?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The charring tissue formation in the ablated lesion during the microwave ablation (MWA) of tumors would induce various unwanted inflammatory responses. This paper aimed to deliver appropriate thermal dose for effective ablations while preventing tissue carbonization by optimizing the treatment protocol during MWA with the set combinations of temperature control and pulsed microwave energy delivery.
Material and methods
The thermal phase transition of ex vivo porcine liver tissues were recorded by differential scanning calorimetry (DSC) to determine the temperature threshold during microwave output control. MWA was performed by an in-house built system with the ease of microwave output parameter adjustment and real-time temperature monitoring. The effects of continuous and pulsed microwave deliveries as well as various intermittent time-set of MWA were evaluated by measuring the dimensions of the coagulation zone and the carbonization zone.
Results
The DSC scans demonstrated that the ex vivo porcine liver tissues have been in a state of endothermic heat during the heating process, where the maximum absorbed heat occurred at the temperature of 105 °C ± 5 °C. The temperature control during MWA resulted in effective coagulative necrosis while preventing tissue carbonization, after setting 100 °C as the upper threshold temperature and 60 °C as the lower threshold. Both the numerical simulation and ex vivo experiments have shown that, upon the optimization of the time-set parameters in the periodic intermittent pulsed microwave output, the tissue carbonization was significantly diminished.
Conclusion
This study developed a straight-forward anti-carbonization strategy in MWA by modulating the pulsing mode and intermittent time. The programmed protocols of intermittent pulsing MWA have demonstrated its potentials toward future expansion of MWA technology in clinical application.
Introduction
Microwave ablation (MWA) is considered as an effective non-surgical alternative for the treatment of malignant tumors, including liver cancer [Citation1–3], lung cancer [Citation4,Citation5], kidney cancer [Citation6], thyroid cancer [Citation7], etc. The general principle of microwave ablation is to selectively deliver the microwave power through an antenna to heat the solid tumors, which could eventually destroy the cancer cells via thermal coagulative necrosis. Owning to their minimally invasiveness, limited complications and proven efficacy, MWA has increasingly been used for treating cancers in clinics [Citation8,Citation9].
During MWA process, direct energy deposition in the confined range of action will lead to excessively high central temperature in tissues around the microwave antenna. The carbonization in the ablated lesion could easily occur, where highly desiccated charring tissues usually appear. The tissue after MWA can be divided into carbonization zone (black part), coagulation zone (light yellow in color), transition zone (red hyperemic band) and normal zone [Citation10,Citation11]. Previous studies have shown that in the MWA of some organs such as the spleen [Citation12], the crushing and avulsion of charring tissues in the course of needle withdrawal can cause bleeding. The carbonization phenomenon may also induce both regional and systemic inflammatory response and other unwanted side effects [Citation13–19].
Appropriate temperature control for ablating tumor effectively while avoiding tissue carbonization is important for clinical MWA. Current anti-carbonization strategies mainly rely on optimizing MWA applicator designs [Citation20] or continuously injecting circulating physiological saline or water flow for cooling purposes [Citation21–23]. Various water-cooled antennas have been developed for preventing overhigh temperature at the front end of the needle rod so as to decrease the carbonizing probability [Citation24].
Some studies have compared MWA outcomes when using pulsed and continuous delivery of power. Hui et al. [Citation25] demonstrated that the method of power application is highly impactful on the size of the ultimate MWA zone and considered that systems with the ability to add short high-power pulses can increase ablation size and reduce procedure time without creating unpredictable shapes. Radosevic et al. [Citation26] studied the differences between continuous and short-pulse mode microwave ablation (MWA) and obtained slightly higher sphericity values under pulsed mode. Bedoya et al. [Citation27] compared the impact of continuous and pulsed energy deliveries on microwave ablation growth and shape, found that pulsed energy delivery created larger ablation zones at low average power compared to continuous energy delivery in the presence of blood perfusion. Although these studies did not directly observe the impact of carbonization, they gave some enlightenment on carbonization control protocol.
There are few studies on reducing carbonization based on the endothermic or exothermic law of tissue during heating. The purpose of this paper is to study the thermal phase transition law of liver tissue during microwave ablation and establish an effective carbonization optimization method on this basis. This paper developed an intermittent time-set technique in MWA, aiming at delivering appropriate thermal dose for effective ablations with decreased tissue carbonization. Known the temperature dependence of thermophysical and dielectric properties of ablated tissues, thermally induced conformational changes and maximum absorbed heat in tissues prior to MWA were firstly measured by differential scanning calorimetry (DSC). The effects of pulsed and continuous deliveries on temperature control, charring tissue formation and ablation efficiency were evaluated, respectively, toward the optimized power and pulsing MWA protocols.
Material and method
Determination of carbonization critical temperature of porcine liver
We cut the porcine liver into small pieces and put it in an electric furnace for heating. The initial upper limit of heating temperature was set to 110 °C, increasing by 5 °C each time. After heating to the threshold temperature, the porcine liver was taken out to observe whether it was carbonized.
Differential scanning calorimetry (DSC) measurement
DSC thermal analysis determines the heat transitions associated with physical properties as a function of time and temperature. In the present study, fresh porcine liver tissues obtained from the slaughterhouse were cut into small pieces between 4 and 12 mg (n = 12). The shape of the sample is close to a cuboid, the length is between 2 and 4 mm, the width is between 2 and 3 mm and the thickness is between 1 and 3 mm. The tissue samples were heated in the DSC (Germany, Netzsch) over a controlled temperature range of 20–170 °C where temperature increased at the rate of 5 °C/min. The excess specific heat capacity was measured at defined temperature increments. The heat quantity absorbed excessively by the liver tissue samples was plotted against temperature by subtracting the reference baseline. The phase transition temperature of porcine liver tissue during heating can be obtained from the DSC thermogram.
