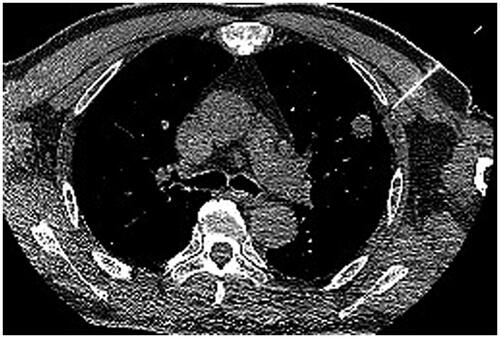
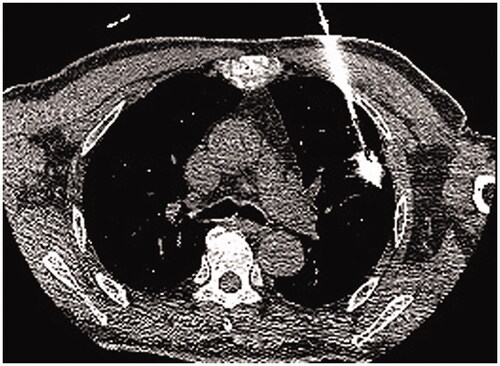
Abstract
Objective
To assess the effect and safety of subpleural multisite anesthesia based on the area of thermal radiation during CT-guided lung malignancy microwave ablation (MWA) on the incidence of moderate or severe pain and the analgesic drug usage.
Materials and methods
Consecutive patients with lung malignancies were retrospectively evaluated between January 2016 and December 2019. Patients undergoing CT-guided lung malignancy MWA were either given in the method of (a) standard subpleural puncture point anesthesia between January 2016 and June 2018 and (b) subpleural multisite anesthesia based on the area of thermal radiation between July 2018 and December 2019. The relationship between local anesthesia mode and moderate or severe pain, and pain medications usage was assessed by using multivariable logistic regression models.
Results
A total of 243 consecutive patients were included in the study. Moderate or severe pain occurred in 84 of 124 (67.7%) patients with subpleural puncture point anesthesia and in 20 of 119 (16.8%) patients with subpleural anesthesia in the area of thermal radiation (p=.001). The intravenous pain medication was required in 56 of 124 (45.2%) patients with subpleural puncture point anesthesia and in 9 of 119 (7.6%) patients with subpleural multisite anesthesia based on the area of thermal radiation (p=.001). Local anesthesia methods (p = 0.001), pleura-to-lesion distance (p=.02) and tumor size (p=.015) were independent risk factors for developing moderate or severe pain. There were no differences in adverse events and local tumor progression rate.
Conclusions
Subpleural multisite anesthesia based on the area of thermal radiation for peripheral lung malignancy MWA can result in lower intraprocedural pain compared with the subpleural puncture point anesthesia. Thus, a subpleural multisite anesthesia technique may be most helpful when performing MWA of peripheral malignancy in patients who are not sedated with general or intravenous anesthesia.
Introduction
Percutaneous CT-guided transthoracic lung microwave ablation (MWA) is an established treatment technique for unresectable or medically inoperable lung malignancies. It provides a promising local tumor control rate and a good safety profile, and has the advantages of being minimally invasive, having only mildly deleterious effects on pulmonary function, needing only a short convalescence, and allowing repeated procedures [Citation1–5]. Pain experienced by patients during thermal ablation of lung cancer is an important concern [Citation6,Citation7]. If intravenous anesthesia or analgesic drugs were not accepted during the procedure, intraoperative pain may oblige operators to alter the ablation algorithm (e.g., decrease microwave power and ablation time) to reduce pain. Such altered algorithms may affect the ablation zone and, ultimately, local efficacy. Intraprocedural pain can be more prominent when treating tumors located close to the pleura [Citation8]. The parietal pleura and chest wall are sensitive to pain because abundant sensory nerve branches originate from the intercostal nerve, contrary to the case in the visceral pleura and lung parenchyma.
