Abstract
Aim
This study aimed to develop a novel tumor-bearing tissue phantom model that can be used for US/CT/MR-guided tumor puncture and thermal ablation.
Methods
The phantom model comprised two parts: a normal tissue-mimicking phantom and a tumor-mimicking phantom. A normal tissue phantom was prepared based on a polyacrylamide gel mixed with thermochromic ink. Moreover, a spherical phantom containing contrast agents was constructed and embedded in the tissue phantom to mimic a tumor lesion. US/CT/MR imaging features and thermochromic property of the phantom model were characterized. Finally, the utility of the phantom model for imaging-guided microwave ablation training was examined.
Results
The tumor phantom containing contrast agents showed hyper-echogenicity, higher CT numbers, and lower T2 signal intensity compared with the normal tissue phantom in US/CT/MR images. Consequently, we could locate the position of the tumor in US/CT/MR imaging and perform an imaging-guided tumor puncture. When the temperature reached the threshold of 60 °C, the phantom exhibited a permanent color change from cream white to magenta. Based on this obvious color change, our phantom model could clearly map the thermal ablation region after thermotherapy.
Conclusions
We developed a novel US/CT/MR-imageable tumor-bearing tissue model that can be used for imaging-guided tumor puncture and thermal ablation. Furthermore, it allows visual assessment of the ablation region by analyzing the obvious color change. Overall, this phantom model could be a good training tool in the field of thermal ablation.
1. Introduction
In recent years, image-guided thermal ablation has been rapidly developed as a minimally invasive therapy and widely used for treating local tumors at different locations, such as liver, breast, thyroid, etc. [Citation1]. However, the continual development of thermo-ablative therapy requires not only the support of advanced technologies but also the operators’ repeated training. Biological tissues, such as beef liver and turkey breast, are commonly used as training model. However, biological tissues have limitations, such as poor uniformity, short shelf life, and biohazardous nature, which hinder its broader application. Tissue-mimicking phantoms can be used as an alternative and a viable way to overcome the challenges described above [Citation2].
Tissue-mimicking phantoms (TMPs) are artificial synthetic materials that are used to mimic biological tissues provide a test environment for equipment-aided calibrations or operator training [Citation3]. Compared to biological tissues, TMPs have many obvious advantages, such as structural uniformity, strong plasticity, and long shelf life [Citation3]. Therefore, an increasing number of researchers have focused on constructing various TMPs and attempted to provide a standardized training model in the field of thermal ablation [Citation2]. For example, Lv et al. [Citation4]. developed a liver phantom based on carrageenan, which could be used to investigate the accuracy of US-US fusion imaging for evaluating the thermal ablation effect. Apart from carrageenan, other popular synthetic materials used for phantom fabrication are agar, gelatin, and polyacrylamide gels [Citation5–7]. Among these, the most common are the polyacrylamide gels, formed by cross-linked acrylamide monomers, and with the advantages of low cost and convenience of fabrication [Citation7,Citation8]. Studies have shown that polyacrylamide gels have many good properties, including favorable stability, high-temperature resistance (up to 120 °C), and similar texture and elastic properties to those of soft tissue [Citation9,Citation10]. Moreover, polyacrylamide gels can be combined with various heat-sensitive indicators (such as BSA and egg white) to provide geometric information of thermal damage after heating. For example, Negussie et al. [Citation11] reported on a thermochromic tissue-mimicking phantom based on polyacrylamide gel with the addition of thermochromic ink that could permanently alter the color from cream white to magenta. Furthermore, Mikhail et al. [Citation9] found the thermochromic polyacrylamide-gel had similar thermal properties to the ex vivo liver, such as the heating and cooling rates. Therefore, polyacrylamide gel is recognized as a very promising tissue-mimicking phantom material.
In this study, we developed a tumor-bearing tissue phantom model based on polyacrylamide gel. This phantom model could be used for US/CT/MR-guided tumor puncture and thermal ablation training.
2. Materials and methods
2.1. Phantom fabrication
All reagents were obtained from Aladdin (Shanghai, China), unless otherwise mentioned.
Our phantom model was based on a polyacrylamide gel with a commercially available thermochromic ink (Kromagen Magenta MB60-NH) that could permanently alter the color from cream white to magenta. According to the manufacturer’s instructions [Citation12], when the temperature is greater than 60 °C, the ink wound reach the maximum color density. In this study, we designed two parts to construct a tumor-bearing tissue model: a normal tissue-mimicking phantom and a tumor-mimicking phantom.