Microwave ablation system setup
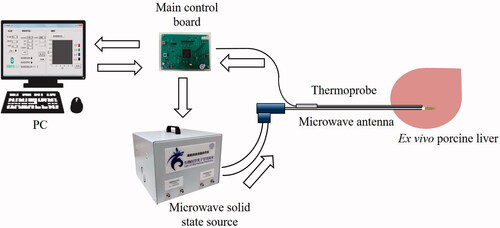
The MWA system () is composed of a 2450 MHz microwave solid state source (Chengdu Wattsine Electronic Technology Co., Ltd., Chengdu, China), a microwave antenna (Nanjing Kangyou Medical Technology Co., Ltd., Nanjing, China), a thermoprobe coupled with the microwave antenna, a personal computer (PC) and an in-house built main control board which can adjust microwave output parameters.
The output power of the microwave generator ranges from 1 to 150 W. The microwave antenna (KY-2450-B1) is 150 mm in length and 1.9 mm in diameter. The microwave antenna has a built-in water-cooling circulation pipe. The normal saline is pumped into the circulation pipeline through a water pump and can flow to a position 1 cm away from the connection between the needle rod and the puncture needle (microwave radiation point). The water circulation flow rate is 30 ml/min, and the average water temperature before and after the circulation is about 25 °C and 28 °C, respectively. The thermoprobe is 130 mm in length and 1.2 mm in diameter, which can measure temperature changes between 0 and 200 °C. The temperature sensor of the thermoprobe is a packaged NTC (negative temperature coefficient) thermistor with a resistance value of 10 K, which is insulated and waterproof. The thermoprobe monitors the temperature of the microwave radiation point, the normal saline cannot flow to this position, so it does not affect the authenticity of the temperature. The main control board accompanying with the software interface allows the setting of heating parameters of microwave ablation and real-time temperature recording.
Microwave ablation experiments in ex vivo liver samples
During the MWA process, the microwave antenna and the thermoprobe were closely attached to each other and temperature changes at the energy radiation point of the microwave antenna (11 mm away from the tip of the needle) was monitored. The antenna coupled with the thermoprobe was inserted into the porcine liver tissues up to 8 cm deep. For better comparison, the ablation power was set at 50 W and 60 W, in consistence with clinical setting, and the cumulative heating time was kept for 300 s in all the experimental conditions.
At the end of each MWA procedure, the tissue was sliced along the antenna tract, revealing a cross section of the ablation zone. The dimensions of the thermally coagulation zone as well as of the central carbonization zone were measured by a ruler. The long and short diameter were measured. Maximum length and width of the carbonization zone were also recorded.
Numerical simulation of MWA
Numerical simulation was performed by COMSOL Multiphysics to assess the heat transfer efficiency and radiation pattern during MWA under various continuous or pulsing power delivery condition. The liver carbonization critical temperature determined by the above-mentioned tissue carbonization experiment was set as the carbonization criterion in the Numerical simulation of MWA.
Electromagnetic wave propagation equation [Citation28,Citation29] and Pennes heat transfer equation [Citation28,Citation29] were coupled and dynamic dielectric and thermal parameters was utilized to establish dynamic MWA simulation model. The construction of ex vivo porcine liver MWA simulation model includes the following steps:
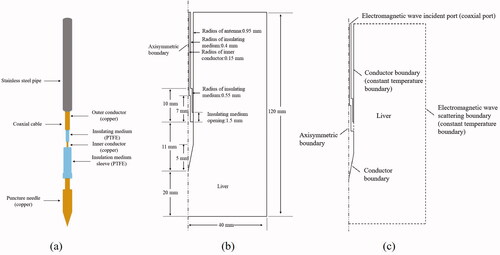
Step 1: Construction of microwave antenna model and ex vivo porcine liver model. The microwave antenna is simulated according to the structure of KY-2450-B1. The microwave antenna () is mainly composed of coaxial cable (inner conductor, insulating medium and outer conductor), stainless steel pipe and puncture needle and polytetrafluoroethylene (PTFE) insulation medium sleeve. The porcine liver tissue is isotropic and uniform, and the shape is set to be cylindrical. According to the symmetry of the two structures, the geometric model of the microwave antenna and porcine liver tissue is reduced to an axisymmetric structure to reduce the calculation time, as shown in .
Figure 2. (a) The structure of KY-2450-B1 microwave antenna. (b) Geometric model of simulated MWA of ex vivo porcine liver. (c) Setting of boundary conditions.
Step 2: Set the simulation parameters. It mainly includes the dielectric property parameters and tissue heat conduction parameters of liver tissue and the material parameters of the microwave antenna. The thermal conductivity () [Citation30–32], density (
) [Citation29,Citation31], relative dielectric constant (
) [Citation33–35], conductivity (
) [Citation33–35] and specific heat capacity (C) [Citation29–31,Citation35] of porcine liver are as follows:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
The dielectric property parameters and tissue heat conduction parameters used in the simulation model are shown in .
Table 1. Parameters used in the simulation model.
Because the outer boundary of the geometric model of liver tissue is far away from the microwave antenna, it has little effect on the central ablation zone. It can be directly set as a constant temperature boundary, that is, 293.15 K [Citation29,Citation36]. The boundary temperature of the inner and outer conductor of the microwave antenna and the stainless steel sleeve was set at 293.15 K [Citation29,Citation36]. The cooling effect of the water-cooling cycle on the coaxial cable can be simplified as the temperature boundary. Therefore, the outer boundary of the insulating medium and the boundary of the water-cooling part of the antenna can be set as the constant temperature boundary, that is, 293.15 K [Citation29,Citation36].
