison with RT alone (up to a factor of 100), indicating that the combined treatment may be a promising approach to enhance the radiation-induced cytotoxic effect [Citation33]. Still, these preclinical models should be considered with caution, as each cell type has a distinct sensitivity to HT (e.g., Dunning R3327 cells being particularly heat resistant [Citation34]), and each cell culture model its own limitations (e.g., spheroid cultures being probably more appropriate than monolayer cultures [Citation35]).
The combined effect of RT and HT was also investigated in athymic nude mice models inoculated with a prostatic carcinoma xenograft. Kaver et al. provided evidence that the combined treatment was the most effective in slowing tumor growth. The median tumor volume doubling time was 35.5 days with combined HT and RT, compared to 18 and 25.5 days for HT or RT alone, respectively [Citation36], confirming at least an additive effect. In another study, Cohen et al. combined HT with a single dose of 12 Gy, in mice transplanted with human prostate cancer cells [Citation37]. HT was delivered by the RF Oncotherm LAB EHY-100 device. The results were consistent with previous experiments, with an additive effect of HT and RT treatments (33.4 days doubling time when treatments were combined, as opposed to 30.4 and 4.5 days with RT and HT alone, respectively). In a rat population transplanted with a prostate anaplastic carcinoma, combined treatments also demonstrated improved therapeutic ratio, with a significant tumor growth delay when multiple HT sessions were delivered before BT (44 days of tumor growth delay when a single HT session was performed before RT compared to 53.7 days when HT was performed both before and after BT) [Citation34].
3.3. Clinical data
3.3.1. Outcomes
Combination of HT and RT has been tested mainly in patients with locally advanced tumors at high risk of LR (PSA > 20 ng/mL, T3–T4 tumors) [Citation38–42] or in patients with an LR either after definitive RT or after radical prostatectomy (RP) [Citation40–44] ().
Table 1. Baseline and treatment characteristics of combined hyperthermia and radiotherapy studies for prostate cancer.
EBRT was the most frequently employed RT modality. Three-dimensional conformal RT (3D-CRT) techniques were used in the majority of the studies using a four-field box technique [Citation39,Citation41,Citation42], while definitive BT was used in three studies [Citation44–47]. Prophylactic lymph node irradiation was in most situations left to the discretion of the clinician, and thus not reported [Citation38,Citation41,Citation42,Citation44,Citation48]. HT was delivered either locally with a transrectal ultrasound [Citation42,Citation48,Citation49], regionally with radiofrequency [Citation38,Citation46], or even interstitially [Citation47].
Deger et al. included in a phase II trial 57 patients diagnosed with localized prostate cancer. Patients were treated with interstitial HT, performed once a week, combined with 3DCRT at the dose of 68.4 Gy in 38 fractions [Citation50]. Cobalt-palladium thermoseeds were placed homogeneously within the prostate gland by a urologist under spinal anesthesia. HT sessions were conducted weekly and performed simultaneously with RT sessions. The 2-year biochemical relapse-free survival (bRFS) rate reached 95%, demonstrating excellent results with respect to the radiation dose delivered, significantly lower than the EBRT doses currently proposed in current guidelines (i.e., total equivalent doses of 78–80 Gy) [Citation1]. These results were confirmed with a longer follow-up, as the median PSA level decreased from 11.6 to 0.5 ng/mL at 48 months. In another study, Hurwitz et al. recruited 37 patients with stage T2b–T3b prostate cancer and a median PSA level of 13 ng/mL [Citation49]. HT treatment was performed using a transrectal ultrasound system. Placement of interstitial temperature probes, for online monitoring, was accomplished via a transperineal route using transrectal ultrasound guidance. The ultrasound power was delivered from a water-cooled 16 element partial-cylindrical intracavitary array. The end point was defined as the attainment of a temperature of 42.0 °C by at least one intra-prostatic temperature sensor for 60 min. Two HT treatments were administered at least one week apart during the first four weeks of a 70 Gy EBRT course. Patients had to receive radiation within 1 h of completion of hyperthermia. Six months of ADT was allowed. The 7-year failure-free survival and overall survival rates reached 61 and 94%, respectively. As an indirect comparison, in a cohort of patients treated for stage T1-T3 prostate cancer, prostate irradiation at a dose of 70 Gy resulted in a 6-year control rate of 43% only [Citation5]. The 2-year disease-free survival benefit of this combined treatment was estimated at 20%, with respect to the short-term ADT arm of the RTOG 92-02 trial (84 vs. 64%).