The development of intraoperative pain during the MWA results in a more technically challenging procedure, and quick-acting analgesic should necessitate hospital admission. Various technical approaches have been published to reduce the incidence of pain, with a focus on reduction of the pleural stimulation. In 2005, Yasui et al. found that epidural anesthesia could not reduce the incidence of severe pain during ablation [Citation9]. In a review of the literature, general anesthesia was found to be used for thermal ablation in approximately half of the centers involved in the studies reviewed. General anesthesia could be associated with various complications and additional expenditure [Citation10]. A number of subsequent studies have reported variable results on the effects of artificial pneumothorax during ablation [Citation11,Citation12]. Artificial pneumothorax is demonstrated to thermal ablation for relieving chest pain and protecting the mediastina and the chest wall [Citation13]; however, studies using this technique may not achieve the satisfactory safety [Citation14–19]. Artificial pneumothorax need compress the pulmonary parenchyma and decrease alveolar air. Decreased alveolar air can alter electrical and thermal conductivity, affecting the ablation zone. Patients with pleural adhesions occurring after pulmonary surgery or lung cancer radiotherapy are also not suitable for this therapy.
Subpleural puncture point anesthesia is a standard local anesthesia technique for treatment of lung malignancy ablation. An alternative approach might be to assess the subpleural area of thermal radiation before the procedure and to increase the extent of subpleural multisite anesthesia. Subpleural multisite anesthesia based on the area of thermal radiation during the procedure may maintain the normal anatomical structure of lung tissue and thorax, thus ensuring the safety of ablation therapy and the accuracy of ablation range. We hypothesized that performing subpleural multisite anesthesia based on the area of thermal radiation would reduce the incidence of pain (including intraoperative intravenous pain medication).
The purpose of this study was to assess the effect and safety of performing subpleural multisite anesthesia based on the area of thermal radiation in reducing moderate or severe pain and pain medication usage associated with peripheral lung malignancy MWA.
Materials and methods
Study population
This study was approved by the institutional ethics committee and all patients provided written informed consent. The therapeutic indications, effectiveness and risk of death were all explained to the patients and their guardians. Data from patients undergoing subpleural anesthesia and CT-guided MWA for primary and secondary lung tumors between January 2016 and December 2019 were prospectively collected. Patients included were those with lung tumors who refused or were considered unable to tolerate surgical resection and radiotherapy by a multidisciplinary team comprising thoracic surgeons, radiation therapists, medical oncologists and interventional radiologists.
Lung malignancies adjacent to the pleura were defined as index tumors at any distance within 1.0 cm from the pleura based on CT scans. The following specific inclusion criteria were considered: (1) lung malignancies adjacent to the plerua; (2) a single tumor (≤3.0 cm); (3) platelet count ≥50 cells × 109/L; (4) prothrombin time ratio ≥70%; (5) Eastern Cooperative Oncology Group (ECOG) performance status score ≤2; (6) forced expiratory volume at 1 s (FEV1) ≥50% predicted; and (7) written informed consent for MWA given. The exclusion criteria were history of uncontrolled angina, arrhythmias, congestive heart failure or implantable cardiac devices and uncontrolled malignant pleural effusion. Finally, 243 patients (124 men and 119 women; mean age 72.8 years; range 46–89 years) were included in the study. The baseline characteristics of patients and tumor characteristics are summarized in . Between January 2016 and June 2018, 124 patients underwent subpleural puncture point anesthesia before MWA. Between July 2018 and December 2019, 119 patients underwent subpleural multisite anesthesia based on the area of thermal radiation before MWA.
Table 1. Patients demographic.