2.1.1. Normal tissue phantom
The protocol for normal tissue phantom fabrication was prepared as follows. Acrylamide and bis-acrylamide were mixed in a ratio of 19:1 and water was added to obtain an aqueous solution with a 7% w/v acrylamide/bis-acrylamide concentration. Then, the thermochromic ink (5% v/v) was added dropwise and stirred well. Ammonium persulfate (0.14% w/v) was added to initiate polymerization in the mixture, and N, N, N’N'-tetramethylethylenediamine (0.1% v/v) was added as a catalyst to accelerate the polymerization reaction. To increase the homogeneity of the phantom, an electric blender was used to continuously stir the mixture at a constant speed and rapidly mix it well. The final solution was immediately poured into a cuboid container (about 8 × 8 × 8 cm) and stored at 4 °C.
2.1.2. Tumor phantom
The fabrication process of the tumor phantom was similar to that of the tissue phantom. Additionally, we added a few imaging contrast agents to the tumor phantom. Specifically, we chose Iohexol (30 mg/mL) as the CT contrast agent and psyllium husk (16 mg/mL) (Anguo Yangrun Biotechnology Co, China) as the US/MR contrast agent. The particle size of psyllium husk (powder form) was controlled using a #45 mesh. The final solution was transferred to a spherical container with about 3 cm diameter and kept in a cold room (4 °C) until use.
2.1.3. Tumor-bearing tissue phantom model
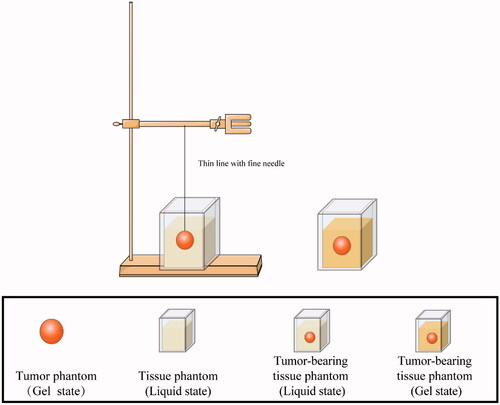
We embedded the spherical tumor phantom (gel state) into a normal tissue phantom (liquid state). A tumor-bearing tissue phantom model was constructed when the normal tissue phantom was completely gelled (). It is worth noting that the phantom model can take any shape as soon as an appropriate mold is available. In this study, the phantom containing contrast agents was referred to as ‘tumor phantom’ and the phantom without contrast agents was referred to as ‘normal tissue phantom’.
2.2. Phantom characterization: imaging and thermochromic properties
An ideal tumor-bearing tissue phantom model for thermal ablation research should satisfy at least two key conditions. First, the operator should be able to position the tumor in this phantom model during imaging-guided puncture training. Second, the phantom model should be thermochromic and could reveal the ablation region after thermal ablation. Therefore, the US/CT/MR imaging features and thermochromic property of our phantom model were examined in this study.
To assess the imaging features, we tested whether the tumor phantom could be recognized and distinguished from the tissue phantom by US/CT/MR imaging. Furthermore, to assess the thermochromic property, a color change of the phantom was observed when the temperature increased. More specifically, we heated the phantom samples in a hot water bath (60 °C), and the color differences between the heated and unheated phantom samples were observed.
2.3. Imaging guided thermal ablation training
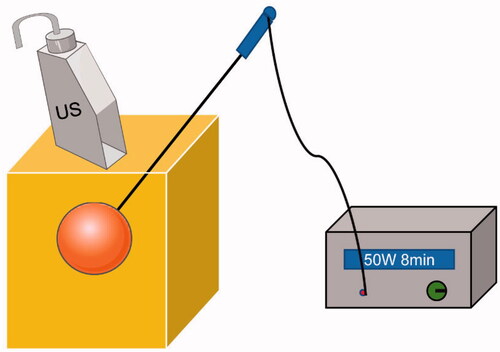
The phantom model was used to perform thermal ablation training at room temperature, which was carried out using an MTC-3 microwave therapy instrument (Forsea Microwave & Electronic Research Institute, Nanjing, China) with a frequency of 2,450 MHz and an output power of 5–100 W. The microwave antenna we used was a 16 G water-cooled MW needle with a 5 mm active tip. As shown in , an ablation needle was advanced to the tumor under imaging guidance. The ablation procedure was subsequently performed at a power output of 50 W for 8 min.
