Abstract
Objective
To investigate the factors influencing the sonication dosage and efficiency of ultrasound-guided high intensity focused ultrasound (USgHIFU) for breast fibroadenomas.
Materials and Methods
Forty-nine patients with 78 breast fibroadenomas who underwent USgHIFU were retrospectively analyzed. The energy efficiency factor (EEF) was set as dependent variable, and the factors possibly influencing the sonication dosage, including age, body mass index (BMI), fibroadenoma size, distance from the shallow margin of the fibroadenoma to skin, distance from the deep margin of the fibroadenoma to pectoralis major, types of near field acoustic pathway, and Adler blood flow classification of ultrasound, were set as independent variables. The correlation between EEF and these independent variables were analyzed, and an optimal scaling regression model was established.
Results
Fibroadenoma size, distance from the shallow margin of the fibroadenoma to skin and type of near field acoustic pathway had significant correlation with EEF (p < 0.05). An EEF (ŷ) dosimetry model of ŷ= −0.496X1 + 0.287X2 + 0.203X3 was established, in which X1 stands for size of fibroadenoma, X2 stands for the distance from shallow margin of the fibroadenoma to skin, and X3 stands for type of near field acoustic pathway. The predicted EEF value was significantly related to actual EEF (R = 0.698, p = 0.000).
Conclusions
Fibroadenoma size, distance from the shallow margin of the fibroadenoma to skin and type of near field acoustic pathway could be used as predictors to evaluate the dosage delivery of USgHIFU treatment for breast fibroadenomas.
Introduction
Fibroadenoma is the most common benign breast tumor in women. Although women of any age can develop this benign tumor, it usually occurs in young women [Citation1,Citation2]. It was reported that about 10% of women have breast fibroadenoma in their lifetime, accounting for 67%−94% of all breast biopsies in women under the age of 20 years [Citation3,Citation4]. Surgical excision is the conventional treatment of choice for fibroadenoma, but patients are worried about the breast scar that may lead to poor cosmetic results. Although vacuum-assisted mammotomy (VAM) was thought to be an alternative treatment in the management of breast fibroadenomas with size smaller than 3 cm, it is still an invasive treatment and the complications of severe bleeding, hematomas, post-operative infections may occur [Citation5].
As a noninvasive treatment, high-intensity focused ultrasound (HIFU) has been used in the treatment of breast tumors. Wu et al. first performed a randomized clinical trial of ultrasound-guided HIFU (USgHIFU) treatment for breast cancer and the results showed that USgHIFU is feasible in the treatment of breast cancer. The pathologic findings showed that HIFU-treated tumor cells underwent complete coagulative necrosis, and tumor vascular vessels were severely damaged [Citation6]. Over the last two decades, studies from several groups have demonstrated the efficiency and safety of HIFU for fibroadenomas. The results from Peek MC’s study showed that the volume of breast fibroadenoma was significantly reduced in 1 year after HIFU treatment [Citation7]. Kovatcheva et al. showed that the 1-year post-HIFU volume reduction rate of fibroadenomas was 72.5% [Citation8].
As a thermoablative approach, the dosage of acoustic energy deposited in the tumor is of the most importance when the effect of treatment is evaluated. However, there was no report about the dosimetric analysis of HIFU treatment for breast fibroadenomas. To quantitatively illustrate dosimetry of HIFU, energy efficiency factor (EEF), defined as the amount of acoustic energy required for ablating 1 mm3 of the biological tissue, was proposed, which was a key factor for the evaluation of energy efficiency [Citation9,Citation10]. Lower EEF represents higher ability and efficiency of ablating the tissue. Therefore, the aim of this retrospective study was to investigate the factors influencing the efficiency of HIFU treatment of breast fibroadenomas.
Materials and methods
This study was approved by the ethics committee of Suining Central Hospital (LLSNCH20200065) and The First Affiliated Hospital of Nanjing Medical University (2020-SR-130). The requirement for informed consent was waived due to the retrospective nature of this study.