Subpleural anesthesia technique
All treatments were performed under CT guidance with 5-mm collimation at 10–50 mA. Spontaneous breathing of patients was maintained via nasal prongs airway (low-flow-rate oxygen inhalation 3–6 L/min) throughout the procedure. Several parameters, including the baseline cardiac rate, rhythm and blood pressure, were recorded before ablation. Electrocardiography, blood pressure and blood oxygen saturation were monitored throughout the procedure, and for at least 6 h after returning to the ward, or until return to normal. Preoperative localization was confirmed by observation of CT images and the scope of CT scan need be narrowed to cover the target lesion. Two attending radiologists with 10 and 8 years of experience in CT-guided lung thermal ablation performed all procedures. A total of 119 patients were treated with subpleural multisite anesthesia based on the area of thermal radiation. The subpleural area and range of thermal radiation were determined by CT localization. The puncture route was planned through CT scanning and thermal area, and the skin puncture point, pleural puncture point, the angle of puncture needle and the distance between skin and pleural puncture point were determined. The skin, subcutaneous tissues and puncture point of parietal pleura were anesthetized layer by layer with 1% lidocaine. When the tip of needle was located in the parietal subpleura and does not break through the parietal pleura, lidocaine was injected under continuous positive pressure to form a subcutaneous uplift into the pleural cavity. The tip slowly breaks through the parietal pleura and enters the subpleural space. A tracer (lidocaine mixed with contrast agent; 10:1) was injected into the pleural space to determine the extent to which the anesthetic covered the subpleural heat area. Multiple overlapping anesthesia were used for the cover of thermal area. A total of 124 patients were treated with subpleural puncture point anesthesia. The skin, subcutaneous tissues and puncture point of parietal pleura were anesthetized layer by layer with 1% lidocaine.
MWA procedure
After successful local anesthetization, the antenna was positioned into the deepest margin of the lesion according to the preoperative‐planned route. The antenna routes were discussed and selected in a way that avoids the intercostal artery, pulmonary bullae. The electrodes were advanced into the tumor along with the planned approach, and adjustments made to correct the path toward the targeted ablation zone if the electrode went off course; care was taken to ensure that the electrode did not pull out lung tissue during readjustment, minimizing the number of lung membrane or lobar fissure repeat punctures. CT scan was used to confirm whether the antenna was properly positioned. MWA could be carried out after connecting the cold circulating pipes and pumps, linking the MWA antenna and MWA machine with a cable, turning on the ablation power in accordance with the preset conditions (generally selected 40–60 W, 6–8 min). CT was routinely performed immediately after the ablation to evaluate technical success and examine possible complications. If viable residual tumors were identified, the ablation zone was re-ablated as previously described. After the ablation procedure, all patients were admitted for overnight observation, according to the routine care standards of our institution. Slight protuberance of subpleural tissue into the chest shown under a CT image was used as a criterion for evaluating degree and area of subpleural drug infiltration. If intolerant pain persisted or worsen despite the subpleural lidocaine injected, intravenous pain medications was administered intravenously. Intraoperative local anesthesia is lidocaine, which has a rapid effect but a short time of action. Postoperative pain management for patients is mainly tramadol injection subcutaneously and/or intravenous infusion of flurbiprofen axetil injection. Intraoperative local anesthesia is not effective, we first consider to increase the dose or expand the subpleural area of anesthesia, if the pain is still not effective, we gave intramuscular tramadol or demerol. The variety and dosage of painkillers, including tramadol (100 mg intramuscular injection), meperidine hydrochloride (100 mg intramuscular injection) and morpholine (15 mg hypodermic injection) and flurbiprofen axetil (50 mg bid continuous intravenous infusion).
Data collection
We recorded patient demographics, treatment positioning, lesion size, lesion location, the distance between skin and pleural puncture point, dosage of local anesthetic, visual analog scale (VAS) of pain, analgesic drug usage and name of performing radiologist. Adverse events that were recorded included the incidence of hemoptysis, presence of a pneumothorax during subpleural anesthesia, and whether chest tube drainage was required [Citation20]. A 10-point score was used for VAS evaluation [Citation21]. In order to collect intraoperative pain information, each patient was asked to be familiar with scoring criteria of VAS. A score of 0–3 is defined as having minor pain and being tolerable. A score of 4–6 was defined as moderate pain. A score of 7–10 was defined as severe and intolerable pain. Local tumor progression was defined as the progressive disease or recurrence within or at the periphery of the completely ablated ablation zone on subsequent CT studies based on the modified response evaluation criteria in solid tumors criteria.