In another study, Anscher et al. included 12 prostate cancer patients with locally advanced disease treated with definitive RT (doses ranging between 65 and 70 Gy) and HT sessions performed once or twice a week with the goal of delivering at least 42.5 °C to the prostate gland. All HT sessions followed the irradiation and were delivered using a Sigma 60 annular phased array (APA) microwave device (82 MHz). Although the desired tumor temperature was obtained in only 3.5% of the HT sessions, mostly due to pain experienced during HT, the patients achieved 36-months local control and disease-free survival rates of 93 and 68%, respectively, higher than an expected local control rate with RT alone of <50% [Citation38]. Van Vulpen et al. also reported the outcomes of patients diagnosed with locally advanced prostate cancer (defined as T3, T4, Nx/0, M0 tumors) [Citation39,Citation40]. HT was delivered weekly, using either a regional or interstitial technique, and a total dose of 70 Gy was delivered to the prostate gland. Regional HT was delivered with the coaxial transverse electrical magnetic (TEM) system and the interstitial HT treatment was delivered with the 27 MHz multi-electrode current source interstitial HT technique (MECS-IHT). Despite the absence of ADT and prophylactic lymph node irradiation, clinical outcomes compared favorably with most published series, with a 70% bRFS rate at 36 months. Yahara et al. explored the combination of RT and HT in a population of patients diagnosed with high- or very high-risk prostate cancer (Gleason score 8–10, PSA > 20 ng/mL, T3b–T4 tumors) [Citation41]. Regional HT was applied after the RT sessions, once or twice a week, using a Thermotron RF-8 system (Yamamoto Vinita, Osaka, Japan). The outcomes after combined treatment were retrospectively compared with a population of patients with the same initial characteristics, treated with RT alone, and suggested a benefit in 3-year bRFS with the addition of HT (78 vs. 72%, p = 0.3).
Kalapurakal et al. reported the outcomes of 13 patients diagnosed with either locally advanced hormone-refractory or locally recurrent prostate cancer [Citation44,Citation45]. Patients received 66.6 Gy in fractions of 1.8 Gy as definitive treatment, and 39.6 Gy in fractions of 1.8 Gy in case of re-irradiation. HT was performed twice weekly, at least 72 h apart, and ∼1 h after the RT session. A radiofrequency BSD-2000 Sigma-60 applicator was used, allowing the temperature delivered to the prostate gland to increase stepwise, as long as the patient could tolerate it. The median progression-free survival (PFS) was 12 months (4–27 months), while the observed local control rate was 77% (only three local failures were observed). Results were encouraging in this population, considering that rectal and bladder invasion was present in 46 and 62% of cases, respectively. Tilly et al. reported the outcomes of 22 patients, treated to 68.4 Gy in conventional fractionation in combination with weekly regional HT, performed either before or after RT sessions [Citation46]. Of these, 15 patients were diagnosed with primary prostate cancer and 7 were diagnosed with an LR after primary RP. With a 6-year follow-up, a 60% bRFS rate was observed for patients treated in the primary setting, while the corresponding rate in patients treated with salvage intent decreased to 43%. Kaplan et al. analyzed six prostate cancer patients with an LR after a 125Iodine BT implant, treated with split-course RT at a total dose of 60 Gy with or without HT [Citation51]. An APA system was used to deliver regional HT. Three out of four patients treated with this multimodal modality were considered disease-free at the last follow-up, while one patient died of a metastatic progression.