In addition, the boundary conditions for electromagnetic wave propagation also need to be set (). The top of the insulating medium between the inner and outer conductors of the coaxial cable is set as the electromagnetic wave incident port, that is, the coaxial type port. The outer boundary of the geometric model of liver is set as the electromagnetic wave scattering boundary condition to avoid the reflection of electromagnetic wave.
Step 3: Set the microwave frequency (2450 MHz), ablation power and ablation time to start MWA simulation. At the same time, the temperature distribution field can be obtained. Arrhenius thermal damage model is a common model used to analyze thermal damage [Citation37]. According to Arrhenius thermal damage model [Citation38], thermal damage can be quantitatively described as thermal damage factor Ω, so the thermal damage accumulated in t (time of duration) is:
(6)
(6)
where A is the frequency factor (s−1), Ea is the activation energy (J · mol−1), R is the gas constant (8.314 J · mol−1 · K−1) and T is the absolute temperature (K). As can be seen from EquationEquation (6)
(6)
(6) , the whole process of tissue thermal damage is described as the integral of Ω, which is determined by A and Ea.
Considering that tissue could get back to its native state after the thermal impulse is ceased, the expression of damage fraction (Fd) can be obtained from EquationEquation (6)(6)
(6)
(7)
(7)
when the damage fraction reaches 1, the degree of thermal injury reaches the maximum [Citation39], and it is considered that the cells have died irreversibly. Therefore, the damage fraction was used to evaluate the coagulation zone of the simulated ablation in this paper. Frequency factor (A) and activation energy (Ea) are set to convert the temperature field into thermal damage field. The A and Ea of liver tissue have fixed values (A = 7.39 × 1039 s−1, Ea = 2.577 × 105 J · mol−1) [Citation40].
If in vivo MWA needs to be simulated, the influence of blood flow should also be considered, the blood perfusion rate is additionally set.
Carbonization optimization experiment
The phase transition temperature from DSC curves was selected as the upper threshold for the temperature control in the optimization of MWA protocols. An aperiodic intermittent microwave output mode based on threshold temperature control is established. On the basis of temperature control, the periodic intermittent pulsed microwave output mode was established to achieve repeatability. The carbonization optimization effects of the two modes were verified by ex vivo porcine liver experiment. Moreover, the periodic intermittent pulsed microwave output mode is simulated and compared with the actual ablation effect to determine the optimal protocol to reduce carbonization.
Results
Carbonization critical temperature of porcine liver
The experimental results showed that the ex vivo porcine liver was carbonized at 130 °C. Therefore, 130 °C is used as the criterion to judge carbonization in the simulation results.
Thermally induced conformational changes in ex vivo porcine liver
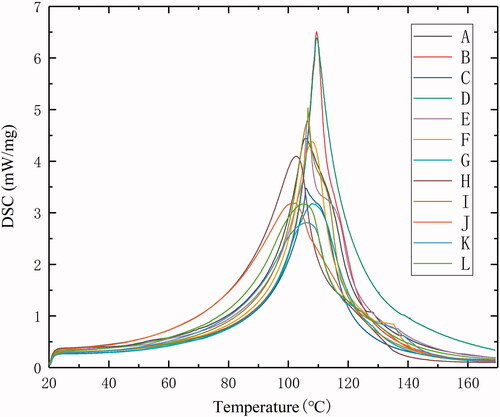
A total of 12 groups (A–L) of porcine liver DSC curves between 20 °C and 170 °C were obtained. has shown the DSC curves of all 12 ex vivo porcine liver samples, and the temperatures at the endothermic peaks and the maximum heat absorption rate for each sample were listed in . It can be found that the porcine liver has been in a state of endothermic heat during the whole process. The heat absorption rate gradually increased first, then started to decrease after reaching the maximum value. The DSC scans demonstrated a series of obvious endotherm between 90 °C and 120 °C and endothermic peaks at 105 °C ± 5 °C. The endothermic transitions of porcine liver tissues during heating process began at approximately from 55 °C to 60 °C, apparently due to protein denaturation. In the clinical treatment of MWA, the standard for complete tumor cell destruction is usually the tissue temperature reaching 60 °C [Citation41]. Therefore, the onset temperature was defined for following MWA temperature control at which the endothermic peaks began (60 °C) and reached the maximum value (∼100 °C).
Table 2. Peak temperature and maximum heat absorption rate.
Ablation results in the ex vivo porcine liver tissues of MWA with temperature control
We firstly assessed the ablation efficiency in ex vivo porcine liver samples using conventional continuous MWA and intermittent heating modes. Different microwave energy was delivered to the liver samples (n = 4 for each condition) by modulating the generator output power at 50 W and 60 W for each mode. Rising temperature in tissues was continuously monitored during ablations in each group by the thermoprobe coupled with the antenna. Temperature changes were recorded in real time and temperature profiles were plotted. In the continuous heating condition, the MWA applicator was inserted into the center of the liver samples and the ablation process was applied to the tissues for 300 s. In the intermittent heating condition, 100 °C was set as the upper threshold temperature and 60 °C was set as the lower threshold temperature. When the measured temperature reached the upper threshold, the ablation was suspended; when the measured temperature was less than the lower threshold, the ablation was restarted. A total cumulative time of microwave output in both continuous and intermittent groups was 300 s.