Statistical analysis
Continuous data were tested by using the Student t test or Mann–Whitney test according to the normality assumption. The Fisher exact test was performed for categorical variables. Comparison between groups was performed using multivariable logistic regression. A logistic regression attempts to predict the probability that an observation falls into one of two categories of a dichotomous dependent variable based on one or more independent variables that can be either continuous or categorical. The model was run for each of the two dichotomous dependent variables of interest: pain and intravenous pain medication. Methods of local anesthesia, patient age, sex, emphysema, pleura-to-lesion distance, tumor size, histological types, length of interface with pleura and lesion location were the independent variables. The cumulative rates of recurrences during the follow-up period were estimated by using the method of Kaplan–Meier and were compared between two groups by using a log-rank test. Prognostic factors for local tumor progression were assessed by using Cox proportional hazard or time dependent Cox proportional hazard models in univariable and multivariable analysis. A p value of less than .05 was considered indicative of statistical significance. All analyses were performed by using commercially available software (SPSS Statistics, version 25.0, IBM SPSS Statistics, Armonk, NY).
Results
Baseline characteristics
A total of 124 patients were performed in the pleural puncture point anesthesia between January 2016 and June 2017 and 119 were performed subpleural anesthesia based on thermal radiation between July 2017 and December 2019. Mean age and sex distribution were similar in both local anesthesia groups. The incidence of tumor adherent to the pleura occurred in 52 of the 124 (41.9%) patients who were performed in the pleural puncture point anesthesia and in 55 of the 119 (46.2%) patients who were performed in the subpleural anesthesia based on thermal radiation (p=.588). 59 of the 124 (47.6%) patients who were performed in the pleural puncture point anesthesia and 62 of the 119 (58.1%) patients who were performed subpleural anesthesia based on thermal radiation had local pleural thickening adhesion (p=.426). Patient demographics are summarized in . Among the 119 patients who were performed in the subpleural anesthesia based on thermal radiation, there were 21 (17.6%) with primary lung cancers and 98 (82.4%) with metastases found at histopathologic examination of the biopsy specimen. Among the 124 patients who were pleural puncture point anesthesia, there were 100 (80.6%) with metastases and 24 (19.4%) with primary lung cancers. There was no significant difference in the study population between the two groups. The characteristics of the lesions are summarized in .
Table 2. Lesion characteristics for patients.
Technique safety
The technique success rate was 99.2% (123/124) in the patient with pleural puncture point anesthesia and 98.3% (117/119) in the patient with subpleural anesthesia based on thermal radiation. There were no major complications associated with both local anesthesia technique. Minor complications included pneumothorax (0.8%/2.5%) and puncture site hematoma (2.4%/4.2%) between two groups.
Pain incidence and risk factor
Moderate and severe pain occurred in 84 of 124 (67.7%) patients who were performed in the pleural puncture point anesthesia and in 20 of 119 (16.8%) patients who were performed in the subpleural multisite anesthesia based on the area of thermal radiation. Intravenous pain medications was required in 56 of 124 (45.2%) patients who were performed in the pleural puncture point anesthesia and in 9 of 119 (7.6%) patients who were performed in the subpleural multisite anesthesia based on the area of thermal radiation. There was significant difference in the incidence of moderate and severe pain (p<.001) and the use of intravenous pain medications (p<.001) between the two pleural anesthesia methods. Three of 119 (2.5%) patients who were performed in the subpleural anesthesia based on thermal radiation combined with intravenous pain medications had a technically unsuccessful ablation due to the development of intolerable pain, which resulted in the procedure stopping. Nine of 124 (7.3%) patients who were performed in the pleural puncture point anesthesia combined with intravenous pain medications had a technically unsuccessful ablation due to the development of intolerable pain during the procedure.
Multivariable logistic regression was performed to ascertain the effects of various demographic, disease and procedure variables on the likelihood of patients experiencing moderate to severe pain. In multivariable analysis, methods of local anesthesia (HR, 2.347; 95% CI, 1.557–4.816; p=.001), tumor size (HR, 1.024; 95% CI, 0.438–2.853; p=.015) and pleura-to-lesion distance (HR, 0.248; 95% CI, 0.126–0.392; p=.02) were independent risk factors in term of moderate to severe pain (). Multivariable logistic regression demonstrated that methods of local anesthesia (HR, 2.402; 95% CI, 1.142–4.902; p=.001) and pleura-to-lesion distance (HR, 2.236; 95% CI, 0.016–4.002; p=.026) were independent risk factors for patients requiring intravenous pain medications ().