3.3.2. Toxicity
Published studies report relatively low rates of treatment-related toxicities when combining HT and EBRT. Grade 2 acute genito-urinary (GU) toxicity rates ranged from 0% [Citation44,Citation45,Citation52] to 55% [Citation46], while grade 3 acute GU toxicities were reported in only three studies [Citation41,Citation42,Citation46]. In the primary setting, this proportion remained anecdotic, ranging from 1% [Citation41] to 4% [Citation42]. In the case of re-irradiation, this incidence reached an 18% rate [Citation46]. Grade 2 acute gastrointestinal (GI) toxicity was more frequently reported, reaching a 48% rate in the study conducted by Anscher et al., mostly consisting of diarrhea [Citation38]. Grade 3 acute GI effects were only observed in patients undergoing re-irradiation for an LR, with a 14% rate of toxicity reported in the study by Van Vulpen et al. [Citation39]. In a study by Yahara et al. no difference was observed in the occurrence of acute GU or GI grade ≥2 toxicities between patients treated with or without regional HT treatment [Citation41]. As long-term toxicity is concerned, no late grade ≥3 toxicity was reported. Only two cases of grade 4 late toxicity were observed in two patients in the salvage setting (hemorrhagic cystitis in a patient with factor XI deficiency and chemotherapy for lymphoma, and rectovesical fistula after disappearance of a large, necrotic tumor, in another patient) [Citation40]. Specific HT toxicity, consisting of skin burn or pain, was inconsistently reported across studies. Tilly et al. reported a 68% and a 9% rate of acute grade 1 and grade 2 skin toxicity, respectively [Citation46]. Yahara et al. reported similar results, with grade 2 skin burn occurring in 6% of the patients [Citation41]. Symptoms presented as a subcutaneous induration, resolving spontaneously after the completion of HT [Citation41]. Within the study led by Kalapurakal et al., no patient developed HT-induced skin burns [Citation45].
Kukielka et al. reported toxicity outcomes after combined interstitial HT and BT [Citation47]. In a heterogeneous study population, the authors included patients treated with HT and exclusive BT (45 Gy in three fractions for low-risk patients) or EBRT with a BT boost (50 Gy + 21 Gy in three fractions). Other patients with a radio-recurrent relapse were treated with 30 Gy in three fractions and HT. Early toxicity profiles showed the safety of the combined approach with HT performed before BT, with a toxicity profile similar to patients treated with exclusive BT. The most frequent GU toxicity consisted in urinary frequency (27%) and was mostly reported in patients treated with BT and HT in the primary definitive setting (67%). No grade 3 urinary toxicities were observed, even in the population of patients being re-irradiated after a previous EBRT course. Additionally, no early rectal complications were observed.
The role of HT combined with EBRT was also evaluated in the HT-Prostate trial (NCT0415905) for patients in biochemical recurrence after RP [Citation53]. In this study, 7–10 sessions of HT were performed in combination with RT at a dose of 70 Gy to the prostate bed. The interim analysis of this trial reported relatively low acute toxicity rates, with a 10 and 4% rate of acute grade 2 GU and GI toxicities, respectively. Forty-two percent of patients experienced acute grade 1 HT-specific toxicity, which consisted mostly of hotspots and skin pain.
3.4. Thermal parameters and technical challenges
Within this review, most treatments were performed using electromagnetic heating, through the use of RF devices [Citation54]. While the majority of studies preferred regional HT (radiative with APA, or capacitive), a few studies preferred a local approach either through interstitial HT (usually coupled with an interstitial BT technique) or transrectal/transurethral ultrasound techniques. APA devices, which consist in positioning multiples antennas around the patient, have been widely used for deep HT treatments of pelvic tumors. This approach brings the great advantages of both remaining non-invasive and providing accurate tumor heating while avoiding normal tissue hotspots. Capacitive HT is another regional approach using RF. Still, it carries the disadvantage of preferentially heating fat subcutaneous tissues, thus it is considered less suitable for Caucasian prostate cancer patients. Application of ultrasound heating for prostate cancer patients can be considered challenging due to near- and far-field risks of thermal build-up. Careful adjustment of acoustic parameters is required. Ideally, multi-planar or 3D temperature monitoring should be available online. Only three studies reported outcomes with ultrasound heating, through a transrectal approach [Citation42,Citation48,Citation49]. Another clinical pilot study reported 3 D-controlled HT with catheter-based ultrasound applicators in conjunction with high-dose-rate (HDR) BT [Citation55].