Subjects
Forty-nine patients with 78 breast fibroadenomas who underwent USgHIFU treatment from two above-mentioned hospitals during January 2021 and October 2021 were retrospectively analyzed.
Inclusion criteria: (1) patients were older than 16 years and in premenopausal status; (2) The breast imaging reporting and data system (BI-RADS) grading ≤3 by ultrasound; (3) diagnosis of breast fibroadenoma was confirmed by core needle biopsy; (4) fibroadenomas with a safe acoustic pathway and the focus can reach the target.
Exclusion criteria: (1) patients with breast malignant tumors; (2) patients who received high dose of radiotherapy for malignant tumors in the breast area; (3) menstruating, pregnant, or lactating women; (4) severe skin scars in the acoustic pathway (protruding from the skin surface, width ≥1 cm).
Pre-HIFU treatment ultrasonography
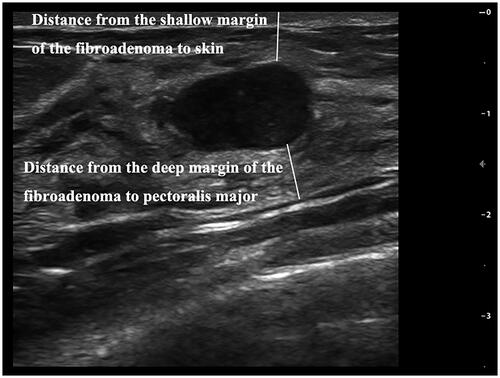
All patients underwent pre-HIFU ultrasonography using a high frequency probe (frequency ≥7.5 mHz) (DC-80S, Mindray Medical, Shenzhen, China). The patient lay supine on the examination bed with arms outstretched or raised, and fully exposing the breasts and armpits. The probe was used for a full breast and axillary scan by rotating clockwise from the nipple to the edge of the breast. The targeted breast fibroadenoma was measured in three dimensions: longitudinal (a), anteroposterior (b) and transverse (c). The fibroadenoma volume was calculated according to the following equation: V = 0.5233 × a × b×c. Distance from the shallow margin of the fibroadenoma to the skin and distance from the deep margin of the fibroadenoma to the pectoralis major were also measured (). BI-RADS classification was performed on the masses found in the scan. Adler blood flow classification was performed by color Doppler flow imaging (CDFI). Then, for the evaluation of USgHIFU acoustic pathway, especially the near field, we divided the acoustic pathway into three types: (1) mainly mammary glandular type: the ratio of fat thickness and glandular tissue thickness between the skin and the shallow margin of the fibroadenoma was <1:2; (2) mixed type: the ratio of fat thickness and glandular tissue thickness between the skin and the shallow margin of the fibroadenoma was between 1:2 and 2:1; (3) mainly fat type: the ratio of fat thickness and glandular tissue thickness between the skin and the shallow margin of the fibroadenoma was >2:1 ().
USgHIFU ablation
The USgHIFU ablation was performed under local anesthesia with ropivacaine (3.25–7.5 mg/mL). The anesthetic was first injected under the skin which was in front of the tumor, and then got into the posterior breast space. Local anesthesia was to relieve discomfort and pain in the targeted area of patients during the procedure.
The procedure of USgHIFU was performed with a US-guided HIFU tumor therapeutic system (Focused Ultrasound Tumor Therapeutic Systmem (Model-JC 200B, Chongqing Haifu Medical Technology Co. Ltd., China) equipped with an ultrasound imaging device (L9-3E, Mindray Medical, Shenzhen, China) for real-time guidance during the procedure. Therapeutic focused ultrasound energy was produced with an 18 cm-diameter transducer with a focal length of 8 cm, operated at a frequency of 1.0 MHz. The acoustic focus dimensions were 5 mm × 1.8 mm × 1.8 mm.