Table 3. Multivariable logistic regression predicting likelihood of moderate and severe pain.
Table 4. Multivariable logistic regression predicting likelihood of intravenous pain medications usage.
Local tumor control and risk factor
During follow-up, local tumor progression occurred in 30 of 124 patients (24.2%) who were performed in the pleural puncture point anesthesia and in 24 of 119 patients (20.2%) who were performed in the subpleural anesthesia based on thermal radiation. The cumulative local tumor progression rates at 1- and 2-years were 16.2% and 25.3%, respectively, in patients who were performed in the pleural puncture point anesthesia, and 10.1% and 21.6%, respectively, in patients who were performed in the subpleural anesthesia based on thermal radiation (p=.042). On multivariate analysis of all study patients (n = 243), tumor size (HR, 2.023; 95% CI, 1.267–3.542; p=.008) and pleura-to-lesion distance (HR, 0.248; 95% CI, 0.152–0.626; p=.001) were significant factors for poor local tumor progression ().
Table 5. Multivariable logistic regression predicting likelihood of local tumor progression.
Discussion
Percutaneous thermal ablation of lung tumors adjacent to the pleura usually causes intolerable pain during the procedure. The pain is associated with thermal or polymodal nociceptors receptors in the parietal pleura and phrenic pleura, which are distributed and supplied segmental by intercostal nerve branches. When the pleura is stimulated, the skin areas where these nerves are distributed can cause pain in the associated area, such as chest, abdomen pain, neck and shoulder pain. Intolerable pain in the muscles and viscera occurs frequently and poses a significant challenge in clinical practice. Our study demonstrates that subpleural multisite anesthesia based on the area of thermal radiation for peripheral lung malignancy MWA resulted in a substantial reduction in the incidence of moderate or severe pain. In addition to the reduced incidence of moderate or severe pain, there was no statistically significant difference in the success rate between the two techniques. Our data suggest that different methods of preoperative subpleural anesthesia are important factors in the development of moderate or severe pain.
We also demonstrated that pleura-to-lesion distance is a significant risk factor for developing a moderate or severe pain, which has been consistently reported in the literature. Okuma et al. [Citation7] showed that location of the lesion at 1 cm or less from the chest wall was significantly related to pain during thermal ablation. This likely relates to the contact area being larger between the thermal radiation region and the pleura with shorter as the distance between the lesion and the pleura. Our study also reports that moderate or severe pain is more likely to occur when a larger lesion is being ablated. Larger lesions will lead to longer ablation time and larger ablation area, and further expand the area of pleural thermal radiation.
Intraoperative pain is the most common complication of thermal ablation. Various methods of relieving intraoperative pain have been used in ablative therapy. General anesthesia was found to be used for radiofrequency ablation in approximately half of the centers involved in the studies reviewed [Citation10]. The adverse effects of mechanical ventilation on lung function during previous general anesthesia procedures have been demonstrated. Pathologically, it is characterized by infiltration of inflammatory cells, hyaline membrane, increased vascular permeability and pulmonary edema, so it may increase the probability of pulmonary infection to some extent [Citation22–24]. Artificial pneumothorax as a new pain relief method has been applied to thermal ablation of lung tumors [Citation15–17]. Lee et al. reported a case in which an artificial pneumothorax was created to prevent pain during ablation of a subpleural tumor [Citation19]. Later, the Medical Center from Japan then further analyzed six patients in whom the artificial pneumothorax was created after the patients complained of severe pain [Citation10]. The mechanism of artificial pneumothorax technique to inject gas between the two layers of pleura to insulate heat on the stimulation of the parietal pleura. Likewise, subpleural infiltration of drugs can block nerve impulse conduction caused by heat stimulation on local pleural. The purpose of both techniques is to reduce thermal stimulation of the parietal pleura and to provide analgesic effects. However, gases move in the lungs with changes in body position and breathing. Artificial pneumothorax technique has some technical difficulties in controlling the relative position of gas between two layers of pleura and ablation zone. Second, because the lung tissue is compressed, it is difficult to adjust the ablation electrode for lesions with larger diameter. Furthermore, visceral pleura damage caused by thermal radiation lead to two pleural layers to fail to adhere closely, ultimately leading to persistent pneumothorax. Due to the relative fixed position between lesion and pleural, our technique has better control and safer on the MWA procedure.