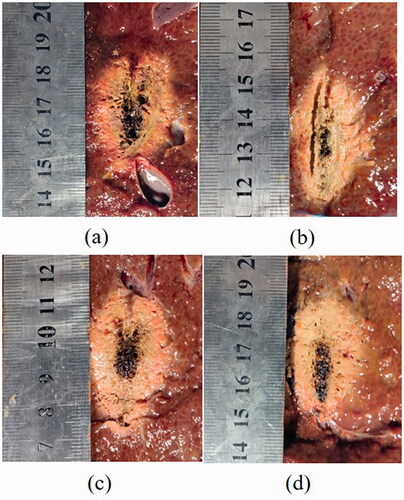
As shown in , yellow inner zones of coagulation were found in both continuous and intermittent MWA groups, suggesting effective ablations can be achieved in all the conditions. Clearly, as microwave energy is transmitted, it creates heat along the cable and length of the shaft, and the radiating portion of the antenna was sufficiently surrounded by the tissue. Larger coagulation zone was generated in the ex vivo porcine livers upon higher microwave energy delivery, i.e., 60 W. However, there are obvious differences in terms of charring tissue formation, dependent on the pulsing mode of MWA. In both power levels, the carbonization formation in the ablation zone of porcine liver in the intermittent heating group () was significantly diminished as compared to the normal ablation experimental group ().
Figure 4. Effective coagulation zone and charring tissue formation of porcine liver after various MWA procedures of (a) continuous mode at 50 W; (b) intermittent mode with temperature control at 50 W; (c) continuous mode at 60 W; and (d) intermittent mode with temperature control with 60 W. The cumulative ablation time of 300 s were maintained for all the treatments.
There was no significant statistical difference between the any two groups of experimental data under each condition (p > .05). As shown in , although the aperiodic intermittent heating mode tended to have no significant difference in coagulation zone compared to continuous heating groups, intermittent deliveries in general resulted in smaller length and width of the carbonization zone as compared to continuous ablation. When the power is 50 W and the effective cumulative time is 300 s, the maximum length and width of carbonization zone decreased from 18.75 mm and 8.25 mm (continuous output) to 9.25 mm and 3.5 mm (aperiodic intermittent pulsed output).
Table 3. Dimensions of coagulation zone and carbonization zone in the ex vivo porcine liver tissues after various MWA treatments when the cumulative time of microwave output is set to 300 s (n = 4 for each condition, actual ablation).
Ablation results in the ex vivo porcine liver of MWA with periodic intermittent time-set pulsing output
As demonstrated above, the MWA outcome was dependent on both temperature and the duration of heating, and appropriate temperature control could result in effective thermal damage without significant carbonization. However, the ablation mode based on temperature control is an aperiodic intermittent pulsed microwave output mode, a thermoprobe is often necessary for continuous temperature monitoring. From the recorded temperature profiles, the time required for porcine liver tissues to rise from 60 °C to 100 °C was relatively stable, which was 10 s ± 1 s. We thus hypothesized that programmed protocols with intermittent time setting could also be applicable for temperature control instead of direct temperature monitoring.
Firstly, three periodic intermittent heating protocols were designed in the present study. In each protocol, samples were continuously heated for 10 s, then the heating process was paused for 20 s, 30 s and 40 s, respectively, and then the tissues were reheated for 10 s, followed by repeating the last two processes. The cumulative ablating time of 300 s was kept identical for each condition, while the total experimental time for each protocol is 900 s, 1200 s and 1500 s, respectively. The microwave generator output power was set at 50 W for all the conditions (n = 4 for each condition).
The carbonization zone (black part) and coagulation zone (light yellow in color) in the actual ablation can be judged with the naked eye. The carbonization zone in the simulated ablation is the zone outlined by a threshold of 130 °C (blue line), the coagulation zone in the simulated ablation is the zone with a damage fraction of 1 (white line). From both simulation results (upper row in ) and experimental results (lower row in ), as expected, the greatest temperatures existed nearest to the microwave antenna, with successively lower temperatures at more distant radial locations. Compared with the continuous ablation mode, periodic intermittent pulsed microwave output can effectively reduce the carbonization composition in the central area of the ablation zone. There was no significant statistical difference between the any two groups of experimental data under each condition (p > .05). As shown in , the maximum length and width of carbonization zone decreased from 21.75 mm and 8 mm (50 W, 300 s) to 18.25 mm and 4 mm (50 W,10 s–40 s–1500 s), respectively, by modulating the pulsing mode and intermittent time of MWA protocols. Given the cumulative heating time fixed (300 s), the longer the intermittent pausing time is, the less carbonization zone would form.
Figure 5. Numerical simulation (upper row) and experimental results (lower row) in the ex vivo porcine liver tissues after various MWA treatments. (a, e) The tissues were treated with continuous ablation; the tissues were treated under intermittent ablation with 10 s heating followed by a pause time of (b, f) 20 s, (c, g) 30 s and (d, h) 40 s for each cycle. The total ablation time in all the conditions is 300 s, and the output power is 50 W.
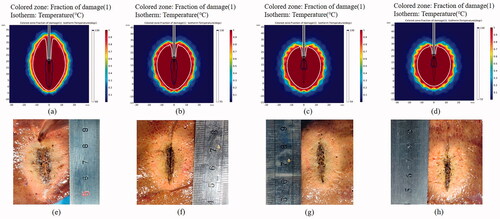
Table 4. Dimensions of coagulation zone and carbonization zone in the ex vivo porcine liver tissues after various MWA treatments under 50 W (n = 4 for each condition, actual ablation).
