Abstract
Background
The use of heat to treat various diseases is called hyperthermia treatment (HT). Since the 1970s, the anti-cancer effects of HT have been investigated. Different HT techniques can be categorized as local, regional and whole-body hyperthermia treatment (WBHT). We aim to provide a summary of recent research done on HT to treat cancer.
Methods
In July 2020 ClinicalTrials.gov were systematically searched for all trials including hyperthermia and cancer registered between 2000 and 2020. Studies were excluded when they did not concern hyperthermal treatment, when they were not oncological studies, when they were observational or other non-interventional studies.
Results
Of 1654 identified trials, 235 were included. Of these 235 studies, 123 described the use of HIPEC (52.3%), 44 other types of regional HT (18.7%), 45 local HT (19.1%) and 15 WBHT (6.4%). A steady increase (720%) in research to hyperthermic intraperitoneal chemotherapy (HIPEC) can be observed in the last decade. Although HIPEC is the most researched HT modality, an evolution in other HT technologies could be observed during the past decade.
Conclusions
Research to HT to treat cancer has expanded fast. Some techniques, for example HIPEC start to be used outside of research context, but overall, more research is needed to establish a clear effect of these HT techniques.
Keywords:
1. Introduction
Heat has been used to treat tumors since ancient times. Even Hippocrates mentioned the use of heat to treat cancer [Citation1]. The use of heat to treat malignancies is called hyperthermia treatment (HT). Since the 1970s, the biological effects of HT have been investigated. Various heating techniques have been introduced in the oncological field ever since. These techniques can be divided in two categories: ablation and HT. Ablation aims to destroy tumor cells directly by using temperatures above 50 °C. HT aims to sensitize tumor cells to chemo- or radiotherapy by using temperatures below 45 °C [Citation2].
The destructive effect of HT can be partially explained by induction of direct apoptosis. The temperature and duration of HT necessary to induce apoptosis can be defined as the thermal effective dose (TED) [Citation3]. The TED differs between cell type and the phase of the cell-cycle. The TED may be altered by induced thermotolerance as well [Citation4]. In addition, HT increases the tumor blood flow, permeability, pO2, pH and tumor nutritional status [Citation5]. These alterations can have an additive effect when HT is compared with other anticancer therapies, since they seem to be complementary.
Many techniques have been developed to deliver heat to a tumor. In general, these can be divided in three main categories; local-, regional- and whole-body hyperthermia treatment (WBHT) [Citation6]. Local HT aims to heat a small part of the body to destroy cancer cells and blood vessels. Examples of local HT include interstitial HT, where a needle is used to administer heat to the tissue [Citation7]; high-intensity frequency ultrasounds (HIFU), which uses ultrasound to deliver the heat [Citation8]; nanoparticle based magnetic HT [Citation9–11] and electroporation [Citation2]. Local HT has been used to treat tumors in different organs, such as head and neck [Citation12], gynecological [Citation13], breast [Citation14, Citation15], brain [Citation16] and thyroid [Citation17].
Regional HT aims to heat a body cavity. It is most often used in combination with radiotherapy or administered as heated chemotherapy [Citation6]. The most frequently used technique, hyperthermic intraperitoneal chemotherapy (HIPEC), aims to create a flow of heated chemotherapy through the abdominal cavity. HIPEC is most often used to treat gastro-intestinal [Citation18] and gynecological tumors [Citation19,Citation20]. Other techniques to heat body cavities are being developed; these include hyperthermic intravesical chemotherapy (HIVEC) [Citation21] and hyperthermic intrathoracic chemotherapy (HITOC) [Citation22]. Last, isolated limb perfusion is a technique that temporarily replaces the blood in a limb with a heated chemotherapeutic agent. It is mainly used to treat soft tissues sarcomas [Citation23] and melanomas [Citation24].
WBHT aims to elevate the temperature of the entire body by heating directly the body or the blood. Temperature may be raised to a fever range (39–40 °C) or to higher temperatures (41–43 °C). WBHT is most often used in combination with chemo and/or radiotherapy [Citation25] for advanced or metastasized cancers of the lung, ovarian, colon, pancreas or sarcomas.