Compared with the current artificial pneumothorax technology, subpleural multipoint anesthesia has the following advantages. Advancing the electrode and artificial pneumothorax may compress the pulmonary parenchyma and decrease alveolar air. Decreased alveolar air may alter electrical and thermal conductivity, affecting the ablation zone. Atelectasis is a collapse of lung tissue, an uneven decrease in the volume or gas content of one or more segments or lobes, and an aggregation of adjacent structures (bronchi, pulmonary vessels and interstitium) toward the atelectasis area. The adverse factors include the non-uniformity of tissue density, resulting in a certain degree of heat transfer in the tissue. Changes in the anatomical position of adjacent dangerous structures (blood vessels, bronchi, etc.) in the lung increase the risk of complications during ablation, such as hemorrhage and bronchopleural leakage. The beneficial factors include the reduction of needle channels and ablation sites and the shortening of ablation time due to the reduced volume of lesions. Subpleural multipoint anesthesia does not alter the normal anatomy of lung tissue. Second, creation of an artificial pneumothorax might not be feasible for patients with considerable pleural adhesion. Subpleural multipoint anesthesia was achieved by the lidocaine diffusion in the pleural space, which was almost unaffected by pleural adhesion. Third, the safety of artificial pneumothorax in lung tumor MWA remains to be further studied. Because microwave needle is different from the radiofrequency claw electrode and compressed lung tissue changed with respiration. The microwave electrode could not fix with tumor tissue. In contrast, subpleural anesthesia did not alter the normal lung anatomy and has little effect on the electrode.
Currently, local anesthesia for lung tumor ablation has performed at a single subpleural puncture site. This technique has the following advantages: first, the anesthesia site of this technology was directly under the pleura. Since the pain was caused by physical stimulation of the pleura, the subpleural anesthesia technology accurately located the source of the pain. Second, multipoint anesthesia actually expanded the pleural point to the pleural surface, aiming at the range of pain stimulation, not only more accurate control of pain, but also uniform coverage of anesthesia to the pleural surface, improving the utilization rate of anesthetics. Third, this technology provided precise theoretical guidance for pleural anesthesia coverage and pain control by pre-operatively planning the ablation heat area and measuring the contact range between the heat area and the pleural surface. Fourth, the technique relied on CT to accurately locate the anesthetic site below the parietal pleura. The pleura of the visceral layer were covered by the interstitial diffusion of anesthetics. The safety of subpleural anesthesia was ensured by introducing 21 G anesthesia needle. It avoided the damage caused by coarse needle puncture to the pleural surface, subcutaneous tissues and capillaries and greatly reduced the incidence of pneumothorax, bleeding and other complications. The technique has high safety and repeatability in adding anesthetic sites during operation. Fifth, CT guidance is currently the mainstream guidance for local treatment of lung tumors. However, the CT machine moved back and forth during treatment, which increased the risk and uncertainty of general anesthesia. Local subpleural multisite anesthesia can be used as a simpler and more flexible treatment for CT-guided ablation of lung tumors.
Our study has the following limitations. The study was retrospective, which is obviously inferior to a randomized control trial to assess this technique. Second, the area of subpleural anesthesia, which is based on the assessment of the area of thermal ablation radiation on CT images, requires injection of anesthesia at multiple subpleural sites to achieve pleural surface coverage. To some extent, subpleural puncture path can be affected by the shape of the pleura and the obstruction of anatomical structures such as ribs, which eventually lead to the insufficient drug coverage on the pleural region. The diffusion effect of drug between tissues can compensate for this deficiency to some extent. Third, lidocaine is currently used as a local anesthetic, the time interval between the effective effects of anesthesia is short. However, patients with longer operation duration, subpleural anesthesia could be injected again during the ablation period, depending on the patient's pain tolerance. Fourth, the technique requires injection at multiple subpleural sites, which increases the rate of complications such as pneumothorax and bleeding. The operator’s experience and meticulous operation can reduce the incidence of complications.