2.4. Observing the ablation region
Because the phantom could permanently turn cream white to magenta when the temperature was over 60 °C, we defined the magenta region in the phantom as the ablation region, referring to the study by Negussie et al.[Citation11]. The phantom model was sliced by a knife after thermotherapy to observe the ablation region, and the thermal effect could be assessed by determining if the ablation region had covered the tumor, referring to the study by Zhou et al. [Citation13].
3. Results
3.1. Optimization of the phantom
3.1.1. Normal tissue phantom
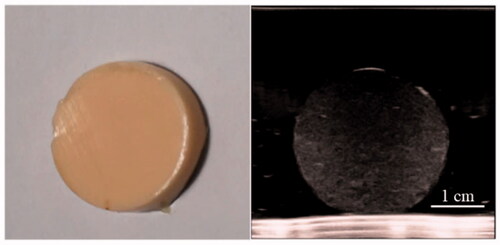
The gross specimen and ultrasound image confirmed the homogenous structure of the normal tissue phantom ().
3.1.2. Tumor phantom
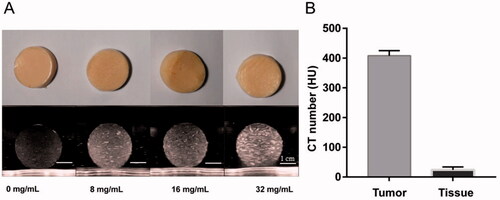
To detect the imaging difference between ‘the tumor’ and ‘the normal tissue phantom,’ we imaged the phantom model using US/CT. As shown in , the echogenicity of the tumor phantom increased with a higher concentration of psyllium husk in US imaging. However, psyllium husk has little impact on the CT number of the phantom. Therefore, we added iohexol as the CT contrast agent to the tumor phantom to increase the CT numbers ().
Figure 4. US and CT images of the disc-shaped phantom samples (with about 3 cm diameter) that were sliced from the spherical tumor phantom. (A) The ultrasound images (Philip EPIQ7, 7.5 MHz linear array probe) are depicted for four different psyllium husk concentration. (B) The CT number of tumor phantom sample was compared with that of normal tissue phantom sample (SAMATOM Force, 40 mA 120 kV).
Finally, we embedded the tumor phantom into a normal tissue phantom, and constructed a tumor-bearing tissue phantom that can be used for both US and CT imaging.
3.2. Us/CT/MR imaging feature and thermochromic property of the phantom model
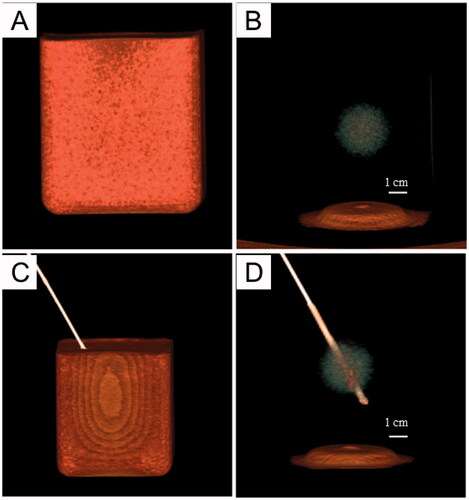
To assess whether our phantom model can be a qualified training model for image-guided puncture and thermal ablation, we first examined the US/CT imaging features. and show that there was a significant difference in echogenicity and CT numbers between the tumor phantom and normal tissue phantom. Therefore, we could locate the tumor using US/CT imaging and perform an imaging-guided tumor puncture.
Figure 5. US imaging of the phantom model: A: Gross specimen of the phantom model. B, C: the US images illustrate the tumor embedded in normal tissue phantom (B: 3.5-5.0 MHz convex array probe, C: 7.5 MHz linear array probe). D: the US image of puncture needle that was advanced into the tumor.