Some trials have shown the correlation between thermal parameters and clinical outcomes. A higher bRFS was found among patients receiving HT over 43 °C for 1 min, with an odds ratio at 4.4 (1.15–16.67, 0.03) [Citation41]. Tilly et al. additionally suggested that Tmax was predictive of PSA control, with a cutoff of 41.2 °C [Citation46], while Emami et al. deemed a 41.5 °C temperature target as satisfactory [Citation56]. Higher thermal parameters were also found to be associated with an improved bRFS in the study led by Yahara et al. [Citation41]. Still, the optimal thermal doses to the prostate carcinoma required for an efficient combination treatment are not exactly known.
Kok et al. estimated that the addition of HT delivered once or twice weekly provide an equivalent delivered dose of 10 Gy higher than RT alone [Citation14]. In the present study, we reported studies using different RT-HT timings, HT being performed before, during, or after RT. While the rationale for performing HT before RT includes increasing tumor oxygenation through vascular dilation, paradoxical effects with increased hypoxia have also been reported at higher temperatures, due to vascular damage. Additionally, some studies demonstrated in animal models increased normal tissue toxicity when HT was performed before RT [Citation57]. As the best results in terms of radiosensitization were found with simultaneous HT and RT [Citation58], this sequence could be favored, still technical realization remains challenging. As to date, no consensus has been reached on combined HT and RT, careful planning and monitoring of heating are advised whatever the sequence used.
Technical challenges exist with regards to treatment delivery, as temperature goals are difficult to be achieved on the prostate gland. In the RF approach using an APA device, disappointing results were published by Anscher et al., as temperature targets were reached in only 3.6% of the patients [Citation38]. Despite the increased conformality for local treatments and the potential to achieve higher temperatures within the prostate gland, even with a TRUS approach, only 36% of the patients reached the temperature target of 42.5 °C in the study led by Fosmire et al. [Citation48]. Recent developments in ultrasound HT, such as magnetic resonance (MR)-guided focused ultrasound hold the potential to improve the therapeutic ratio, by delivering uniform isotherms inside the target, allowing at the same time a greater sparing of surrounding healthy tissues [Citation59].
Lastly, the comparability between studies of HT-induced prostate temperatures is limited by several factors. A great disparity exists in temperature monitoring between studies, which could be performed either with urethral [Citation39,Citation40,Citation46,Citation50], rectal [Citation41,Citation47,Citation49], or bladder probes [Citation52]. Additionally, evaluation of thermal parameters in prostate HT remains hampered by a lack of information about both vasculature and perfusion [Citation60], which can lead to an overestimation of the prostate temperature of 1–2°. As invasive thermometry remains the clinical gold standard technique, MR-based thermometry holds the potential to improve treatment outcomes, by providing full 3D temperature distribution registered with anatomical imaging [Citation61]. Still, proton resonance frequency shift (PRFS) MR thermometry has the major drawback to be highly sensitive to tissue motion, which can be limiting for organs, such as the prostate gland. Indeed, it is prone to non-periodic motion originating from the neighboring rectal wall, due to the presence of moving gas pockets [Citation62], gradual bladder filling, or cough. This matter is being extensively investigated in the field of prostate RT (prostate intrafraction motion is estimated to be around 3 mm in both infero-superior and antero-posterior axis [Citation63,Citation64]), data are still lacking for considerably longer HT sessions. Good accuracy of MR thermometry was obtained in prostate thermotherapy using the TUSLA system from Profound Medical (Mississauga, Canada), over sessions of 11–52 min [Citation65]. Although MR thermometry was performed to monitor ablative temperatures (above 57 °C), it allowed to obtain temperature radii around the prostate down to 37 °C, as part of a safety strategy. To date, the system was used in 224 patients [Citation66]. However, the setup carries the drawback of being invasive, as it includes a rigid urethral applicator of ultrasound to stabilize the prostate gland. Overall, using appropriate parameters for PRFS MR data acquisition and post-processing filtering, a global precision of thermometry near to 1 °C was obtained. Dynamic MRI thermometry has been evaluated by different teams, for abdominal organs, with satisfactory results [Citation67–71].
