Abstract
Purpose
To evaluate the overall survival (OS), local progression-free survival (PFS) and prognostic factors of patients with colorectal cancer liver metastases (CRLM) undergoing microwave ablation (MWA).
Method
A total of 132 patients were retrospectively enrolled who had been treated between 2010 and 2018. For the evaluation of survival rates, all patients were divided according to their indications (curative n = 57 and debulking (patients with additional non-target extrahepatic metastases) n = 75). In total, 257 ablations were evaluated for prognostic factors: number of liver metastases, primary tumor origin (PTO), diameter and volume of metastases, duration and energy of ablation.
Results
The OS was 32.1 months with 93.2% of patients free from recurrence at 28.3 months (median follow-up time). The one- year and three-year OS were 82.72% and 41.66%, respectively. The OS and recurrence-free survival of the curative group were statistically significantly higher than the debulking group (p < .001). Statistically significant prognostic factors for OS included the location of the primary tumor (p < .038) and the number of metastases (all p < .017). Metastasis diameter and volume and ablation duration and energy had no significant correlation with survival (p > .05).
Conclusions
Satisfactory OS and local tumor PFS can be achieved in patients with CRLM using MWA with the number of metastases and the location of the primary tumor influencing the outcome of patients. The metastasis’s size and the duration and energy used for ablation were not of significant prognostic value.
Introduction
Complete surgical resection represents the ‘gold standard’ technique for curative treatment of colorectal cancer liver metastases (CRLM) and liver tumors. Recent studies showed favorable results for combined partial hepatectomy and ablation compared with partial hepatectomy only [Citation1]. In addition for patients who are not eligible for surgical intervention because of comorbidities, anatomical location and the local treatment history of patients, the use of other therapeutic option is a valuable adjunct [Citation2,Citation3]. Also in these cases, local ablation techniques such as RFA and MWA offer a treatment alternative or could be used side by side with the surgical Intervention. These techniques have shown high effectiveness in different trials [Citation4] and are applied for various indications with notable success rates, also mentioned in the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology guidelines [Citation5–13]. The NCCN recommends thermal ablation alone or in combination with surgery as long as all visible disease can be eradicated.
MWA is currently widely used for treatment of hepatic malignancy. It has several advantages over RFA including higher intratumoral temperatures and the possibility to achieve larger volumes within shorter ablation times. Moreover, the dependence of the electrical conductivity of the tissue is lower allowing a more targeted ablation [Citation9,Citation14–21].
Although MWA of hepatic malignancy has been thoroughly investigated in the medical literature, studies addressing long-term survival rates and different prognostic factors on a relatively large number of patients and specially addressing factors influencing prognosis are still insufficient. Hence, the aim of this work is to evaluate the long-term survival rates, progression-free survival and prognostic factors of patients with CRLM who were treated by MWA.
Materials and methods
The current study was approved by the university hospital Ethical committee (Reference number 403/17). All patients gave an informed consent before ablation including a permission for the anonymous usage of their data for possible research purposes. For the standardization of reporting criteria, the terminology from Ahmed et al. (Radiology 2014) was used [Citation15].
Patient selection
A total of 132 consecutive patients (72 men and 60 women; mean age: 61.9 ± 7.3; 35.3–88.5) who received MWA of CRLM between 2010 and 2018 were retrospectively evaluated. Included for ablation were patients with five or less liver metastases with a maximal diameter of 5 cm or less. As a further inclusion criteria for the study is the availability of at least one follow-up MRI study at least three months after ablation and one before the treatment. Excluded from the study were patients who did not return to follow-up and patients who did not receive an MRI examination before the ablation. Eight Patients had to be excluded due to incomplete imaging and a total of 132 patients had all criteria required to be part of this study. The treatment decision was taken based on the decision of a multidisciplinary tumor board and all patients included were deemed unresectable or refused the surgical treatment option.