ClinicalTrials.gov is a website established in 2000 and maintained by the National Library of Medicine and the National Institutes of Health [Citation26]. Since 2008, investigators must upload the study protocol of their clinical trial before conducting any clinical trial. Study progress and results related to the trial can be uploaded as well; however, this is not mandatory. Due to the obligation of uploading the clinical trial study protocol, drafting databases from all listed studies on a specific topic is possible.
Cihoric et al. [Citation27] published an initial article about the research of HT, using the ClinicalTrials.gov database [Citation28]. He mainly concluded a growing interest in HT as a cancer treatment modality and the large amount of HIPEC studies within the HT research. Since the publication of this article, an extensive number of new research in HT has been conducted. This systematic review aims to update and summarizes the clinical studies available on clinicaltrials.gov carried out on HT for cancer treatment.
2. Material and methods
2.1. Study protocol
In this study, we try to provide an overview of all clinical trials that have been conducted on HT in cancer patients posted to ClinicalTrials.gov.
2.2. Search strategy and eligibility criteria (inclusion/exclusion)
For this systematic review, we conducted a search on the ClinicalTrials.gov website. Using ‘Cancer’ and ‘Hyperthermia’ as Medical Subject Headings (MeSH) terms. For this systematic review, we searched for studies focusing on the efficacy of any form of HT in humans with all types of cancer. Studies were excluded when they did not concern HT, when they were not oncological studies, when they were observational or other non-interventional studies.
2.3. Data extraction and screening methods
One author (HP) screened the files retrieved from ClinicalTrials.gov. Using the information provided on the website (title, study description, study design, arms and interventions and outcome measures), inclusion for this systematic review was decided. Then, using the information provided by the ClinicalTrials.gov website, the studies were categorized by: NCT number, phase, recruitment status, last update date, funder type, age of the patients, enrollment, tumor type and stage, HT technique, temperature, duration and frequency of the HT administered, other simultaneous treatments and whether the results were published to ClinicalTrials.gov. This information was summarized in a Microsoft Excel version 16.4 database.
3. Results
The great diversity among the methodology studies and insufficient information was an impeditive for achieving profound statistical analysis; therefore, a narrative report of the included studies was performed.
3.1. Data extraction
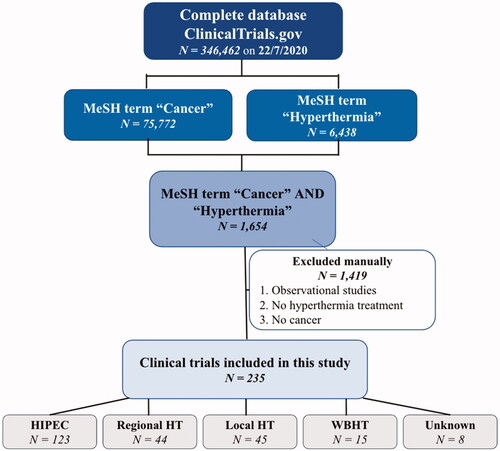
On 22 July 2020, records of 346,462 registered studies were retrieved from ClinicalTrials.gov. Studies were refined using the MeSH terms for database search. Cancer as MeSH term provided 75,772 hits, hyperthermia as MeSH term gave 6438 hits. The combination of these two provided 1654 hits. Hereafter, non-interventional studies, studies which did not concern HT nor cancer treatment were excluded manually. Eventually, 235 studies were included, which were classified into five types based on the HT used: HIPEC (n = 123), Regional HT (n = 44), Local HT (n = 45), WBHT (n = 15) and unknown (n = 8). The search strategy and exclusion of trials are summarized in . Later, included trials were characterized by location, enrollment, type of hyperthermic intervention (e.g., local, regional or WBHT), temperature, duration and frequency of the HT administered.