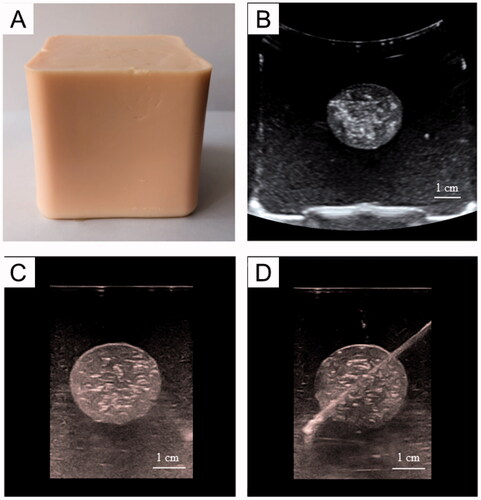
Figure 6. 3 D-CT imaging of the tumor-bearing tissue phantom (SAMATOM Force, 40 mA 120 kV). A: 3 D-CT imaging of the phantom model: B: the 3 D-CT image illustrates the tumor embedded in normal tissue phantom. C, D: the 3 D-CT images of puncture needle that was advanced into the tumor.
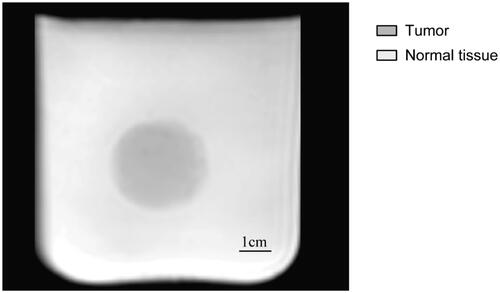
Considering that the psyllium husk could change not only the echogenicity of the gel in US imaging but also the MR signal intensity in MR images, as reported by Hofstetter et al. [Citation14], we further performed MR imaging on our phantom. As shown in , the tumor phantom containing psyllium husks showed lower T2 signal intensity compared to the normal tissue phantom, and the tumor phantom could be visualized and differentiated from the tissue phantom in MR images, which could provide the basis for MR-guided tumor puncture and thermal ablation training.
Figure 7. MR imaging of the tumor-bearing tissue phantom (3 T Philips Ingenia Elition X, T2-weighted MR imaging, TR: 842.7 TE:80.0, slice thickness 6 mm, field of view (FOV) = 250 × 250 mm2, matrix = 320 × 320). The T2 signal intensity in the tumor region is lower than that in the tissue region.
To examine the thermochromic property of the phantom, we conducted a heating experiment. As shown in , the color of our phantom samples changed from cream-white to magenta after heating.
3.3. Imaging-guided thermal ablation training
To further examine the applicability of our phantom model, we performed ultrasound-guided microwave ablation training. depicts the successful advancement of the ablation needle into the tumor under ultrasound guidance and the conduction of the MWA procedure.
Figure 9. (A) US-guided tumor puncture. (B) The gross specimen of the phantom model after MWA. Bar = 1 cm.
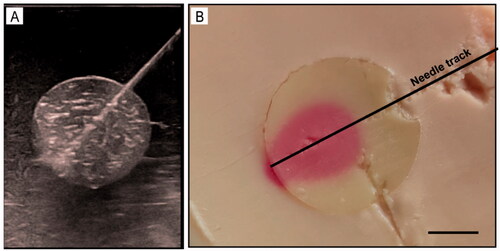
After MWA ended, the phantom was sliced along the largest section of the ablation region. As shown in , the heated and unheated regions showed a uniform color distribution, which illustrates that our phantom presented good homogeneity. Furthermore, we found that the ablated region was present in magenta, which was consistent with the results of the above heating experiment, and indicated that the temperature of the ablated region was about 60 °C. More importantly, owing to the sharp color contrast between the ablated and unablated regions and the well-defined boundary between ‘the tumor’ and surrounding normal tissue, the ablation effect could be assessed visually in this phantom model.
4. Discussion
In this study, we combined polyacrylamide gel with commercially available thermochromic ink and contrast agents to present a novel triple-modal (US/CT/MR) imageable tumor-bearing tissue model that could be used for imaging-guided tumor puncture and thermal ablation training.