Patients were divided into two different cohorts () based on the clinical indication of ablation namely a curative group (n = 57 (43.2%)), and a debulking group (n = 75 (56.8%)). The aim of curative treatment was a complete eradication of all metastases in the liver to achieve a disease-free state in patients with no extrahepatic metastases. The aim of the debulking clinical indication was to completely eradicate the index metastases within the liver in patients with known non-target extrahepatic tumor metastases in the body to prolong the survival of the patients. The endpoint of ablation for each group was the same since in both groups the ablation was performed with the aim of eradicating all liver metastases. Patients of the curative group who developed metastases outside the liver following curative ablation of the liver metastases were treated according to the decision of the tumor board based on the location of new metastases and the general condition of the patients.
Table 1. Demographic data of patients in both groups.
MWA technique
All MWA were performed by means of computed tomography (CT)-guided percutaneous approach under light sedation. Two experienced radiologists with more than 20- and 10-years of experience in ablation techniques performed all ablation procedures. Before ablation, a contrast-enhanced MRI was performed within two weeks before the ablation procedure and was used for treatment planning. MWA was performed using two different systems, both systems were used in a chronological order: the 2.4 GHz Amica system (Amica™ HS Hospital Service, Aprilia, Italy) and the 2.4 GHz Microsulis system (Microsulis Medical Limited, Waterlooville, UK). The Amica system became available in 2013 and has been used until 2018. The Microsulis system was used between 2010 and January 2013. The ablation power was set at 80 W and duration was determined by the operator on an individual basis (5–10 min), guided by tumor size and manufacturers’ specifications. Technical MWA success was defined as a correct CT-guided positioning of the antennas inside the metastasis and complete ablation of the metastasis with a sufficient safety margin.
After MWA, each patient was monitored for 8 h to detect any periprocedural complications (according to the Society of interventional Radiology Standards (SIR) Classification), and subsequently discharged.
Imaging protocol
All patients underwent contrast-enhanced MRI study of the abdomen before (up to two weeks) and after the procedure for control (within 24 h). Follow-up MRI scans were performed every three months for the first year, and every six months thereafter. All MRI examinations were performed using a 1.5-Tesla scanner (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) and included T2-weighted sequences in axial and coronal orientation, diffusion-weighted sequences and T1-weighted sequences before and after contrast administration in axial and sagittal orientation.
Image evaluation
All image evaluations of the study were performed by two radiologists with more than 20 and five years of experience in MRI of the abdomen in consensus. For each patient, the number of CRLM and the primary tumor origin was recorded and classified in four main locations: colon, rectum, sigma and cecum. In addition, the following factors were included in the evaluation: system used, ablation time and energy.
CRLM and ablation zone volumes were measured in at least two orthogonal diameters. Ablation zone size was defined as the largest ablation diameter estimated at the immediate post-procedural MRI study. Ablation margin was considered sufficient when it was at least 10 mm larger than CRLM at the three-month MRI study. This study evaluated the ablation margins using the Liu et al. criteria [Citation20] where the ablation margin is defined as the difference between the maximum diameter of the ablation zone and the maximum diameter of the metastasis before ablation divided by two. The VolumeViewer ™ (GE Healthcare, Little Chalfont, Great Britain) was used for the measurements. Local tumor progression was defined as a relapse within 10 mm of a previously ablated area seen on any of the follow-up MRI studies after at least on post-ablation contrast-enhanced study has documented adequate ablation.
Statistical analysis
All analyses were done using BiAS software. The Shapiro–Wilk test and histograms were used to evaluate the normality of data distribution. Normal distributed data were expressed as means ± standard deviation, non-normal data as medians and interquartile ranges (IQR). Normal distributed data were analyzed using t-test, whereas non-normal data were compared with Wilcoxon signed-rank test. Categorical variables were compared using Fisher’s exact test. Kaplan–Meier analysis and the log rank test were used to evaluate overall survival and the median overall survival, which were calculated from the date of treatment to date of death or last follow-up. Patients alive at last follow-up were censored. Time to event was calculated as the interval from treatment to the date of first event (LTP or death without recurrence) or last follow-up. A p value < .05 was considered significant.