3.2. General characteristics of the clinical trials
After initial evaluation, we included 235 studies on HT. Of these 235 studies, 123 described the use of HIPEC (52.3%), 44 other types of regional HT (18.7%), 45 local HT (19.1%) and 15 WBHT (6.4%). The type of HT could not be classified in 8 trials (3.4%). General trial characteristics are summarized in . In terms of trial stages, 40 trials (17%) were exploratory or in phase I, 95 trials (40.4%) were in phase II and 56 trials (23.8%) were in phase III. Forty-four trials (18.7%) did not specify the phase of the study. Most of the studies were sponsored by public institutions or hospitals (n = 189) and a minority (n = 46) were sponsored by a private initiative. Most trials have been categorized as completed (n = 100). We categorized all recruiting, not yet recruiting and suspended studies as ongoing (n = 65). The others (not completed, withdrawn or terminated) were marked as not completed (n = 27). Forty-three studies are stated as unknown by ClinicalTrials.gov.
Table 1. General trial characteristics.
3.3. Evolution of hyperthermia studies
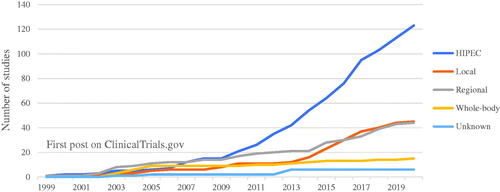
As illustrated in , a large increase of HIPEC studies can be observed since 2010. Prior to 2010, 15 studies were found. After 2010, 108 new studies were published on HIPEC, an increase of 720%. Since 2014, a smaller increase in local and regional HT studies can be seen. WBHT is the least researched type of HT. Up until now, only 15 studies could be identified on ClinicalTrials.gov.
Figure 2. Evolution of studies posted to ClinicalTrials.gov. Cumulative quantity of studies uploaded on ClinicalTrials.gov from 1999 to 2019. These studies are grouped by the type of hyperthermic treatment (HIPEC, Local, Regional, Whole-body hyperthermia or Unknown). The group unknown consisted of studies where the specific type of hyperthermia was not defined.
3.4. Use of hyperthermia treatment in different types of cancer
HIPEC is the most frequently analyzed type of HT for gastro-intestinal tumors (n = 68), especially gastric and colorectal tumors (). In addition, HIPEC is also the most used technique for gynecological cancers especially ovarian and fallopian tube malignancies.
Figure 3. Types of hyperthermia for treatment in cancer in different organ systems. Organ systems were grouped as gastrointestinal (GI), Gynecological (GYN), Urological (URO), Respiratory (RESP), Other and Multiple Systems (MS). All cancer types that did not fit under the other categories were categorized under other. These included: (bone, brain, breast, head and neck, Melanoma retinoblastoma and sarcoma). All studies who looked at tumors in different organ systems, were classified under multiple systems.
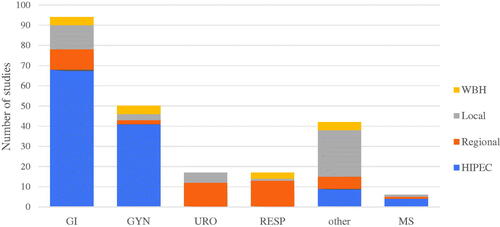
Local and regional HT (n = 12, n = 10, respectively) are used for other types of gastro-intestinal cancer like pancreatic and liver tumors. Tumors in the urological and respiratory tracts are most often treated with regional hyperthermic techniques. For malignancies in these locations, special techniques have been developed (e.g., HITOC-technique) for the respiratory tract and the intravesical hyperthermic chemotherapy for bladder cancer. Local HT techniques can be administered almost anywhere in the body. New types of local HT have been described in a wide arrange of organ systems. WBHT is used for advanced malignancies. Although it is used to treat a variety of tumor types, gynecological, respiratory and pancreatic malignancies have been investigated the most profoundly.
3.5. Location of hyperthermia treatment studies
Trials investigating HT-related cancer treatment are predominantly performed in the US, China and Europe (). In Europe, most studies are done in western Europe, especially Belgium, the Netherlands, France, Italy and Germany ().
Table 2. Distribution of hyperthermia studies around the world and in Europe.
3.6. Efficacy in hyperthermia treatment studies
Results from twelve phase III studies included in our analysis were identified. Six from the local-regional HT category and six including HIPEC. Five out of six local-regional HT studies showed increased overall survival (OS) and/or complete response for adding HT to standard chemo- and/or radiotherapy (). In the HIPEC category four out of six showed increased OS for the addition of HIPEC to standard of care treatment ().