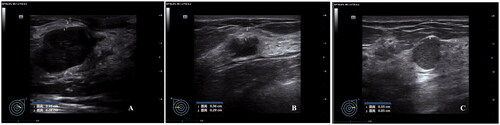
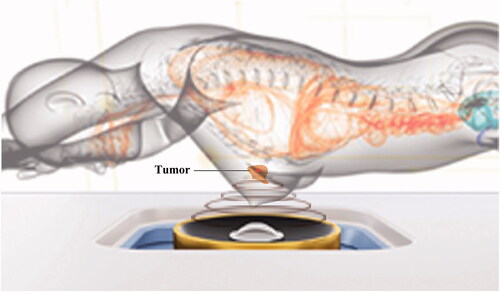
The patients were positioned prone on the treatment bed, with the targeted breast immersed into a degassed water reservoir (). The target tumor and adjacent structures were monitored with real-time ultrasonographic imaging. The sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. The distance between treated slices was 3 mm. Point scan energy delivery was used during HIFU. The treatment power started from 100 W, and it was increased stepwise after the start of the procedure. Treatment power of 100–300 W was used for different sites of breast fibroadenomas. The focus was manually moved and the adjustment of energy was based on the hyperechoic grayscale change on the real-time ultrasonographic imaging or the feedback of feeling from the patients. The treatment was terminated when the significant grayscale changed area covered the whole fibroadenoma (). After HIFU treatment, an ice bag was put on the skin of the treated breast fibroadenoma for 30 min.
Figure 4. The significant gray scale changes during HIFU treatment for breast fibroadenomas. A. Pre-HIFU ultrasound showed a hypoechoic breast fibroadenoma (red ring). B. A significant gray scale changes was observed during HIFU (white arrow). C. The significant gray scale changed area covered the whole fibroadenoma.
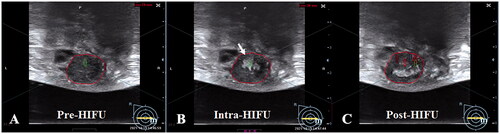
The following treatment parameters were recorded: (1) treatment time (min), defined as the time from the first sonication to the last sonication; (2) sonication energy (J), defined as the total energy used during the treatment; (3) sonication time per hour (s/h), defined as sonication time (s) used per hour (h) to illustrate the therapeutic rhythm; (4) and time of hyperechoic grayscale change emerging (s), defined as sonication time (s) from the first sonication output to the hyperechoic grayscale change emerging. For safety evaluation, patients were requested to report any discomfort or pain during the treatment. Visual analogue scale (VAS) was used to evaluate the pain during HIFU [Citation11]. Adverse events, such as skin burn, fever and pectoralis major injury, were monitored within 72 h after USgHIFU treatment.
Contrast-enhanced ultrasound
To evaluate the therapeutic results, contrast-enhanced ultrasound using a microbubble agent (SonoVue, Bracco, Milan, Italy) was performed before and immediately after HIFU treatment. Briefly, a vial of 59 mg of SonoVue microbubble powder was reconstituted with 5 ml of normal saline. Then, the vial was vigorously shaken to let the microbubble powder fully dissolved. The contrast-enhanced ultrasound imaging was observed and recorded after intravenous injection 2 ml of SonoVue solution followed by 5 ml normal saline flushing. The contrast-enhanced ultrasound results were analyzed independently by two senior ultrasound doctors. Nonperfused volume (NPV), representing coagulative necrosis volume, was first measured in 3 dimensions and then calculated according to the above-mentioned equation. The NPV ratio was defined as NPV/fibroadenoma volume × 100%.
Dosimetric analysis
For sonication efficiency evaluation and dosimetric analysis, EEF was calculated with the following equation: EEF (J/mm3)=η·E/V, in which η stands for the transducer focusing coefficient, E (J) stands for the sonication energy and V (mm3) stands for NPV.