Polyacrylamide gel is recognized as a very promising tissue-mimicking phantom material with the advantages of favorable stability, low cost, and ease of fabricate [Citation10]. In this study, we constructed a normal tissue-mimicking phantom based on polyacrylamide gel. Interestingly, the puncture through the tissue phantom felt similar to a puncture through soft tissue (such as that of the liver). Indeed, studies have shown that polyacrylamide gel has a similar mass density and flexibilities to those of the liver, along with the similar heat capacity and electrical conductivity [Citation9,Citation11,Citation13].Therefore, a polyacrylamide gel-based phantom is an ideal artificial synthetic material for mimicking soft tissue, specifically the liver. Furthermore, we designed a spherical phantom to mimic the tumor lesion embedded in the soft tissue phantom. To improve the visualization of the ‘tumor’ in US/CT/MR images, we added contrast agents into the tumor phantom. Specially, we chose the psyllium husk as one of the contrast agents, which could provide both US and MR imaging characteristics. Thus, complex phantom recipes were avoided. Notably, the order of addition of contrast agents is important. In our experience, it is better to add iohexol before thermochromic ink, so that we can easily confirm whether iohexol is fully dissolved in the mixture visually. However, psyllium husk is better added after APS and TEMED. Otherwise, it may result in lack of homogeneity of the phantom. Finally, we planted ‘the tumor’ into a normal tissue phantom and a tumor-bearing tissue model was constructed that can be used for multimodal imaging.
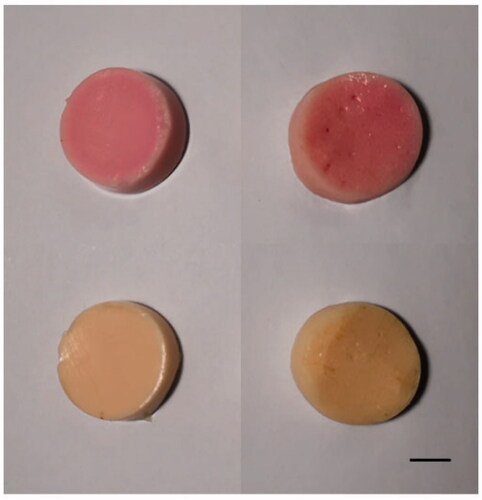
An ideal phantom model for thermal ablation should also provide a visualization of the ablation region. Therefore, many researchers have attempted to modify TMPs by the addition of various temperature-sensitive materials such as bovine serum albumin (BSA), egg white, or color-changing ink [Citation10,Citation15–17]. In this study, we adopted a commercially available thermochromic ink (Kromagen Magenta MB60-NH) as the temperature indicator. According to the manufacturer [Citation12], this ink can permanently change color from cream white to magenta when the temperature is above 60 °C, and it will reach the maximum color density at around 60-65 °C. The threshold temperature for irreversible coagulative necrosis of human soft tissue is approximately 60 °C [Citation18]. Therefore, this thermochromic ink could provide geometric information of the coagulative necrosis region after thermal ablation in our phantom. Furthermore, Negussie et al. [Citation11] found a relatively sharp decrease in temperature at the margin of the ablation region, and the thermochromic ink in phantom could produce a corresponding gradual color change in this region. Further, Mikhail et al. [Citation9] found it was possible to estimate the accurate temperature value (40-67 °C) at the ablation margin by comparing the phantom color to a phantom standard calibration series. Therefore, our phantom model may also theoretically provide thermal information below 60 °C. Referring to the study by Mikhail et al. [Citation9], we also made a phantom standard calibration series (see Supplementary information ) that may contribute to the temperature estimation below 60 °C in our phantom by visual comparison between the phantom color to the standard calibration series. Overall, thermochromic ink is a very popular temperature indicator and has been widely used to modify various phantoms for thermotherapy [Citation3,Citation9,Citation11,Citation13,Citation19].
Imaging-guided thermal ablation techniques have been widely used for treating local liver tumors in recent years [Citation20]. An ideal tumor-bearing tissue-mimicking phantom is critically important for clinical training, calibration, and optimization of hyperthermia equipment [Citation21–23]. Although previous studies [Citation10,Citation11,Citation16,Citation24] have developed some phantoms that can provide a visualization of the thermal region, most of them lack favorable imaging characteristics. Sugimoto et al. developed a liver phantom that contained four simulated tumors, which had favorable US imaging characteristics and could be used for US-guided puncture training [Citation25]. However, this phantom is unsuitable for thermal ablation training due to the lack of thermochromic materials. Li et al. [Citation26] constructed a tumor-bearing liver phantom that clearly showed the coagulative necrosis region after imaging-guided thermal ablation. However, the operation process was complex because the carrageenan melted into a liquid and needed to be replaced in a short time after thermal ablation. Therefore, further optimization is required to solve this problem. In this study, our phantom could provide triple-modal imaging (US/CT/MR) characteristics, which could provide the basis for US/CT/MR guided tumor puncture and thermal ablation. To further demonstrate the availability of our phantom model, we performed imaging-guided MWA training. The results showed that we could locate the position of the tumor and conducted an imaging-guided tumor puncture and MWA therapy. Furthermore, due to the obvious color change between the ablated and unablated region, we could visually assess the ablation region. As shown in , the mono-point ablation region could not completely cover the tumor in this phantom model. Increasing the ablation point, ablation power, or ablation time is a viable way to achieve complete ablation. As reported by Zhou et al. [Citation13], the thermal ablation region could increase with the increase in ablation time or ablation power in the polyacrylamide-based phantom, which is similar to that in the biological tissue.