Table 3. All phase III trials including (A) local and regional hyperthermia and (B) HIPEC.
4. Discussion
Within this review, we aimed to provide an update for clinical research on the anticancer effect of HT. By analyzing 235 trials on ClinicalTrials.gov, we constructed a database to extrapolate trends that could explain the evolution of the HT research field. Our main findings include: 1) there are an expanding number of clinical trials on oncological HT and 2) HIPEC is by far the most researched and developed technique within the HT field.
It is remarkable that more than half of studies included were focused on HIPEC. Although this technique was already discovered in the 80 s, a steady increase in research to HIPEC can be observed in the last decade. HIPEC is currently already being used in the clinical setting. For instance, HIPEC can be considered a standardized treatment option after neoadjuvant chemotherapy and interval CRS for Stage 3 primary epithelial ovarian cancer, Fallopian tube cancer or primary peritoneal carcinoma. In other oncological patients, there is insufficient evidence to consider HIPEC as a treatment modality, however in case of primary malignant mesothelioma or disseminated mucinous neoplasms, patients should be assessed by a HIPEC specialty center to determine whether they can receive HIPEC in a clinical trial setting [Citation40]. The additive effect of HIPEC compared to normothermic intraperitoneal chemotherapy is still unclear and is currently being investigated in the HyNOVA trial [Citation41].
For other oncological patients, there is insufficient or even negative evidence for HIPEC as a treatment. Quénet et al. [Citation36] demonstrated there was no additive effect of HIPEC for the treatment of peritoneal metastasis in patients with colon cancer. HIPEC research is more advanced compared to other HT modalities, with more Phase 3 trials than other modalities. Most of these advanced HIPEC research is performed in China, due to a high prevalence of gastric cancer there probably due to the consumption of very hot tea [Citation42,Citation43].
In recent years, an increase interest to local and regional hyperthermia it has also been observed. Both account for approximately 20% of HT research. Regional HT is most often used for bladder and lung cancer due to development of the HIVEC [Citation44] and HITOC techniques. HIVEC trials have proven that the technique is safe and indicate an improved outcome, but more research is needed [Citation45] in order to establish clear outcomes and indications. HITOC can be used in specific oncological settings. In Germany, recommendations for this technique have been described [Citation46]. Indications for HITOC include malignant pleural mesothelioma, pleural dissemination of thymic tumors and specific cases of pleural carcinosis. The first clinical trials show promising results. For these two techniques, more research is being conducted in order to establish clear evidence of their efficacy.
Only a few studies on WBHT (n = 15) could be identified. Novel WBHT techniques for cancer treatment have been explored in recent years. More elaborate trials are needed to be conducted to be able to conclude the effectiveness and safety of WBHT for cancer treatment.
Nearly all HT research has been done in North America, Europe, and China. China primarily focuses on HIPEC for its appliance in GI malignancies. Most of clinical research in WBHT has been conducted in North America and Europe. Both regions have also focused more on development of novel technologies European research is for a large part driven by certain institutions like UNICANCER [Citation47] in France and the University of Erlangen-Nürnberg in Germany [Citation48]. Because of these specific institutions or research groups a lot of European research is concentrated in certain countries like France, Italy, Belgium, The Netherlands and Germany.
The research on HT is different in comparison to classical drug research. Research to medical devices requires a smaller enrollment. Furthermore, this research does not follow the phases of classical drug discovery methods. Therefore, research to HT which relies most often on medical devices, could experience a rapid growth in a few years.
5. Conclusion
HT covers a lot of techniques and technologies. Recently, the research to HT in the oncological setting has increased exponentially. The grade of research done to these techniques differs considerably with HIPEC as the most researched technique. In general, although many studies show an increased therapeutic effect of HT and in some cases these techniques already can be used in the clinical setting, more advanced research needs to be done in this field. Especially more novel technologies, like for example HIVEC, HITOC and WBHT, need to be investigated more in order to establish the therapeutic effect of these techniques.