4. Discussion
Strategies able to enhance the therapeutic ratio for prostate cancer patients with locally advanced disease or recurrent tumors treated with curative RT are eagerly required.
In men with high-risk or local advanced prostate cancer, dose-escalation performed either on the whole prostate using EBRT [Citation3,Citation5,Citation72] and/or BT [Citation73] techniques, or with a focal boosting on the dominant intraprostatic lesion [Citation74], represents one of the mostly investigated strategies used to improve tumor control. Improvements in bRFS rates have been demonstrated with dose escalation, although this benefit has often been counterbalanced by an increase in GU or GI toxicity rates [Citation48,Citation58]. In the specific situation of locally-advanced tumors, definitive RT combined with long-term ADT remains the cornerstone treatment [Citation75], providing a 5-year RFS of 74% in historical trials [Citation76]. The addition of HT to conventional hormone-RT may represent a valuable alternative in this setting. In a population of patients diagnosed mostly with locally-advanced tumors (61% rate of T3-T4 tumors), Yahara et al. reported an improved disease control in both bRFS and cancer-specific survival at 5-year with the addition of HT [Citation41]. Based on the promising results of studies presented in , prospective trials exploring the role of combined HT and RT vs. RT alone are eagerly awaited in this population of patients.
Non-surgical salvage local therapies offer a chance of a curative local approach in radiorecurrent prostate cancer. Re-irradiation, either performed with EBRT or BT, has been associated with approximately a 50% bRFS at 5-year [Citation77], sometimes at the cost of long-term severe radiation-induced toxicities [Citation78]. Although literature data are lacking, the combination of HT and RT holds the potential to improve disease control without the need to escalate the RT dose, often limited in these situations by the risk of inducing severe toxicities to the nearby organs. The ongoing HETERERO trial (NCT04889742) will probably provide in the next future new insights on the role of this treatment combination. Additionally, the combination of HT with BT is also currently being evaluated in the salvage setting, within two ongoing single-arms prospective trials (NCT02899221 and NCT03238066) ().
Table 2. Ongoing clinical trials of combined hyperthermia and radiotherapy for prostate cancer.
Prostate bed RT with or without ADT is the main treatment for patients with a biochemical recurrence after RP. The combination of HT with RT offers in this setting an appealing treatment alternative to ensure durable local control without increasing the radiation-induced toxicity expected with dose escalation. As interim results have been published, the HT-Prostate phase II trial (NCT0415905) is still recruiting patients with biochemical relapse after radical prostatectomy, with a primary endpoint of acute tolerance [Citation53]. As prostate bed RT is associated with high rates of GU toxicities, both in the adjuvant and salvage setting (54% and 70% of grade ≥2 toxicity reported within the recently published ANZUP RAVES trial [Citation79]), the combination of HT with RT may also represent a compelling strategy to de-escalate the RT dose while assuring the same bRFS rate of standard prostate bed RT doses. Similarly, the presence of a macroscopic LR within the prostate bed may represent another clinical situation for which the long-term local control can potentially be enhanced by this combined strategy.
The limitations of this systematic review include the retrospective and non-randomized nature of the reported studies, as well as their time of publication. Indeed, many of the studies performed on prostate cancers were conducted in the 1990s, and most of the RT treatments were performed with 2D or 3DCRT techniques, at doses lower than our current standards. All these biases thus could have negatively affected the external validity of these studies. Moreover, the lack of data on ADT use and duration in many studies may have hampered our conclusions on long-term disease control of this combined strategy.
5. Conclusions
Assumed the expected dose-benefit of 10 or more Gy, the addition of HT to RT represents a promising treatment strategy to improve outcomes without increasing treatment-related toxicities of prostate cancer patients treated with curative RT. Patients at higher risk of LR, including those with high- or very high risk disease, with macroscopic LR after RP, or with radiorecurrent tumors, represent probably the optimal candidates for this combined treatment. By implementing new technological developments both in HT and RT delivery techniques, results of prospective randomized trials are awaited to define the role of this treatment strategy in the therapeutic management of prostate cancer.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Agarwal P, Sadetsky N, Konety B, et al. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112(2):307–314.