Results
Patient/tumor characteristics
A total of 132 patients, 57 patients underwent a curative procedure and 75 patients received MWA within the context of a debulking clinical indication. About 257 tumor ablations were performed with a mean number of 2.4 ± 1.6 ablations per patient. The median follow-up time after MWA was 28.3 months (range 6.7–102 months) without significant differences between the curative and debulking cohorts (p = .526). Overall CRLM had a mean diameter of 18.6 ± 5.7 mm, and a mean volume of 4.5 ± 1.2 cm3. Metastasis diameter and volume had no correlation with the survival rates (p > .05).
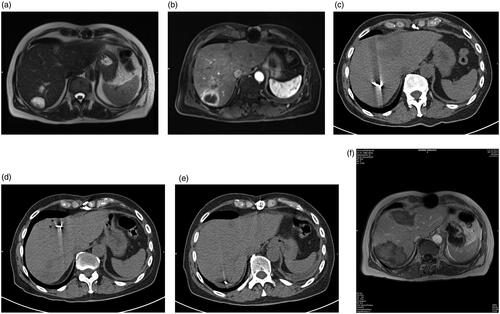
Figure 1. (a) A 63-year-old male CRLM patient with the primary tumor origin in the colon. The patient did not have extrahepatic metastases and was assigned to the patient group with curative clinical indication. T2-weighted (a) and T1-weighted contrast-enhanced (b) MRI images before MWA show three hepatic metastases in segments 4a, and 7. CT images (c, d and e) show the position of the Antenna during the ablation procedure. Follow-up T1-weighted contrast-enhanced MRI at six months (f) show complete ablation and no recurrence at the site of ablation.
Treatment parameters
Technical success was achieved in all procedures (257/257, 100%). An ablation margin of 10 mm or more was achieved in 94.2% of ablations (242/257), and mean minimal ablative margin was 9.7 ± 2.9 mm.
Mean diameter and volume of metastases were not significantly different between the curative and debulking cohorts (p > .216). No significant differences between the curative and debulking cohorts (p = .526) were noted regarding the cumulated energy used between the curative (Mean energy output in (J) 48590) and debulking (Mean energy output in (J) 46660) groups of ablation.
Mean ablation time was 8.62 ± 3.9 min, and cumulated energy output was 47910 ± 19840 J (range 2700–178800 J). Neither the ablation time nor the energy output showed a significant correlation with the median overall survival (all p values >.05).
Complications
The cumulative complication rate was 1.5% (2/132), accounting only for minor postprocedural complications (i.e., local bleeding that stopped spontaneously without further treatment and patients were kept overnight for observation only (Minor complication category B according to the SIR classification of complications).
Survival rates and progression free survival analysis
At three-month MRI follow-up, the mean post-ablation volume was 36.4 ± 13.6 cm3 without significant differences between curative and debulking cohorts (p = .798) ().
Table 2. Overview of survival according to the volume, diameter and energy output.
Median overall survival was 32.1 months (range 4.4–102 months) (IQR 22.5–42.3), with 93.2% of patients free from recurrence (including absent local recurrence in the ablation area and absent new metastases in the liver) at 28.3 months (range 6.7–102 months) (IQR 19.3–39.7). The one- year and three- year overall survival in all patients was 82.72% and 41.66% respectively.
During follow-up, nine patients showed a LTP (9/132; 6.8%) and 13 developed new intrahepatic metastases (13/132; 9.8%). In five of nine LTPs, there were sufficient ablative margins of 5 mm or more.