Conclusion
In conclusion, subpleural multisite anesthesia based on the area of thermal radiation for percutaneous CT-guided lung malignancy MWA could help to reduce the incidence of moderate to severe pain; it also may help to reduce the use of intraoperative intravenous pain medication. This technique is a simple and safety approach to reduce the incidence of moderate to severe pain for peripheral lung malignancy MWA. When planning the lung MWA near the pleura without the use of general or intravenous sedation or pain medication, the subpleural local anesthetic technique may improve outcomes and pain management.
Disclosure statement
The authors report no declarations of interest.
References
- Hess A, Palussière J, Goyers JF, et al. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology. 2011;258(2):635–642.
- Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004;230(1):125–134.
- Lencioni R, Crocetti L, R. Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective intention-to-treat, multicentre clinical trial (the RAPTURE study. Lancet Oncol. 2008;9(7):621–628.
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987–991.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Hiraki T, Gobara H, Fujiwara H, et al. Lung cancer ablation: complications. Semin Intervent Radiol. 2013;30(02):169–175.
- Okuma T, Matsuoka T, Yamamoto A, et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol. 2008;31(1):122–130.
- Dravid RM, Paul RE. Interpleural block - part 1. Anaesthesia. 2007;62(10):1039–1049.
- Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231(3):850–857.
- Rose SC, Thistlethwaite PA, Sewell PE, et al. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17(6):927–951.
- de Baère T, Dromain C, Lapeyre M, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology. 2005;236(2):666–670.
- Thornton RH, Solomon SB, Dupuy DE, et al. Phrenic nerve injury resulting from percutaneous ablation of lung malignancy. AJR Am J Roentgenol. 2008;191(2):565–568.
- Solomon SB, Thornton RH, Dupuy DE, et al. Protection of the mediastinum and chest wall with an artificial pneumothorax during lung ablations. J Vasc Interv Radiol. 2008;19(4):610–615.
- Zuo T, Lin W, Liu F, et al. Artificial pneumothorax improves radiofrequency ablation of pulmonary metastases of hepatocellular carcinoma close to mediastinum. BMC Cancer. 2021;21(1):505 6
- Jia H, Tian J, Liu B, et al. Efficacy and safety of artificial pneumothorax with position adjustment for CT-guided percutaneous transthoracic microwave ablation of small subpleural lung tumors. Thorac Cancer. 2019;10(8):1710–1716.
- Hou X, Zhuang X, Zhang H, et al. Artificial pneumothorax: a safe and simple method to relieve pain during microwave ablation of subpleural lung malignancy. Minim Invasive Ther Allied Technol. 2017;26(4):220–226.
- Yang X, Zhang K, Ye X, et al. Artificial pneumothorax for pain relief during microwave ablation of subpleural lung tumors. Indian J Cancer. 2015;52(6):e80–3.
- Hiraki T, Gobara H, Shibamoto K, et al. Technique for creation of artificial pneumothorax for pain relief during radiofrequency ablation of peripheral lung tumors: report of seven cases. J Vasc Interv Radiol. 2011;22(4):503–506.
- Lee EW, Suh RD, Zeidler MR, et al. Radiofrequency ablation of subpleural lung malignancy: reduced pain using an artificially created pneumothorax. Cardiovasc Intervent Radiol. 2009;32(4):833–836.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), mcgill pain questionnaire (mpq), short‐ form mcgill pain questionnaire journal pre-proof 18 (sf‐ mpq), chronic pain grade scale (cpgs), short form‐ 36 bodily pain scale (sf‐ 36 bps), and measure of intermittent and constant osteoarthritis pain (icoap. ).Arthritis Care Res. 2011;63(S11):S240–S252.
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646.
- Neto AS, da Costa LGV, Hemmes SNT, et al.; LAS VEGAS. The las vegas risk score for prediction of postoperative pulmonary complications: an observational study. Eur J Anaesthesiol. 2018;35(9):691–701.