Statistical analysis
SPSS software (SPSS 21.0, IBM, USA) was used for statistical analysis. The one-sample Kolmogorov-Smirnov test was used to obtain statistics of the probability distribution for quantitative data. Normally distributed data were reported as mean ± SD. Skewly distributed data were reported as median and inter-quartile range (P25, P75). Spearman’s rank correlation test was used for the correlation analysis. An optimal scaling regression model was established by analyzing the characteristics of the data. Statistical significance was defined as a P value <0.05.
Results
Baseline characteristics of patients with breast fibroadenomas
A total of 49 patients with 78 breast fibroadenomas were treated by USgHIFU. As shown in , the average age of the patients was 30.27 ± 9.02 years. The average body mass index (BMI) was 21.25 ± 2.30 kg/m2. Among these patients, 33 had solitary fibroadenoma and 16 had multiple fibroadenomas. The average size of the 78 fibroadenomas was 16.48 ± 6.43 mm, and the median fibroadenoma volume was 810.66 (Interquartile range: 377.10, 2010.47) mm3. The average distance from shallow margin of the fibroadenoma to skin was 13.07 ± 5.70 mm, and the median distance from deep margin of the fibroadenoma to pectoralis major was 3.15 (Interquartile range: 0.08, 7.65) mm. For the types of near field of acoustic pathway, 11 patients with mainly mammary glandular type, 44 with mainly fat type and 23 with mixed type. For the Adler classification, the number of fibroadenomas with grade 0, I, II and III was 20, 44, 14 and 0, respectively ().
Table 1. Baseline characteristics of Patients with fibroadenoma (n = 49).
Table 2. Characteristics of fibroadenoma lesions (n = 78).
Evaluation of USgHIFU ablation
As shown in , the median treatment time was 8.00 (Interquartile range: 5.00, 15.00) min. The median sonication energy used was 10,375.00 (Interquartile range: 5085.00, 19,580.00) J, and the average sonication time per hour was 378.80 ± 85.90 s/h. The median significant hyperechoic grayscale change time was 12.00 (Interquartile range: 6.00, 28.50) s. The median EEF was 13.68 (Interquartile range: 7.88, 23.75) J/mm3 and the median NPV ratio was 92.94% (Interquartile range: 71.49%, 116.57%).
Table 3. USgHIFU treatment results for fibroadenomas (n = 78).
Adverse events and complications
Under local anesthesia, all the patients tolerated the treatment well. The average VAS score during the treatment was 4.20 ± 2.10 points. No skin burn was found after treatment. No fever or pectoralis major injury was observed after HIFU treatment.
Establishment of EEF dosimetric model
In this model, EEF was set as dependent variable, and a total of 7 factors were selected and established as independent variables, including age, BMI, fibroadenoma size, distance from shallow margin of the fibroadenoma to skin, distance from deep margin of the fibroadenoma to the pectoralis major, types of near field of acoustic pathway, and Adler blood flow classification of ultrasound. As shown in , among these seven factors, there were three predictors had statistically significant correlation with EEF: fibroadenoma size (negative correlation), distance from shallow margin of the fibroadenoma to skin (positive correlation), and type of near field of acoustic pathway (negative correlation).
Table 4. Correlation between predictors and EEF.
We then included these three predictors in the optimal scaling regression analysis to get the predicted EEF value. In this regression analysis, “Numeric” was selected as the optimal scaling level for EEF, and “Nominal” was selected as the optimal scaling level for fibroadenoma size, distance from the shallow margin of the fibroadenoma to skin, and type of near field of acoustic pathway. The results of the regression analysis showed that the optimal scaling regression model was acceptable with the adjusted R2 being 0.358 (). From analysis of variance, it was shown that the fitting model after transformation is statistically significant (p = 0.000, ). The coefficients of these three factors were shown in and the quantification of EEF and three factors were shown in . Based on these results, the optimal scaling regression equation was established as ŷ= −0.496X1+0.287X2+0.203X3, in which ŷ stands for a quantified value of EEF, X1 stands for fibroadenoma size, X2 stands for distance from shallow margin of the fibroadenoma to the skin, and X3 stands for type of near field of acoustic pathway. The importance of X1, X2 and X3 were 0.691, 0.218 and 0.091, respectively (). The tolerance of each statistically significant predictor was >0.1, which indicated that there was no collinearity among the predictors (). Therefore, the model matched well with the requirement of the statistics.