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473.
- Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239.
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105.
- Pasalic D, Kuban DA, Allen PK, et al. Dose escalation for prostate adenocarcinoma: a long-term update on the outcomes of a phase 3, single institution randomized clinical trial. Int J Radiat Oncol Biol Phys. 2019;104(4):790–797.
- Dal Pra A, Panje C, Zilli T, et al. Salvage radiotherapy for macroscopic local recurrences after radical prostatectomy: a national survey on patterns of practice. Strahlenther Onkol. 2018;194(1):9–16.
- Zilli T, Jorcano S, Peguret N, et al. Results of dose-adapted salvage radiotherapy after radical prostatectomy based on an endorectal MRI target definition model. Am J Clin Oncol. 2017;40(2):194–199.
- Le Guevelou J, Achard V, Mainta I, et al. PET/CT-Based salvage radiotherapy for recurrent prostate cancer after radical prostatectomy: Impact on treatment management and future directions. Front Oncol. 2021;11:742093.
- Weg E, Pei X, Kollmeier M, et al. Dose-escalated intensity modulated radiation therapy for prostate cancer: 15-year outcomes data. Adv Radiat Oncol. 2019;4(3):492–499.
- Bolla M, Verry C, Long J. High-risk prostate cancer: combination of high-dose, high-precision radiotherapy and androgen deprivation therapy. Curr Opin Urol. 2013;23(4):349–354.
- Ghadjar P, Hayoz S, Bernhard J, et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: the SAKK 09/10 randomized phase 3 trial. Eur Urol. 2021;80(3):306–315.
- Hanks G, Pajak T, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the radiation therapy oncology group protocol 92-02. J Clin Oncol. 2003;21(21):3972–3978.
- Kok H, Crezee J, Franken N, et al. Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int J Radiat Oncol Biol Phys. 2014;88(3):739–745.
- Datta N, Ordóñez S, Gaipl U, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753.
- Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev. 2010;2010(3):CD006377.
- Ohno S, Sumiyoshi Y, Mori M, et al. Hyperthermia for rectal cancer. Surgery. 2002;131(1 Suppl):S121–S127.
- Snider J, Datta N, Vujaskovic Z. Hyperthermia and radiotherapy in bladder cancer. Int J Hyperth. 2016;32(4):398–406.
- van der Zee J, González González D, van Rhoon G, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355(9210):1119–1125.
- Yea J, Park J, Oh S, et al. Chemoradiotherapy with hyperthermia versus chemoradiotherapy alone in locally advanced cervical cancer: a systematic review and Meta-analysis. Int J Hyperthermia. 2021;38(1):1333–1340.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
- Winslow TB, Eranki A, Ullas S, et al. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperth. 2015;31(6):693–701.
- Sun X, Xing L, Ling C, et al. The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: relevance for radiotherapy, vascular targeting and imaging. Int J Hyperth. 2010;26(3):224–231.
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77(4):399–408.
- Krawczyk P, Eppink B, Essers J, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108(24):9851–9856.
- Horsman M, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 2007;19(6):418–426.
- Overgaard J. The heat is (still) on-the past and future of hyperthermic radiation oncology. Radiother Oncol J Oncol. 2013;109:185–187.
- Nielsen OS. Effect of fractionated hyperthermia on hypoxic cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;39(1):73–82.
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977;123(2):511–514.
- Lee S, Son B, Park G, et al. Immunogenic effect of hyperthermia on enhancing radiotherapeutic efficacy. IJMS. 2018;19(9):2795.
- Janati Esfahani A, Mahdavi S, Shiran M, et al. The role of radiofrequency hyperthermia in the radiosensitization of a human prostate cancer cell line. Cell J Spring. 2017;19(Suppl 1):86–95.
- Mahdavi S, Janati Esfahani A, Khoei S, et al. Capacitive hyperthermia as an alternative to brachytherapy in DNA damages of human prostate cancer cell line (DU-145). Int J Radiat Biol. 2019;95(2):193–200.