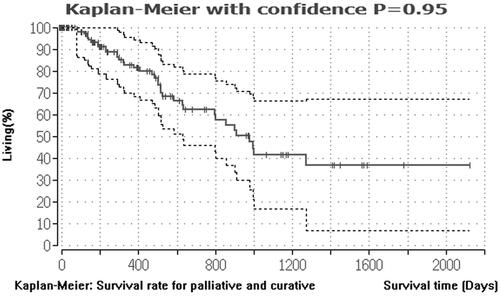
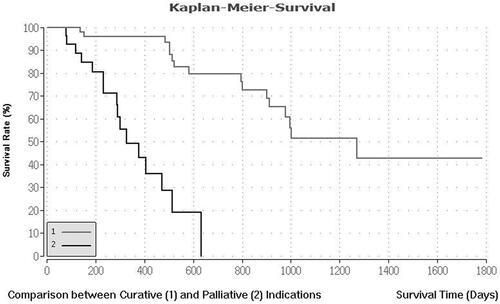
On a cohort-based analysis, the median overall survival and recurrence-free survival of the curative group were higher than the debulking group, with 41.7 months (range 14.5–102 months) and 34.3 months (range 10.5–74.7 months) for the curative group versus 12.9 months (range 3.1–24 months) and 7.3 months (range 3.1–16.8 months) for the debulking group, respectively. The one- and three-year survival of the curative group was 96.92% and 54.47% in the curative group and 55.14% and 14.95% in the debulking group, respectively. The difference in survival and recurrence free survival between both groups was statistically significant (p < .001). and .
Figure 2. Median survival rate for curative and palliative indication. This figure describes the median survival rate for 57 CRLM patients with curative and 75 CRLM patients with palliative treatment indications. The Kaplan–Meier estimator was created using the BiAS program. The survival rate is shown in percent on the y-axis. The x-axis describes the survival times in days.
Prognostic factors analysis
The primary origin of CRLM was the colon in 50 patients (37.9%), the rectum in 43 patients (32.6%), the sigma in 39 patients (29.5%) and the cecum in 3 patients (2.3%).
On a primary-tumor location analysis, one-year and three-year overall survival showed significant differences, being 83.43% and 56.64% for colon, 78.75% and 35.44% for rectum, 84.29% and 37.92% for sigma (all p < .038) ().
Table 3. Overview of survival according to the primary tumor origin.
Median overall survival for patients treated for one single CRLM was significantly higher than patients with two or more metastases (all p < .017). In particular patients with one, two/three, four/five metastases had a median overall survival of 45.4 (range 18.8–102 months), 25.5 (range 7.3–52.2 months) and 13.0 months (range 3.1–19.5 months), respectively.
Discussion
The aim of the current study was to evaluate the long-term overall survival in patients treated with MWA for CRLM with special emphasis on the clinical indication and intention of ablation and the different prognostic factors influencing the survival rate and overall survival.
A statistically significant difference (p < .001) in the OS of patients was noted based on the clinical indication (whether curative or debulking). The OS for the curative clinical indication (41.7 months) was about 220% higher than the debulking indication (12.9 months). The difference is clearly due to the fact that patients treated with debulking clinical indication represent a more advanced stage of the disease than those treated with curative intention.
Including all patients with debulking and curative clinical indications, the overall one-year and three-year survival times following MWA were 82.72% and 41.66%, respectively. Groeschl et al. [Citation22] evaluated 39 patients with CRLM and reported a similar one- and three-year survival times of 92% and 35%. Compared with RFA, similar results were described in the literature, for example Sofocleous et al. [Citation23,Citation24] reported a one- and three-year survival rate of 91% and 41% and Jiang et al. [Citation25] 86.7% and 49.5%, another study by Solbiati et al. [Citation24] reported a relatively higher one- and three-year survival rate (98% and 69.3% respectively) compared with the current study results. This is probably attributed to the metastasis size selection where Solbiati et al. [Citation24] included only small metastases with a size up to a maximal diameter of 4 cm. A study by Wang et al. [Citation26] reported relatively lower survival rates compared with the current study with overall one and three-year survival times of 72.2% and 37.2% respectively. The reason for such a discrepancy is probably attributed to the selection criteria where Wang et al. [Citation26] included cases with extensive metastatic disease in their study. Shady et al. [Citation27] reported a median overall survival of 36 months following RFA of colorectal cancer liver metastases which is comparable to the current study results of 32.1 months. The positive impact of ablations on the OS has been shown in unresectable metastases treated with RFA [Citation27].