In order to further reduce carbonization, we further optimized the intermittent MWA time-set protocols by shortening the ablation time and pause time. Samples were continuously heated for 5 s, then the heating was paused for 10 s, 15 s and 20 s, respectively, followed by repeating the process (n = 4 for each condition). With the same duty cycle, the output power and the total heating time of 300 s, the carbonization composition of the ablation zone was further decreased, evidenced by both simulated results (, upper row) and experimental results (, lower row).
Figure 6. Numerical simulation (upper row) and experimental results (lower row) in the ex vivo porcine liver tissues after various MWA treatments. The tissues were treated under intermittent ablation with 5 s heating followed by a pause time of (a, d) 10 s, (b, e) 15 s and (c, f) 20 s for each cycle. The total ablation time in all the conditions is 300 s, and the output power is 50 W.
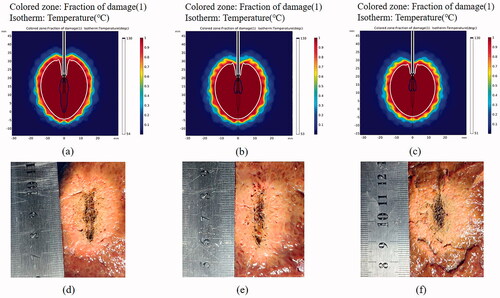
As shown in , there was no significance of the measured long and short diameter of the thermally coagulation zone between groups with the same settings (p > .05). Although the periodic intermittent pulsed mode tended to have slightly smaller ablations compared to continuous heating groups, pulsed deliveries in general resulted in smaller length and width of the carbonization zone as compared to continuous ablation. In particular, the maximum length and width of carbonization zone decreased from 21.75 mm and 8 mm (50 W, 300 s) to 12 mm and 4 mm (50 W, 5 s–20 s–1500 s), respectively, by modulating the pulsing mode and intermittent time of MWA protocols. Under the same duty cycle, by shortening the ablation time and pause time, the maximum length of the carbonization zone is decreased from 18.25 mm (50 W, 10 s–40 s–1500 s) to 12 mm (50 W, 5 s–20 s–1500 s).
To our interest, the ablation axial ratio was significantly increased in the periodic intermittent pulsed ablation group. In the continuous ablation (50 W, 300 s), the long and short diameter of the coagulation zone were 36.75 mm and 25.75 mm, respectively, and the axial ratio was 0.701. On the contrary, in the periodic intermittent pulsed mode (50 W, 5 s–20 s–1500 s), the long and short diameter of the coagulation zone was 34.5 mm and 29.5 mm, respectively, and axial ratio was 0.855, making the coagulation zone close to spherical shape.
Discussion
Carbonization critical temperature
In the process of MWA, charring tissue formation close to the microwave antenna would lead to an increase of inflammatory possibilities. As the ultrahigh temperature is considered as the major contributor to the tissue carbonization, temperature control has been often involved in the MWA procedure to avoid carbonization. However, there are controversy reports regarding the typical carbonization temperature dependent on the properties of samples, heating transition and ablating duration. For instance, Rhim et al. reported that when the temperature exceeded 110 °C, the tissue would be carbonized [Citation42]. Ghanaati et al. [Citation43] believed that the tissue carbonization would occur when the temperature exceeds 300 °C. Therefore, it is critical to determine the carbonization temperature toward the optimization of MWA protocols for achieving improved therapeutic outcomes. After heating verification, 130 °C was used as the carbonization critical temperature of porcine liver in this paper.
Advantages of periodic intermittent pulsed microwave output mode
In the present study, the heat transition properties of liver tissues induced by increasing temperature was determined by the DSC thermal analysis. The temperatures at which the tissue conformational changes firstly occurred and the maximum absorbed heat achieved were obtained. From our results, 60 °C and 100 °C were used as threshold temperatures to reduce carbonization during MWA, in consistency with previous reports suggesting that the irreversible cellular damage and protein coagulation occur by heating tissues at 60 °C [Citation40,Citation44,Citation45].
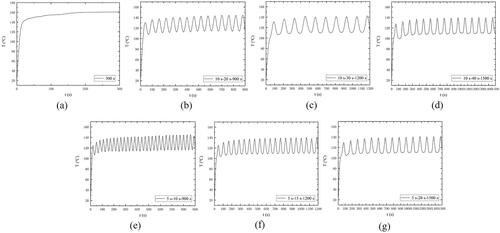
With the combination of numerical simulation and ex vivo experiments, temperature control during MWA can be achieved by adjusting the microwave power and time of irradiation without the re-design of antenna structure. From our ex vivo porcine liver ablation results, pulsed deliveries were characterized by comparable coagulation zone and smaller carbonization compared to continuous ablations with similar average power. As the ablating temperature was closely monitored during the MWA process and total heating time and output power remained unchanged, differences between groups may actually have been more attributable to heat-mass transfer. Comparing the different experimental conditions, the significant decrease of the central carbonization zone obtained in the cases with the pulsing microwave energy delivery could be possibly accounted for more efficient thermal exchange with the surrounding environment. From the temperature curves in the actual ablation (), it can be seen that compared with the continuous microwave output mode, the periodic intermittent pulsed microwave output mode can reduce the temperature of the energy radiation point by about 20 °C. Under the same ablation power (50 W) and cumulative ablation time (300 s), the maximum temperature in the periodic intermittent pulsed microwave output mode (5 s–20 s–1500 s) was 141 °C, and the maximum temperature in the continuous microwave output mode was 161 °C.