Figure 5. Quantifications of EEF and three independent predictors for EEF dosimetric model. A. Quantification of EEF actual value range; B. Quantification of actual value range of the size of fibroadenoma. C. Quantification of actual value range of distance from the shallow margin of the fibroadenoma to skin. D. Quantification of type of near field of acoustic pathway.
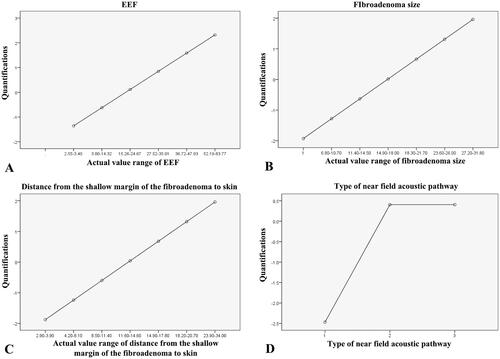
Table 5. Optimal scaling regression model*.
Table 6. Analysis of variance*.
Table 7. Coefficient and tolerance of optimal scaling regression model of EEF.
Validation of EEF dosimetric model
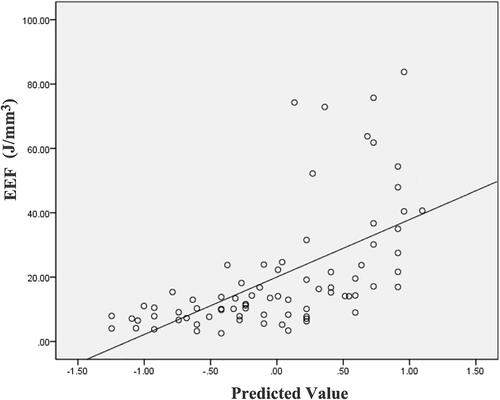
After the ŷ value was solved from the equation, the predicted EEF value was obtained and listed in . A scatter plot showed that the EEF distribution was correlated well with the predicted values (). There was a significant correlation between the actual EEF and the predicted EEF (R = 0.698, p = 0.000).
Table 8. Optimal scaling level of EEF.
Discussion
EEF can be considered as a quantitative index to reflect the difficulty level of HIFU treatment for tumors. In this study, three independent predictors, including fibroadenoma size, distance from shallow margin of the fibroadenoma to the skin, and type of near field of acoustic pathway, were found to have correlation with dependent variable EEF. The other four independent predictors, including age, BMI, distance from deep margin of the fibroadenoma to pectoralis major and Adler blood flow classification of ultrasound, were not correlated with EEF.
The previous studies have shown that the blood flow inside the tumor may affect the deposition of ultrasonic energy inside tumor, because the cooling effect of blood flow could quickly take away the ultrasonic energy, making it difficult for the local temperature to reach the threshold leading to tumor necrosis [Citation9,Citation12]. However, we did not find any significant correlation between Adler blood flow classification of ultrasound and EEF in this study. It might be explained by that the blood supply of breast fibroadenoma is usually poor, as more than 50% of breast fibroadenomas have no feeding vessels, and the blood supply for these fibroadenomas was from the surrounding breast tissue into the fibroadenoma [Citation13,Citation14]. In this study, Adler blood flow classification, being a semi-quantitative system, was used to determine the blood supply of fibroadenomas. Sixty-four of 78 lesions (82.1%) were classified as Grade 0 or I, accounting for the majority of the lesions, which was in accordance with the previous finding that benign tumors usually had Grade 0 or I of Adler blood flow classification. Since the blood flow classification among the fibroadenomas in this study was too concentrated in Grade 0 or I, and was not evenly distributed in different grades, this may be the cause of no significant correlation between Adler blood flow classification of ultrasound and EEF.