For the assessment of a local ablative technique like the MWA, the local progression free survival is an important parameter. The current study results regarding the local progression free survival are positive. The median follow-up observation after the treatments was 28.3 months. In this time, 93.2% of all patients achieved a successful local progression free survival (123/132). In nine patients, a LTP was observed. In their study, Peng et al. [Citation28] reported a median liver progression-free survival of 11.5 months and identified carcinoembryonic antigen as an important prognostic factor for prediction of early recurrence. The current study did not include the carcinoembryonic antigen as one of the prognostic factors comparable to stereotactic patient survival and the duration or amount of energy used for ablation, whereas Joo et al. [Citation29] reported better local control when using higher radiation doses.
Correa-Gallego et al. [Citation17] evaluated 127 lesions who were treated with MWA and 127 lesions with RFA. Patients with the MWA treatment had a local tumor progression rate of 6%. These results are similar to the current study reported local tumor progression rate of 6.8%. A study from Lannitti DA et al. [Citation30] investigated patients with unresectable primary tumors and liver metastases. In this study, a total of 224 tumors were treated with MWA. They detected six local tumor progressions (2.7%) after a mean follow-up of 19 months. This value is relatively better than the reported LTP of 6.8% in the current study, the difference might be attributed to the fact that slightly more than half of the lesions were either ablated during open or laparoscopic surgical procedures which might facilitate the ablation procedure monitoring and performance as compared with the percutaneous route of monitoring. In their meta-analysis, Meijerink et al. [Citation1] compared the rule of RFA, MWA, partial hepatectomy and chemotherapy and concluded that RFA with systemic chemotherapy was superior to chemotherapy alone and that partial hepatectomy was superior to RFA alone but not to RFA with partial hepatectomy or to MWA. Similar results were reported by Ruers et al. [Citation4] who reported a statistically significant improvement in overall survival in patients treated with combined systemic chemotherapy and aggressive local ablation with or without surgery and those who received chemotherapy only. Another meta-analysis by van Amerongen et al. [Citation31] concluded that RFA of colorectal cancer liver metastases is associated with lower incidence of complications but also with higher incidence of recurrence and lower survival rate compared with surgery.
An observation of the current study that need further discussion is that the initial tumor size and volume did not significantly affect the overall survival of the patients. This is probably attributed to two factors, first the relatively small initial size of the ablated tumors (mean size was 18.6 mm) and the second reason is that according to the institutional protocol used in the current study tend to oversize the volume of microwave induced coagulation in such tumors based on experience liver metastases of colorectal cancer are characterized by a higher rate of local tumor recurrence.
A recent study included 54 patients with CRLM and a LTP rate of 10% [Citation32]. Other studies had local tumor recurrences between 2% and 9.6%. The number of patients was comparatively small (31 and 39 Patients) [Citation33,Citation34].
Of the studied prognostic factors, only the location of the primary tumor within the colon and the number of metastatic lesions in the liver were of prognostic value on the OS of the patients. The exact reason for the impact of location of the primary tumor on the OS remains unclear. Still the lower survival in case of rectal origin might be attributed to the dual venous drainage system in the portal and systemic circulation making metastases outside the liver more frequent than in other locations of the colon. Yamashita et al. [Citation35] and Zhou et al. [Citation36] evaluated the impact of tumor location as a prognostic factor following ablation of liver metastases. In both studies they divided the colon into right sided and left sided colon and concluded that right-sided colon has a worse prognosis. The current study used an anatomically based classification and not based on the embryonic origin as in the previously mentioned studies. Hence, a comparison to the current study results is not possible. In addition, Jiang et al. [Citation25] concluded that the size of the metastases (>3 cm), the presence of multiple hepatic metastases, the presence of the tumor in the right side and the presence of extrahepatic metastases are being associated with poor overall survival. The current study results confirm the results of Jiang et al. [Citation25] regarding the association of the presence of multiple hepatic metastases and extrahepatic metastases (the debulking group of patients) with a poorer overall survival. As previously mentioned, the current study used an anatomical classification of the colon; hence, a direct comparison regarding the impact of location is not possible. Regarding the impact of the tumor size, this has been identified to be of non-statistical significance regarding the overall survival. The discrepancy here might be attributed to two factors, first the relatively small size of the metastases (mean metastasis diameter 18.6 mm), hence a large number of the current study patients was below the 30 mm size and the second reason is that about one-third of the metastases in Jiang et al. study [Citation25] were located in a perivascular location (n = 104) where a sufficient ablation margin is difficult to achieve especially that Jiang et al. [Citation25] study performed their ablation using RFA which suffers more from heat sink effect compared with MWA.