- Rajaee Z, Khoei S, Mahdavi S, et al. Evaluation of the effect of hyperthermia and electron radiation on prostate cancer stem cells. Radiat Environ Biophys. 2018;57(2):133–142.
- Peschke P, Hahn E, Wolber G, et al. Interstitial radiation and hyperthermia in the dunning R3327 prostate tumour model: therapeutic efficacy depends on radiation dose-rate, sequence and frequency of heating. Int J Radiat Biol. 1996;70(5):609–616.
- Larsen C. [Spheroids: a reference model for in vitro culture of solid tumors?]. Bull Cancer. 2018;105(1):25–34.
- Kaver I, Koontz W, Wilson J, et al. The effect of radiation therapy and hyperthermia on a human prostatic carcinoma cell line grown in athymic nude mice. J Urol. 1991;145(3):654–656.
- Cohen J, Anvari A, Samanta S, et al. Mild hyperthermia as a localized radiosensitizer for deep-seated tumors: investigation in an orthotopic prostate cancer model in mice. Br J Radiol. 2019;92(1095):20180759.
- Anscher M, Samulski T, Leopold K, et al. Phase I/II study of external radio frequency phased array hyperthermia and external beam radiotherapy in the treatment of prostate cancer: technique and results of intraprostatic temperature measurements. Int J Radiat Oncol Biol Phys. 1992;24(3):489–495.
- Van Vulpen M, De Leeuw A, Raaymakers B, et al. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: preliminary results. BJU Int. 2004;93(1):36–41.
- Van Vulpen M, De Leeuw JRJ, Van Gellekom MPR, et al. A prospective quality of life study in patients with locally advanced prostate cancer, treated with radiotherapy with or without regional or interstitial hyperthermia. Int J Hyperthermia. 2003;19(4):402–413.
- Yahara K, Ohguri T, Yamaguchi S, et al. Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: thermal parameters correlated with biochemical relapse-free survival. Int J Hyperth. 2015;31(6):600–608.
- Algan O, Fosmire H, Hynynen K, et al. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer. 2000;89(2):399–403.
- Prionas S, Kapp D, Goffinet D, et al. Thermometry of interstitial hyperthermia given as an adjuvant to brachytherapy for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1994;28(1):151–162.
- Kalapurakal J, Mittal B, Sathiaseelan V. Re-irradiation and external hyperthermia in locally advanced, radiation recurrent, hormone refractory prostate cancer: a preliminary report. Br J Radiol. 2001;74(884):745–751.
- Kalapurakal J, Pierce M, Chen A, et al. Efficacy of irradiation and external hyperthermia in locally advanced, hormone-refractory or radiation recurrent prostate cancer: a preliminary report. Int J Radiat Oncol Biol Phys. 2003;57(3):654–664.
- Tilly W, Gellermann J, Graf R, et al. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol. 2005;181(1):35–41.
- Kukiełka A, Hetnał M, Brandys P, et al. Interstitial hyperthermia of the prostate in combination with brachytherapy: an evaluation of feasibility and early tolerance. Strahlenther Onkol. 2013;189(6):467–475.
- Fosmire H, Hynynen K, Drach G, et al. Feasibility and toxicity of transrectal ultrasound hyperthermia in the treatment of locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1993;26(2):253–259.
- Hurwitz M, Hansen J, Prokopios-Davos S, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber cancer institute study 94-153. Cancer. 2011;117(3):510–516.
- Deger S, Boehmer D, Türk I, et al. Interstitial hyperthermia using self-regulating thermoseeds combined with conformal radiation therapy. Eur Urol. 2002;42(2):147–153.
- Kaplan I, Kapp D, Bagshaw M. Secondary external-beam radiotherapy and hyperthermia for local recurrence after 125-iodine implantation in adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1991;20(3):551–554.
- Maluta S, Dall'Oglio S, Romano M, et al. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: preliminary results of a prospective phase II study. Int J Hyperthermia. 2007;23(5):451–456.
- Beck M, Ghadjar P, Mehrhof F, et al. Salvage-Radiation therapy and regional hyperthermia for biochemically recurrent prostate cancer after radical prostatectomy (results of the planned interim analysis). Cancers Basel. 2021;13(5):1133.