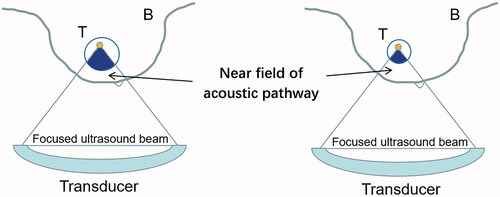
Breast is a superficial organ attached to the pectoralis major. During HIFU treatment for fibroadenomas, the tissues inside the near field of acoustic pathway of treating breast tumors are relatively simple. Thus, fibroadenoma was thought to be especially suitable for HIFU ablation because ultrasonic beams only need to pass the skin, fat tissue and normal breast glandular and duct tissue to reach the fibroadenoma. In this study, the tissues in the near field of acoustic pathway were categorized as three types (). We found that the type of near field of acoustic pathway had significant correlation with EEF and could be included into the optimal scaling regression model. It demonstrated that mainly fat type in the near field of acoustic pathway was associated with lower EEF, compared with the mixed type and mainly mammary glandular type. Mixed type had lower EEF than that of mainly mammary glandular type. This could be interpreted that efficiency was from high to low with mainly fat tissue, mixed tissues and mainly mammary glandular tissue in the near field of acoustic pathway when the breast fibroadenomas were treated with USgHIFU. During the process of ultrasonic beams of USgHIFU focused at the focal region and induced the thermoalabtive effect causing coagulative necrosis of tumor, the tissues inside the near field of the acoustic pathway may absorb, reflect and scatter the ultrasonic beams, which may cause ultrasonic energy attenuation and then consequently affect the focused ultrasonic energy deposition into the targeted tumor. A previous study showed that the attenuation coefficient of fat was lower than the breast parenchyma, including the mammary glandular and duct tissues [Citation15]. It demonstrated that the fat tissue would let more focused ultrasonic beams pass through with lower attenuation, while the mammary glandular tissue would let less focused ultrasonic beams pass through with higher attenuation.
Our study showed that the size of fibroadenoma and EEF were negatively correlated (). It indicated that ablating a unit of tumor tissue volume in a large breast fibroadenoma required less ultrasonic energy in comparison with ablation of small breast fibroadenoma. Our results were in accordance with the previous studies of HIFU treatment for uterine fibroids [Citation16–18]. An in vitro experimental study showed that the EEF for a section of tissue ablation is smaller than that for a line of tissue ablation, and the EEF for the volumetric tissue ablation is smaller than that for a section of tissue ablation [Citation19]. Thus, this phenomenon may be explained by that the necrotic area will dynamically influence the acoustic environment, which lowers the EEF. This phenomenon may be also explained by that more acoustic energy was delivered in a given time by minimizing the interval between sonications when treating a larger breast fibroadenoma, and thus preventing the treated area from cooling down. In addition, the fibroadenoma with large size may have degeneration such as infarction, calcification and hyalinization, which could also facilitate the deposition of ultrasonic energy.
An in vitro study showed that the volume of necrosis decreased as sonication depths within bovine liver tissue increased [Citation20]. A previous retrospective study also demonstrated that the longer the distance from fibroid ventral side (shallow margin) to the skin was, and the more ultrasonic energy was required to ablate the uterine fibroid [Citation16]. In this study, we found the distance from shallow margin of the fibroadenoma to the skin and EEF were positively correlated. Similarly, we found that the ultrasonic energy needed to ablate the same volume of the breast fibroadenoma is increased along with the increase of the distance from shallow margin of the fibroadenoma to the skin, which was in consequence of more tissue in the acoustic pathway leading to higher energy attenuation and less energy deposition in the focus (). Therefore, the distance from shallow margin of the fibroadenoma was one of the predicting factors influencing efficiency of USgHIFU ablation.
Figure 7. Schematic diagram of fibroadenoma with different sizes in the near field of acoustic pathway.