According to the previous studies [Citation10,Citation21,Citation27,Citation28], polyacrylamide gel has favorable physical properties (such as thermal and dielectric properties) and could be available for various thermoablative modalities. Especially, the conductivity and permittivity of PAG were well consistent with those of the biological soft tissue at frequencies between 500 MHz and 3 GHz [Citation27]. Therefore, our phantom also has the potential for use in other thermoablative modalities besides MWA, such as radiofrequency ablation (RFA)[Citation9,Citation10,Citation27], high-intensity focused ultrasound (HIFU)[Citation3,Citation21], and laser interstitial thermal therapy (LITT)[Citation11,Citation28]. According to the working mechanism of these thermoablative modalities, the phantom could be further optimized. For example, sodium chloride can be added to optimize the phantom’s electrical conductivity for RFA [Citation10,Citation27]. Corn sirup or glass beads can be added to optimize the phantom’s acoustic properties for HIFU [Citation7,Citation29]. Overall, all of the above characteristics suggest that our phantom has the potential to be an ideal training model for imaging-guided thermal ablation.
This study had several limitations. First, the thermal and dielectric properties of the phantom were not directly measured in this study. Earlier studies [Citation9,Citation11,Citation17,Citation27] have shown that the thermal properties (such as heat capacity and thermal diffusivity), electrical conductivity, and permittivity of the PAG-based phantom are comparable to those of biological soft tissue. However, whether the ablation area in this phantom is comparable to that in real tissue remains to be further studied. Second, MR imaging-guided tumor puncture and thermal ablation should be further tested using MR-compatible thermal devices. Third, the ablation region of our phantom could not be detected using US/CT/MR (Supplementary information ). Therefore, further optimization is necessary.
5. Conclusion
In this study, we developed a tumor-bearing tissue phantom model that can be used for US/CT/MR-guided tumor puncture and MWA training. Although there are some shortcomings to our phantom model, it can be further optimized and has the potential to become not only an ideal training tool for US/CT/MR guided tumor puncture and thermal ablation, but also a practical platform for many novel clinic techniques in the field of thermotherapy, such as US-CT, CT-MR, US-MR, US-CT-MR fusion imaging-guided thermal ablation techniques and US/CT/MR-based preoperative plans techniques.
Supplemental Material
Download PDF (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- McGarry CK, Grattan LJ, Ivory AM, et al. Tissue mimicking materials for imaging and therapy phantoms: a review. Phys Med Biol. 2020;65(23). DOI:10.1088/1361-6560/abbd17.
- Eranki A, Mikhail AS, Negussie AH, et al. Tissue-mimicking thermochromic phantom for characterization of HIFU devices and applications. Int J Hyperth. 2019; 36(1):518–529.
- Lv S, Long Y, Su Z, et al. Investigating the accuracy of ultrasound-ultrasound fusion imaging for evaluating the ablation effect via special phantom-simulated liver tumors. Ultrasound Med Biol. 2019;45(11):3067–3074.
- Kim J, Kim M, Park Y, et al. Development of an agar phantom adaptable for visualization of thermal distribution caused by focused ultrasound. IEEE International Ultrasonics Symposium (IUS); 2011 Oct 18–21; Orlando, FL2011. p. 1372–1375.
- Dunmire B, Kucewicz JC, Mitchell SB, et al. Characterizing an agar/gelatin phantom for image guided dosing and feedback control of high-intensity focused ultrasound]. Ultrasound Med Biol. 2013;39(2):300–311.
- Choi MJ, Guntur SR, Lee KIL, et al. A tissue mimicking polyacrylamide hydrogel phantom for visualizing thermal lesions generated by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39(3):439–448.