- Kok HP, Cressman ENK, Ceelen W, et al. Heating technology for malignant tumors: a review. Int J Hyperthermia. 2020;37(1):711–741.
- Diederich CJ, Wootton J, Prakash P, et al. Catheter-based ultrasound hyperthermia with HDR brachytherapy for treatment of locally advanced cancer of the prostate and cervix. Proc SPIE Int Soc Opt Eng. 2011;7901:79010O.
- Emami B, Scott C, Perez C, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the radiation therapy group. Int J Radiat Oncol Biol Phys. 1996;34(5):1097–1104.
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980;6(11):1507–1517.
- Overgaard J. Fractionated radiation and hyperthermia: experimental and clinical studies. Cancer. 1981;48(5):1116–1123.
- Guillemin P, Gui L, Lorton O, et al. Mild hyperthermia by MR-guided focused ultrasound in an ex vivo model of osteolytic bone tumour: optimization of the spatio-temporal control of the delivered temperature. J Transl Med. 2019;17(1):350.
- Van den Berg C, Van de Kamer J, De Leeuw A, et al. Towards patient specific thermal modelling of the prostate. Phys Med Biol. 2006;51(4):809–825.
- Feddersen T, Hernandez-Tamames J, Franckena M, et al. Clinical performance and future potential of magnetic resonance thermometry in hyperthermia. Cancers Basel. 2020;13(1):31.
- Schmitt A, Mougenot C, Chopra R. Spatiotemporal filtering of MR-temperature artifacts arising from bowel motion during transurethral MR-HIFU. Med Phys. 2014;41(11):113302.
- de Muinck Keizer DM, Pathmanathan AU, Andreychenko A, et al. Fiducial marker based intra-fraction motion assessment on cine-MR for MR-linac treatment of prostate cancer. Phys Med Biol. 2019;64(7):07NT02.
- Jaccard M, Ehrbar S, Miralbell R, et al. Single-fraction prostate stereotactic body radiotherapy: dose reconstruction with electromagnetic intrafraction motion tracking. Radiother Oncol. 2021;156:145–152.
- Anttinen M, Mäkelä P, Suomi V, et al. Feasibility of MRI-guided transurethral ultrasound for lesion-targeted ablation of prostate cancer. Scand J Urol. 2019;53(5):295–302.
- Dora C, Clarke GM, Frey G, et al. MRI-guided transurethral ultrasound ablation of prostate cancer: a systematic review. J Endourol. 2022.
- de Senneville BD, Ries M, Maclair G, et al. MR-guided thermotherapy of abdominal organs using a robust PCA-based motion descriptor. IEEE Trans Med Imaging. 2011;30(11):1987–1995.
- de Senneville BD, El Hamidi A, Moonen C. A direct PCA-based approach for real-time description of physiological organ deformations. IEEE Trans Med Imaging. 2015;34(4):974–982.
- Salomir R, Viallon M, Kickhefel A, et al. Reference-free PRFS MR-thermometry using near-harmonic 2-D reconstruction of the background phase. IEEE Trans Med Imaging. 2012;31(2):287–301.
- Kickhefel A, Rosenberg C, Roland J, et al. A pilot study for clinical feasibility of the near-harmonic 2D referenceless PRFS thermometry in liver under free breathing using MR-guided LITT ablation data. Int J Hyperthermia. 2012;28(3):250–266.
- Zou C, Tie C, Pan M, et al. Referenceless MR thermometry-a comparison of five methods. Phys Med Biol. 2017;62(1):1–16.
- Heemsbergen W, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110(1):104–109.
- Morris W, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285.
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39(7):787–796.
- Moris L, Cumberbatch MG, Van den Broeck T, et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an international multidisciplinary systematic review. Eur Urol. 2020;77(5):614–627.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106.
- Ingrosso G, Becherini C, Lancia A, et al. Nonsurgical salvage local therapies for radiorecurrent prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2020;3(2):183–197.
- Valle LF, Lehrer EJ, Markovic D, et al. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER). Eur Urol. 2021;80(3):280–292.
- Kneebone A, Fraser-Browne C, Duchesne G, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21(10):1331–1340.