Figure 7. Temperature at the microwave energy radiation point during various MWA treatments of ex vivo porcine liver. (a) The tissues were treated with continuous ablation; The tissues were treated under intermittent ablation with 10 s heating followed by a pause time of (b) 20 s, (c) 30 s and (d) 40 s for each cycle; The tissues were treated under intermittent ablation with 5 s heating followed by a pause time of (e) 10 s, (f) 15 s and (g) 20 s for each cycle. The total ablation time in all the conditions is 300 s, and the output power is 50 W.
Tissue contraction during high-temperature MWA has a great impact on the size and shape of the coagulation zone [Citation46]. The degree of tissue contraction is related to the size of the carbonization zone. Moreover, the longitudinal contraction (along the microwave antenna direction) is always less than the radial contraction (orthogonal to microwave the antenna direction) [Citation47], which will cause the coagulation zone to appear more elongated along the microwave antenna [Citation48]. In addition, the carbonization tissue is characterized by an asymmetric linear contraction (about 30% and 19% in the radial and longitudinal directions, respectively), while the coagulation tissue is characterized by an isotropic linear contraction (about 11%, independent of the direction) [Citation49]. As can be seen from and , compared with the continuous microwave output mode, the periodic intermittent pulsed microwave output mode can effectively reduce carbonization and increase the axial ratio of the coagulation zone. This is precisely because the periodic intermittent pulsed microwave output mode can reduce the temperature of the center ablation zone, so as to reduce carbonization, resulting in lower radial contraction, and finally increase the axis ratio of the coagulation zone.
Comparison between simulated ablation and actual ablation
The main purpose of simulation is to verify the feasibility of reducing carbonization in periodic intermittent pulsed microwave output mode. Only when the same results are obtained in both simulation and actual experiment, can the effectiveness of intermittent pulsed ablation mode in reducing carbonization be preliminarily proved.
In the fixed microwave output mode, the results of ex vivo model simulation and experimental ablation are consistent. The shape of the coagulation zone and the carbonization zone are basically the same. However, since some parameter changes are simplified and some ideal boundaries are set in the simulation model, the simulated coagulation zone is slightly different from the actual coagulation zone, the average error of the long diameter and the short diameter is within 3 mm. As shown in , the maximum errors of the long diameter and short diameter of the coagulation zone obtained by simulated ablation and actual ablation are 2.75 mm and 2.5 mm, respectively, and the minimum errors are 0.75 mm and 0.5 mm, respectively. The maximum absolute percentage errors of long diameter and short diameter are 6.96% and 9.8%, respectively, and the minimum absolute percentage errors of long diameter and short diameter are 2.13% and 1.66%, respectively. For example, in the same periodic intermittent pulsed microwave output mode (50 W, 5 s–20 s–1500 s), the long and short diameter of the coagulation zone of simulated ablation are 35.25 mm and 30 mm, respectively, and the long and short diameter of the coagulation zone of actual ablation are 34.5 mm and 29.5 mm, respectively. The maximum length and width of carbonization zone of simulated ablation are 9.5 mm and 4 mm, respectively, and the maximum length and width of carbonization zone of actual ablation are 12 mm and 4 mm, respectively.
Table 5. Dimensions of coagulation zone of simulated ablation and actual ablation of the ex vivo porcine liver tissues after various MWA treatments under 50 W.
Simulation results and ex vivo porcine liver experiments have confirmed that the periodic intermittent pulsed microwave output mode can obtain less carbonization than the continuous microwave output mode. In the case of fixed microwave power (50 W) and cumulative ablation time (300 s), one fifth of the duty cycle (5 s–20 s–1500 s) can obtain a less carbonization than one third of the duty cycle (5 s–10 s–900 s). This result was also verified in the ex vivo porcine liver experiments. The maximum length and width of carbonization zone decreased from 20.5 mm and 4.25 mm (5 s–10 s–900 s) to 12 mm and 4 mm (5 s–20 s–1500 s).
Limitation of the study
A limitation of this study was that the in vivo ablation verification was not performed. In this study, the design of simulation model and actual ablation were performed based on the ex vivo porcine liver. The characteristics of ex vivo porcine liver tissue are similar to that of human liver [Citation50]. The biggest difference between ex vivo animal tissue experiments and in vivo experiments is that there is a rich blood supply in living animal tissues. Because blood perfusion has a certain influence on the ablation zone, the MWA zone of living animal tissues is generally smaller than ex vivo experiments. However, in order to analyze the carbonization results under different microwave output ablation modes, the experiment often needs to be repeated many times, and the in vivo tissue experiment is difficult to carry out on a large scale, so the ex vivo tissue experiment is still very necessary.
Since the value of blood perfusion rate and its change with temperature are affected by individual differences and other factors, different researchers use different values in the simulation. In addition, considering the effect of thermal injury on blood flow, it can be simplified that the blood perfusion rate of tissues above 333.15 K is 0. We use the average value reported in the literature for simulation, that is, when the temperature is less than 333.15 K, the blood perfusion rate is 0.016 s−1 [Citation31]. According to our simulation results (), it is found that dimensions of the simulated coagulation zone with blood perfusion is smaller than that without blood perfusion in the same periodic intermittent pulsed microwave output mode, the short diameter of the coagulation zone is 30% smaller when blood perfusion is included in simulations. However, after blood perfusion is added, the dimension of carbonization zone in the periodic intermittent pulsed microwave output mode is much smaller than that in the continuous ablation mode. In the continuous ablation (50 W, 300 s), the maximum length and maximum width of the carbonization zone can be 21 mm and 6 mm, respectively. In the one fifth of the duty cycle (50 W, 5 s–20 s–1500 s) periodic intermittent pulsed microwave output mode, there is only a little carbonization near the microwave antenna, the maximum length and maximum width of the carbonization zone can be 3 mm and 2.5 mm, respectively, as shown in . Meanwhile, the axial ratio of the coagulation zone of in vivo periodic intermittent pulsed microwave output is greater than that of the continuous microwave output. In the continuous ablation (50 W, 300 s), the long and short diameter of the coagulation zone were 37.5 mm and 21 mm, respectively, and the axial ratio was 0.560. On the contrary, in the one fifth of the duty cycle (50 W, 5 s–20 s–1500 s) intermittent pulsed microwave output mode, the long and short diameter of the coagulation zone was 29 mm and 19 mm, respectively, and axial ratio was 0.655.