- Bini MG, Ignesti A, Millanta L, et al. The polyacrylamide as a phantom material for electromagnetic hyperthermia studies. IEEE Trans Biomed Eng. 1984;31(3):317.
- Mikhail AS, Negussie AH, Graham C, et al. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med Phys. 2016;43(7):4304–4311.
- Zhang B-L, Hu B, Kuang S-L, et al. A polyacrylamide gel phantom for radiofrequency ablation. Int J Hyperth. 2008;24(7):568–576.
- Negussie AH, Partanen A, Mikhail AS, et al. Thermochromic tissue-mimicking phantom for optimisation of thermal tumour ablation. Int J Hyperthermia. 2016;32(3):239–243.
- TLC Hall. Permanent change thermochromic ink. https://spotsee.io/wp-content/uploads/2021/05/PDS078-Rev01.pdf
- Zhou Y, Zhao L, Zhong X, et al. A thermochromic tissue-mimicking phantom model for verification of ablation plans in thermal ablation. Ann Transl Med. 2021;9(4):354.
- Hofstetter LW, Fausett L, Mueller A, et al. Development and characterization of a tissue mimicking psyllium husk gelatin phantom for ultrasound and magnetic resonance imaging. Int J Hyperthermia. 2020;37(1):283–290.
- Park SK, Guntur S, Il Lee K, et al. Reusable ultrasonic tissue mimicking hydrogels containing nonionic Surface-Active agents for visualizing thermal lesions]. IEEE Trans Biomed Eng. 2010;57(1):194–202.
- Takegami K, Kaneko Y, Watanabe T, et al. Polyacrylamide gel containing egg white as new model for irradiation experiments using focused ultrasound. Ultrasound Med Biol. 2004;30(10):1419–1422.
- Dabbagh A, Abdullah BJJ, Abu Kasim NH, et al. Reusable heat-sensitive phantom for precise estimation of thermal profile in hyperthermia application. Int J Hyperthermia. 2014;30(1):66–74.
- Wood BJ, Ramkaransingh JR, Fojo T, et al. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;94(2):443–451.
- Ambrogio S, Baesso RM, Gomis A, et al. A polyvinyl alcohol-based thermochromic material for ultrasound therapy phantoms. Ultrasound Med Biol. 2020;46(11):3135–3144.
- Crocetti L, Lencioni R. Thermal ablation of hepatocellular carcinoma. Cancer Imaging. 2008;8(1):19–26.
- McDonald M, Lochhead S, Chopra R, et al. Multi-modality tissue-mimicking phantom for thermal therapy. Phys Med Biol. 2004;49(13):2767–2778.
- Arora D, Cooley D, Perry T, et al. Direct thermal dose control of constrained focused ultrasound treatments: phantom and in vivo evaluation. Phys Med Biol. 2005;50(8):1919–1935.
- Bouchard LS, Bronskill MJ. Magnetic resonance imaging of thermal coagulation effects in a phantom for calibrating thermal therapy devices. Med Phys. 2000;27(5):1141–1145.
- Dabbagh A, Abdullah BJJ, Ramasindarum C, et al. Tissue-Mimicking gel phantoms for thermal therapy studies. Ultrason Imaging. 2014;36(4):291–316.
- Sugimoto K, Moriyasu F, Shiraishi J, et al. A phantom study comparing Ultrasound-Guided liver tumor puncture using new Real-Time 3D ultrasound and conventional 2D ultrasound. Am J Roentgenol. 2011;196(6):W753–W7.
- Li K, Su Z, Xu E, et al. Evaluation of the ablation margin of hepatocellular carcinoma using CEUS-CT/MR image fusion in a phantom model and in patients. BMC Cancer. 2017;17(1)61.
- Surowiec A, Shrivastava PN, Astrahan M, et al. Utilization of a multilayer polyacrylamide phantom for evaluation of hyperthermia applicators. Int J Hyperth. 1992;8(6):795–807.
- Geoghegan R, Santamaria A, Priester A, et al. A tissue-mimicking prostate phantom for 980 nm laser interstitial thermal therapy. Int J Hyperth. 2019;36(1):993–1002.
- Guntur SR, Choi MJ. An improved Tissue-Mimicking polyacrylamide hydrogel phantom for visualizing thermal lesions with High-Intensity focused ultrasound. Ultrasound Med Biol. 2014;40(11):2680–2691.