A sufficient ablation margin of 10 mm or more was achieved in 94.2% of ablations and the mean minimal ablative margin was 9.7 mm. Kaye et al. [Citation37] had with a 3D assessment of margins a similar output and results. In their study, Shady et al. [Citation27] reported a much lower percentage of patients with ablative margins 10 mm or more as compared with the current study. The discrepancy is probably related to the difference in the method used to measure the ablative margin. An improved overall survival was achieved with minimal margin larger than 5 mm [Citation38–40]. An ablation margin of more than 5 mm has been even identified as the most dominant prognostic factor for local tumor progression free survival [Citation40]. An ablation margin more than 10 mm has been shown to be associated with absence of LTP according to a study by Kurilova et al. [Citation41]. A sub-analysis of the patient cohort of the current study to assess local tumor control based on the size of the ablation margin was not possible due to the relatively small number of metastases with ablation margin less than 10 mm since 94.2% of the ablated metastases were ablated with margins of 10 mm or more. Hence, statistical analysis was not possible.
Factors like the pre-ablation size and volume of the ablated metastases, the duration of ablation and the energy used for ablation were not of significant prognostic impact on the OS. Several other prognostic factors have been described in the literature [Citation42] e.g., Kras mutation with its negative prognostic value on patient survival [Citation43], the metastasis Standardized uptake value (SUV ratio) on PET-CT [Citation44], the RAS mutation status [Citation45,Citation46], the Ki-67 [Citation47], the fluorescent tissue assessment of the ablation zone [Citation48] and the use of diffuse reflectance spectroscopy for detection of completeness of ablation during and after RF ablation [Citation49]. The current study did not evaluate the prognostic value of these biomarkers and invasive techniques on the ablation results since the current study main aim was to evaluate the clinical setting and the effect of the indication on the outcome following ablation. Other prognostic factors like Charlson score, ASA Score, perfusion status of the metastases and molecular profiles were also not evaluated in the current study and need further evaluation in dedicated studies.
New innovations like computer-assisted ablation were not implemented in the current study since at the beginning of the study time frame such innovations were not available. Still it is worth mentioning that such advancements carry the potential of improving the results of ablation and reducing the incidence of recurrence [Citation50].
There are several limitations in the current study, first the retrospective nature of the study and the usage of two different ablation systems in a chronological order. Although a comparison between the results of both systems might be beneficial, it should be kept in mind that the chronological order of using both systems constitutes an obstacle for this comparison because of the difference in learning curve.
In conclusion MWA of colorectal cancer liver metastases is a treatment option for surgically unresectable hepatic metastases or for those who refuse the surgical treatment option. Satisfactory overall survival and local tumor progression free survival can be achieved in patients with colorectal cancer liver metastases treated with microwave ablation. Prognostic factors influencing the overall survival of patients following MWA include the number of metastases and the location of the primary tumor. The diameter and volume of the ablated metastasis and the duration and energy used for ablation were not of significant prognostic value for survival.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):788–1204.
- Hoffmann R, Rempp H, Clasen S. [Microwave tumor ablation. New devices, new applications?]. Radiologe. 2012;52(1):22–28.
- Tsai S, Pawlik TM. Outcomes of ablation versus resection for colorectal liver metastases: are we comparing apples with oranges? Ann Surg Oncol. 2009;16(9):2422–2428.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
- Scholefield JH, Eng C, editors. Colorectal Cancer. Diagnosis and Clinical Management. 1st ed., John Wiley & Sons, Ltd; 2014.
- Audisio RA. Atlas of procedures in surgical oncology with critical, evidence-based commentary notes (With Dvd-Rom). Singapore: World Scientific Publishing Company; 2009.
- Benson AB, 3rd, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(3):370–398.