Disclosure statement
At the time of writing, all the authors were associated with the company ElmediX, which is actively developing a whole-body hyperthermia device.
References
- Habash RWY. Therapeutic hyperthermia. Handbook of clinical neurology. Vol. 157. Amsterdam, Netherlands: Elsevier B.V.; 2018. p. 853–868.
- Petra Kok H, Cressman K, Ceelen EN, et al. Heating technology for malignant tumors: a review. Int J Hyperthermia. 2020;37(1):711–741.
- E Mouratidis PX, Rivens I, Civale J, et al. International journal of hyperthermia “relationship between thermal dose and cell death for ‘rapid’ ablative and ‘slow’ hyperthermic heating” ‘slow’ hyperthermic heating. Int J Hyperthermia. 2019;36(1):228–242.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Critic Rev Oncol/Hematol. 2002;43(1):33–56.
- Dunne M, Regenold M, Allen C. Hyperthermia can alter tumor physiology and improve chemo-and radio-therapy efficacy. Adv Drug Deliv Rev. 2020;163–164:98–124.
- Kok HP, Cressman ENK, Ceelen W, et al. Heating technology for malignant tumors: a review. Int J Hyperthermia. 2020;37(1):711–741.
- Dobšíček Trefná H, Schmidt M, van Rhoon GC, et al. Quality assurance guidelines for interstitial hyperthermia. Int J Hyperthermia. 2019;36(1):276–293.
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31(3):302–309.
- Meneses-Brassea BP, Borrego EA, Blazer DS, et al. Ni-Cu nanoparticles and their feasibility for magnetic hyperthermia. Nanomaterials. 2020;10(10):1988.
- Rahban D, Doostan M, Salimi A. Cancer therapy; prospects for application of nanoparticles for Magnetic-Based hyperthermia. Cancer Invest. 2020;38(8–9):507–521.
- Medina-Ramírez IE, Díaz A, Olmos L, et al. Cancer investigation development and assessment of Nano-Technologies for cancer treatment: cytotoxicity and hyperthermia laboratory studies. Cancer Invest. 2020;38(1):61–84.
- Gao S, Zheng M, Ren X, et al. Local hyperthermia in head and neck cancer: mechanism, application and advance. Oncotarget. 2016;7(35):57367–57378.
- Minnaar CA, Maposa I, Kotzen JA, et al. Effects of modulated Electro-Hyperthermia (mEHT) on two and three-year survival of locally advanced cervical cancer patients. Cancers (Basel). 2022;14(3):656.
- Dharmaiah S, Zeng J, Rao VS, et al. International journal of hyperthermia clinical and dosimetric evaluation of recurrent breast cancer patients treated with hyperthermia and radiation clinical and dosimetric evaluation of recurrent breast cancer patients treated with hyperthermia and radiation. Int J Hyperthermia. 2019;36(1):985–991.
- Bakker A, van der Zee J, van Tienhoven G, et al. International journal of hyperthermia temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review. Int J Hyperthermia. 2019;36(1):1023–1038.
- Hwan Byun Y, Shin Gwak H, Kwon JW, et al. International journal of hyperthermia local recurrence of brain metastasis reduced by intra-operative hyperthermia treatment. Int J Hyperthermia. 2019;35(1):168–175.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Zhang X, Wu Q, Wei M, et al. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: a systematic review and Meta-analysis of comparative studies. Int J Colorectal Dis. 2020;35:1831–1839.
- Tsuyoshi H, Inoue D, Kurokawa T, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J Obstet Gynaecol Res. 2020;46:1661–1671.
- Vermorken JB, van Dam P, Brand A. HIPEC in advanced epithelial ovarian cancer: why is there controversy? Curr Opin Oncol. 2020;32(5):451–458.
- Sousa A, Piñeiro I, Rodríguez S, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32(4):374–380.
- Zhou H, Wu W, Tang X, et al. Effect of hyperthermic intrathoracic chemotherapy (HITHOC) on the malignant pleural effusion. Medicine. 2017;96(1):e5532.