As shown in , the “importance” of X1, X2 and X3 were 0.691, 0.218 and 0.091, respectively. The “importance” reflected the weighting of the independent variable in the model. The larger the value, the more important the variable was in predicting the dependent variable. In this study, the value of the “importance” of fibroadenoma size was the highest, accounting for 69.1% in the model; distance from shallow margin of the fibroadenoma to the skin was the second, accounting for 21.8% in the model; the type of near field of acoustic pathway accounts for only 9.1% in this model. Our results revealed that fibroadenoma size was the most dominant factor to predict EEF for breast fibroadenomas, while the role of other 2 factors was sub-dominant. For the validation of the model, since the correlation coefficient between the quantified value of predicted EEF and the quantified value of actual EEF was 0.698 (p = 0.000), this EEF dosimetric model was reliable in predicting the efficiency of USgHIFU ablation of fibroadenomas.
This study is limited because it is a retrospective study and dosage delivery or the termination of treatment might be influenced by different doctors performing the procedure. This study is also limited since ultrasonography was the only recommended screening method for breast fibroadenoma before USgHIFU, whether the feature of fibroadenomas on other imaging screening, such as mammography or magnetic resonance imaging (MRI), had an influence on EEF was not shown in this study. In this study, some bias may have occurred because many cases showed EEF was much higher than the linear regression. Future studies with large number of patients could be prospectively designed with different imaging examinations, in order to explore more factors that may be associated with the efficiency of USgHIFU treatment for fibroadenomas.
Conclusions
In conclusion, our preliminary study demonstrated that fibroadenoma size, distance from the shallow margin of the fibroadenoma to skin and types of near field of acoustic pathway could be used as predictors to evaluate the dosage delivery for USgHIFU treatment of fibroadenoma, with fibroadenoma size as the most important factor. The established regression model of ŷ= −0.496X1+0.287X2+0.203X3 could be used to predict EEF.
Disclosure statement
Zhibiao Wang and Lian Zhang are senior consultants to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):743–285.
- Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg. 2014;3(2):142–148.
- Chang DS, McGrath MH. Management of benign tumors of the adolescent breast. Plast Reconstr Surg. 2007;120(1):13e–19e.
- Neinstein LS. Breast disease in adolescents and young women. Pediatr. Clin. North Am. 1999;46(3):607–629.
- Schaefer FKW, Order BM, Eckmann-Scholz C, et al. Interventional bleeding, hematoma and scar-formation after vacuum-biopsy under stereotactic guidance: Mammotome(®)-system 11 g/8 g vs. ATEC(®)-system 12 g/9 g. Eur J Radiol. 2012;81(5):e739-45–e745.
- Wu F, Wang ZB, Cao YD, et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89(12):2227–2233.
- Peek MC, Ahmed M, Scudder J, HIFU-F Trialists’ Group, et al. High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia. 2016;32(8):881–888.
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound. 2015;3(1):1.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19(4):724–732.
- Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. 2018;50(4):1694–1715.
- Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82(1):e43–e49.
- Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med. 1998;13(9):640–645.
- Strano S, Gombos EC, Friedland O, et al. Color Doppler imaging of fibroadenomas of the breast with histopathologic correlation. J Clin Ultrasound. 2004;32(7):317–322.
- D'Astous FT, Foster FS. Frequency dependence of ultrasound attenuation and backscatter in breast tissue. Ultrasound Med Biol. 1986;12(10):795–808.
- Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine (Baltimore). 2015;94(13):e650.
- Liu ZQ, Gong CM, Liu YC, et al. Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2018;34(1):77–86.
- Yang MJ, Yu RQ, Chen JY, et al. Comparison of dose and effectiveness of a single-session ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids with different sizes. Front Oncol. 2021;11:725193.
- Li F, Wang Z, Du Y, et al. Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2006;23:839–843.
- Wang ZB, Bai J, Li FQ, et al. Study of a "biological focal region" of high-intensity focused ultrasound. Ultrasound Med Biol. 2003;29(5):749–754.