- Brown G. Colorectal cancer. Leiden: Cambridge University Press; 2007.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40.
- Jenkins JE. Colorectal cancer: risk, Diagnosis, and treatments. New York: Nova Science Publishers, 2011.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Vogl TJ, Mack M, Eichler K, et al. Interventional thermal ablation of malignant liver tumors and liver metastases: comparison of radiofrequency ablation (RFA), laser-induced thermotherapy (LITT) and microwave ablation (MWA). Hessisches Aerzteblatt. 2011;10:10.
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28(12):3429–3434.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Boyvat F. Local ablation for Hepatocellular Carcinoma. Exp Clin Transplant. 2014;12(Suppl 1):55–59.
- Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21(13):4278–4283.
- Ding J, Jing X, Liu J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68(6):608–615.
- Ierardi AM, Floridi C, Fontana F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med. 2013;118(6):949–961.
- Liu C-H, Arellano RS, Uppot RN, et al. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol. 2010;20(4):877–885.
- Martin RCG, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17(1):171–178.
- Groeschl RT, Wong RK, Quebbeman EJ, et al. Recurrence after microwave ablation of liver malignancies: a single institution experience. HPB. 2013;15(5):365–371.
- Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Jiang B, Luo H, Yan K, et al. Ten-year outcomes of percutaneous radiofrequency ablation for colorectal cancer liver metastases in perivascular vs. non-perivascular locations: a propensity-score matched study. Front Oncol. 2020;10:553556.
- Wang Y, Zheng J, Chen H, et al. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int J Hyperthermia. 2018;34(6):853–862.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Peng S, Huang P, Yu H, et al. Prognostic value of carcinoembryonic antigen level in patients with colorectal cancer liver metastasis treated with percutaneous microwave ablation under ultrasound guidance. Medicine. 2018;97(10):e0044.
- Joo JH, Park J-h, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):876–883.
- Iannitti DA, Martin RC, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II trial. HPB. 2007;9(2):120–124.
- van Amerongen MJ, Jenniskens SFM, van den Boezem PB, et al. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases – a meta-analysis. HPB. 2017;19(9):749–756.
- Takahashi H, Kahramangil B, Kose E, et al. A comparison of microwave thermosphere versus radiofrequency thermal ablation in the treatment of colorectal liver metastases. HPB. 2018;20(12):1157–1162.
- Bhardwaj N, Strickland AD, Ahmad F, et al. Microwave ablation for unresectable hepatic tumours: clinical results using a novel microwave probe and generator. Eur J Surg Oncol. 2010;36(3):264–268.
- Lorentzen T, Skjoldbye B, Nolsoe C. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med. 2011;32(5):492–496.
- Yamashita S, Odisio BC, Huang SY, et al. Embryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastases. Eur J Surg Oncol. 2017;43(6):1040–1049.
- Zhou F, Yu X, Liang P, et al. Does primary tumor location impact the prognosis of colorectal liver metastases patients after microwave ablation? Lessons from 10 years‘ experience. Oncotarget. 2017;8(59):100791–100800.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29(5):2698–2705.
- Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275 e1.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clinical Colorectal Cancer. 2021;20(2):e82–e95.
- Han K, Kim JH, Yang SG, et al. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology. 2021;298(1):212–218.
- Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8(39):66117–66127.
- Cornelis FH, Petre EN, Vakiani E, et al. Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med. 2018;59(9):1360–1365.
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–2734.
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768.
- Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–4269.
- Sotirchos VS, Fujisawa S, Vakiani E, et al. Fluorescent tissue assessment of colorectal cancer liver metastases ablation zone: a potential real-time biomarker of complete tumor ablation. Ann Surg Oncol. 2019;26(6):1833–1840.
- Tanis E, Spliethoff JW, Evers DJ, et al. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur J Surg Oncol. 2016;42(2):251–259.
- Beermann M, Lindeberg J, Engstrand J, et al. 1000 Consecutive ablation sessions in the era of computer assisted image guidance – lessons learned. Eur J Radiol Open. 2019;6:1–8.