Figure 8. In vivo porcine liver ablation simulation effect (blood perfusion added) after various MWA treatments. (a) The tissues were treated with continuous ablation; the tissues were treated under intermittent ablation with 10 s heating followed by a pause time of (b) 20 s, (c) 30 s and (d) 40 s for each cycle; The tissues were treated under intermittent ablation with 5 s heating followed by a pause time of (e) 10 s, (f) 15 s and (g) 20 s for each cycle. The total ablation time in all the conditions is 300 s, and the output power is 50 W.
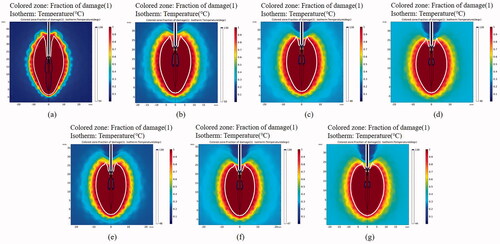
Table 6. Dimensions of coagulation zone and carbonization zone obtained by in vivo porcine liver tissues ablation simulation (blood perfusion added) after various MWA treatments under 50 W.
Our study first focuses on verifying the feasibility of intermittent pulsed microwave output mode to reduce carbonization. Next, a more accurate simulation model with blood perfusion will be established and the repeatability of intermittent ablation mode to reduce carbonization will be further verified on living animals (in vivo).
Conclusion
The heat transition properties of ex vivo porcine liver tissues during heating process were studied to determine the boundary temperature for effective ablation zone while preventing tissue carbonization. Temperature control was also achieved with set combinations of microwave energy delivery and irradiation time duration. As compared to the conventional continuous ablation mode, periodic intermittent pulsed microwave output could effectively reduce the charring tissue formation, this ablation mode is of great value for the tiny carbonization ablation of small tumors. More in-depth understanding of the thermal-induced bio-physical changes of ablating tissues in response to the temperature distribution profiles as the function of time is required to elucidate the underlying mechanisms and link to the findings in in vivo study. At the same time, how to reduce carbonization while maintaining a comparable ablation zone should also be studied.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ryu T, Takami Y, Wada Y, et al. Oncological outcomes after hepatic resection and/or surgical microwave ablation for liver metastasis from gastric cancer. Asian J Surg. 2019;42(1):100–105.
- De Cobelli F, Marra P, Ratti F, et al. Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions: new advances in interventional oncology: state of the art. Med Oncol. 2017;34(4):49.
- Meloni MF, Chiang J, Laeseke PF, et al. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 2017;33(1):15–24.
- Al-Hakim RA, Abtin FG, Genshaft SJ, et al. Defining new metrics in microwave ablation of pulmonary tumors: ablation work and ablation resistance score. J Vasc Interv Radiol. 2016;27(9):1380–1386.
- Vogl TJ, Roman A, Nour Eldin NEA, et al. A comparison between 915 MHz and 2450 MHz microwave ablation systems for the treatment of small diameter lung metastases. Diagn Interv Radiol. 2018;24(1):31–37.
- Cornelis FH, Marcelin C, Bernhard JC. Microwave ablation of renal tumors: a narrative review of technical considerations and clinical results. Diagn Interv Imaging. 2017;98(4):287–297.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30(2):150–157.
- Dou JP, Liang P, Yu J. Microwave ablation for liver tumors. Abdom Radiol. 2016;41(4):650–658.
- Wells SA, Hinshaw JL, Lubner MG, et al. Liver ablation: best practice. Radiol Clin North Am. 2015;53(5):933–971.
- Jin X, Li Y, Liu W, et al. Study on the relationship between reduced scattering coefficient and Young's modulus of tumors in microwave ablation. Minim Invasive Ther Allied Technol. 2021;30(6):347–355.
- Zhao J, Qian Z, Liu J, et al. Real-time assessment of microwave ablation efficacy by NIR spectroscopic technique. J Innov Opt Health Sci. 2014;07(01):1350053.
- Fei G, Yang G, Jing S, et al. Experimental study of destruction to porcine spleen in vivo by microwave ablation. World J Gastroenterol. 2011;17(45):5014–5020.
- Davidson EB, Davis MS, Campbell GA, et al. Comparison of CO2 laser and sharp dissection techniques for excision of elongated soft palates in brachycephalic dogs. The International Symposium on Biomedical Optics. 2001, San Jose, CA, United States. Washington: SPIE Digital Library, vol. 4244. p. 575–582.
- Salwa Y, Edgard J, Sami ET, et al. Histological study of induced incisions on rabbits’ tongues with three diode lasers with different wavelengths in continuous mode. Scientifica. 2018;2018:1–8.
- Liu F, Yu X, Liang P, et al. Ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous haemangiomas. Int J Hyperthermia. 2018;34(7):1061–1066.
- Cui R, Yu J, Gu Y, et al. Microwave ablation assisted by three-dimensional visualization system as local therapy for relapsed hepatoblastoma: a small pilot study. Abdom Radiol. 2019;44(8):2909–2915.