- Martin-Tellez KS, van Houdt WJ, van Coevorden F, et al. Anti-tumour treatment isolated limb perfusion for soft tissue sarcoma: current practices and future directions. A survey of experts and a review of literature. Cancer Treat Rev. 2020;88:102058.
- Song Y, Bruce AN, Fraker DL, et al. Isolated limb perfusion and infusion in the treatment of melanoma and soft tissue sarcoma in the era of modern systemic therapies background and objectives: isolated limb perfusion (ILP) and infusion (ILI) are. J Surg Oncol. 2019;120(3):540–549.
- Robins HI, Longo WL, Steeves RA, et al. A pilot study of whole body hyperthermia and local irradiation for advanced non-small cell lung cancer confined to the thorax. Int J Radiat Oncol Biol Phys. 1988;15(2):427–431.
- Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database-update and key issues. N Engl J Med. 2011;364(9):852–860.
- Cihoric N, Tsikkinis A, van Rhoon G, et al. International journal of hyperthermia hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015;31(6):609–614.
- National Library of Medicine (U.S.) [accessed 10 Mar 2021]. https://clinicaltrials.gov/ct2/home
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492.
- Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guérin or institutional standard in patients with recurrence of Non-muscle-invasive bladder cancer following induction or maintenance Bacillus Calmette-Guérin Therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol. 2019;75(1):63–71.
- Chi MS, Yang KL, Chang YC, et al. Comparing the effectiveness of combined external beam radiation and hyperthermia versus external beam radiation alone in treating patients with painful bony metastases: a phase 3 prospective, randomized, controlled trial. Int J Radiat Oncol Biol Phys. 2018;100(1):78–87.
- Ott OJ, Schmidt M, Semrau S, et al. Chemoradiotherapy with and without deep regional hyperthermia for squamous cell carcinoma of the anus. Strahlenther Onkol. 2019;195(7):607–614.
- Zolciak-Siwinska A, Piotrkowicz N, Jonska-Gmyrek J, et al. HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy-a phase III study. Radiother Oncol. 2013;109(2):194–199.
- Lim MC, Chang SJ, Park B, et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374.
- Liu L, Sun L, Zhang N, et al. A novel method of bedside hyperthermic intraperitoneal chemotherapy as adjuvant therapy for stage-III gastric cancer. Int J Hyperthermia. 2022;39(1):239–245.
- Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266.
- Goéré D, Glehen O, Quenet F, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21(9):1147–1154.
- Lei Z, Wang Y, Wang J, et al. Evaluation of cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for stage III epithelial ovarian cancer. JAMA Netw Open. 2020;3(8):e2013940.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Auer RC, Biagi J, Conner J, et al. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a clinical practice guideline. Curr Oncol. 2020;27(3):146–154.
- Farrell R, Burling M, Lee YC, et al. Clinical trial protocol for HyNOVA: hyperthermic and normothermic intraperitoneal chemotherapy following interval cytoreductive surgery for stage III epithelial OVArian, fallopian tube and primary peritoneal cancer (ANZGOG1901/2020). J Gynecol Oncol. 2022;33(1):e1.
- Huang Y, Chen H, Zhou L, et al. Association between green tea intake and risk of gastric cancer: a systematic review and dose-response meta-analysis of observational studies. Public Health Nutr. 2017;20(17):3183–3192.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Ba M, Cui S, Long H, et al. Development of a high-precision bladder hyperthermic intracavitary chemotherapy device for bladder cancer and pharmacokinetic study. BMC Urol. 2019;19(1):126.
- Tan WP, Longo TA, Inman BA. Heated intravesical chemotherapy: biology and clinical utility. Urol Clin North Am. 2020;47(1):55–72.
- Markowiak T, Larisch C, Hofmann HS, et al. Hyperthermic intrathoracic chemotherapy (HITHOC): narrative review of the current literature, recommendations and future studies. Ann Transl Med. 2021;9(11):955–955.
- UNICANCER. [accessed 18 Mar 2021]. http://www.unicancer.fr/
- Faculty of Medicine at Friedrich-Alexander University Erlangen-Nürnberg > Faculty of Medicine. [accessed 18 Mar 2021]. https://www.med.fau.eu/