- Zhong L, Sun S, Shi J, et al. Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis. 2017;9(3):590–597.
- Hansch A, Pfeil A, Neumann R, et al. Renal ablation in patients with end-stage renal disease. Vasa. 2011;40(4):308–314.
- Cheng Z, Liang P, Yu X, et al. Percutaneous microwave ablation for hepatic cavernous hemangiomas: a preliminary clinical result. Eur J Oncol. 2015;19(4):231–239.
- Umehara H, Seki T, Inokuchi R, et al. Microwave coagulation using a perfusion microwave electrode: preliminary experimental study using ex vivo and in vivo liver. Exp Ther Med. 2012;3(2):214–220.
- Lee JH, Kim YS, Lee D, et al. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010;34(7):1488–1493.
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81(5):905–910.
- Kim DW. Sonography-guided ethanol ablation of a remnant solid component after radio-frequency ablation of benign solid thyroid nodules: a preliminary study . AJNR Am J Neuroradiol. 2012;33(6):1139–1143.
- Granchi S, Vannacci E, Breschi L, et al. Advantages of cooled fiber for monitoring laser tissue ablation through temporal and spectral analysis of RF ultrasound signal: a case study. Ultrasonics. 2018;82:49–56.
- Hui T, Brace CL, Hinshaw JL, et al. Microwave ablation of the liver in a live porcine model: the impact of power, time and total energy on ablation zone size and shape. Int J Hyperthermia. 2020;37(1):668–676.
- Radosevic A, Prieto D, Burdío F, et al. Short pulsed microwave ablation: computer modeling and ex vivo experiments. Int J Hyperther. 2021;38(1):409–420.
- Bedoya M, Rio AD, Chiang J, et al. Microwave ablation energy delivery: influence of power pulsing on ablation results in an ex vivo and in vivo liver model. Med Phys. 2014;41(12):123301.
- Jiang Y, Zhao J, Li W, et al. A coaxial slot antenna with frequency of 433 MHz for microwave ablation therapies: design, simulation, and experimental research. Med Biol Eng Comput. 2017;55(11):2027–2036.
- Zhao J, Wang J, Mu Y, et al. Construct of a practical simulation model of microwave ablation. China Med Dev. 2019;36(4):36–43.
- Guntur SR, Lee KI, Paeng DG, et al. Temperature-dependent thermal properties of ex vivo liver undergoing thermal ablation. Ultrasound Med Biol. 2013;39(10):1771–1784.
- Hall SK, Ooi EH, Payne SJ. Cell death, perfusion and electrical parameters are critical in models of hepatic radiofrequency ablation. Int J Hyperthermia. 2015;31(5):538–550.
- Lopresto V, Argentieri A, Pinto R, et al. Temperature dependence of thermal properties of ex vivo liver tissue up to ablative temperatures. Phys Med Biol. 2019;64(10):105016.
- Lopresto V, Pinto R, Cavagnaro M. Experimental characterisation of the thermal lesion induced by microwave ablation. Int J Hyperthermia. 2014;30(2):110–118.
- Ji Z, Brace CL. Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys Med Biol. 2011;56(16):5249–5264.
- Sebek J, Albin N, Bortel R, et al. Sensitivity of microwave ablation models to tissue biophysical properties: a first step toward probabilistic modeling and treatment planning. Med Phys. 2016;43(5):2649–2661.
- Etoz S, Brace CL. Analysis of microwave ablation antenna optimization techniques. Int J RF Microw Comput Aided Eng. 2018;28(3):e21224.
- Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia. 2013;29(4):262–280.
- Zhao J, Zhao Q, Jiang Y, et al. Feasibility study of modeling liver thermal damage using minimally invasive optical method adequate for in situ measurement. J Biophotonics. 2018;11(6):e201700302.
- Jasiński M. Investigation of tissue thermal damage process with application of direct sensitivity method. Mol Cell Biomech. 2013;10(3):183–199.
- Chang IA, Nguyen UD. Thermal modeling of lesion growth with radiofrequency ablation devices. Biomed Eng Online. 2004;3(1):27.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72(1):124–131.
- Rhim H, Goldberg SN, Dodd GD, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21(suppl_1):S17–S35.
- Ghanaati H, Alavian SM, Firouznia K, et al. Tailoring of interventional procedures for HCC Patients-Review article. Iran J Radiol. 2011;7(3):129–143.
- Wu H, Chen B, Peng B. Effects of intratumoral injection of immunoactivator after microwave ablation on antitumor immunity in a mouse model of hepatocellular carcinoma. Exp Ther Med. 2018;15(2):1914–1917.
- Shao Q, Han Z, Ni X, et al. Feasible temperature of percutaneous microwave ablation of dog liver abutting the bowel. Int J Hyperther. 2011;27(2):124–131.
- Liu D, Brace CL. Numerical simulation of microwave ablation incorporating tissue contraction based on thermal dose. Phys Med Biol. 2017;62(6):2070–2086.
- Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia. 2014;30(7):419–428.
- Liu D, Brace CL. Evaluation of tissue deformation during radiofrequency and microwave ablation procedures: influence of output energy delivery. Med Phys. 2019;46(9):4127–4134.
- Lopresto V, Strigari L, Farina L, et al. CT-based investigation of the contraction of ex vivo tissue undergoing microwave thermal ablation. Phys Med Biol. 2018;63(5):055019.
- Jones C, Badger SA, Ellis G. The role of microwave ablation in the management of hepatic colorectal metastases. Surgeon. 2011;9(1):33–37.