Abstract
Objective
To evaluate the biliary complication rates and efficacy of peribiliary tumor ablation using irreversible electroporation (IRE) or radiofrequency ablation (RFA).
Material and methods
This is a retrospective study of 42 consecutive patients with 44 peribiliary tumors (≤5 mm distance between the tumor margin and the primary or secondary bile duct). Data were collected between January 2014 and September 2020 from patients who underwent percutaneous liver ablation using IRE (n = 13) or RFA (n = 31).
Results
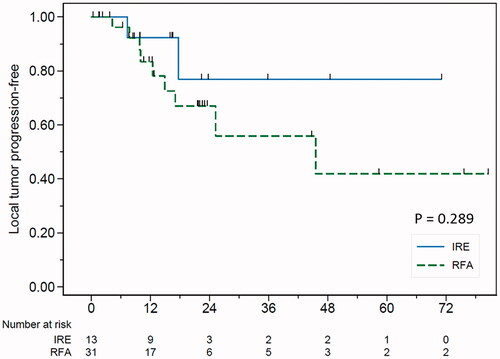
The median length of follow-up was 23.1 months. The mean tumor size was 17.2 ± 5.2 mm in IRE vs. 18.4 ± 7.0 mm in RFA (p= .56). Complete tumor ablation was achieved in 100% with a significantly larger ablation zone in the IRE group (3.8 ± 0.3 cm vs. 2.6 ± 0.6 cm, p<.001). Significant biliary complications occurred in one patient (7.7%) of the IRE group and in five patients (16.1%) of the RFA group. Significant risk factors for biliary complications included the RFA procedure (HR 9.71, p=.032) and proximity of the tumor to the bile duct (HR 0.63, p=.048). The local tumor progression (LTP) rates were 7.7% (IRE) vs. 21.5% (RFA) at 1 year, 23.1% (IRE) vs. 32.7% (RFA) at 2 years and 23.1% (IRE) vs. 44% (RFA) at 3 years, respectively (p=.289).
Conclusions
The IRE and RFA procedures are safe and effective to treat peribiliary liver tumors. However, the RFA may have a higher risk of significant bile duct injury than IRE. The shorter distance between the bile duct and the tumor is a strong risk factor for biliary complications.
Introduction
Liver cancer is the sixth most common cancer diagnosis in the world and the world’s fourth leading cause of death. About 75–85% of liver cancers are hepatocellular carcinoma [Citation1]. In Thailand, the incidence is highest in men and it is the third most common cause of cancer in Thai women [Citation2]. The management of peribiliary liver tumors, with their complex anatomy and deep location, is challenging. According to the BCLC guidelines [Citation3], if liver cancer patients are not eligible for transplantation or surgery, imaging-guided tumor ablation, including thermal ablation and non-thermal ablation, are alternative treatment options. Thermal ablation techniques such as radiofrequency ablation (RFA) and microwave ablation (MWA) have been widely used but have limitations. Complications include thermal injury and mechanical damage to adjacent major blood vessels or bile ducts [Citation4]. Additionally, the proximity to major vessels has been associated with a heat-sink effect, where blood flow drags thermal energy away from the tumor, resulting in decreased ablation intensity. Suboptimal ablation and high tumor recurrency rates are also concerns [Citation5]. However, Teratani et al. [Citation6] reported no statistically significant differences in RFA ablation efficacy at high-risk locations adjacent to large vessels, but reported concerns about the rate of complications. Moreover, Kang et al. [Citation7] also found no significant differences in long-term outcomes of perivascular tumor in RFA ablation.
Irreversible electroporation (IRE) destroys a tumor by inducing apoptosis using a high voltage electrical field to create the ablation zone [Citation8,Citation9]. Based on this mechanism, IRE should be an ideal tool for local treatment of peribiliary liver tumors [Citation10]. However, the limitations of IRE have been well-documented. For example, excessively high voltage or high frequency of pulses can cause local heating, induce thermal coagulation, and lead to bile duct stricture [Citation11]. Moreover, a study by Distelmaier et al. [Citation12] reported that IRE-induced sufficient local heating to cause thermal damage to the bile duct in 24% of ablations, incomplete ablation in 10% of ablations, and local tumor recurrence in 38% of ablations.
To our knowledge, no study has compared outcomes between IRE and RFA in terms of biliary complications and efficacy in the treatment of these tumors in high-risk locations. Therefore, we investigated the biliary complication rate, residual tumor rate and local tumor progression (LTP) between IRE and RFA for the treatment of peribiliary liver tumors.
Material and methods
Patient population
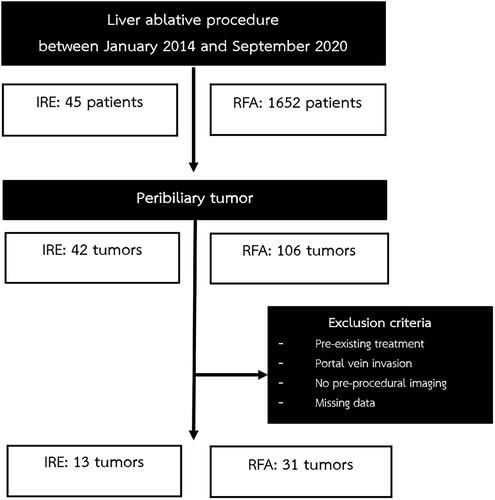
Electronic records were searched for liver ablation patients between January 2014 and September 2020. A total of 1697 records were identified and 42 consecutive patients with peribiliary tumors that underwent percutaneous RFA and/or IRE were included. The decision to offer percutaneous ablative treatment was made after consultation with a multidisciplinary team. The choice between IRE and RFA was based on operator preference. Patients with preexisting treatment, portal vein invasion, missing data and those that were lost to follow-up were excluded. The study was approved by the Siriraj hospital institutional review board (SIRB Protocol No: 038/2563 IRB 3). Informed consent was not required due to the retrospective and anonymized design of the study ( and ).
Table 1. Demographic data and tumor characteristics.
IRE procedure
All IRE procedures were done under general anesthesia with muscular relaxation. IRE was performed by four interventional radiologists that had at least 2 years of experience in liver ablation procedures. The number of IRE electrodes used (NanoKnife; AngioDynamics, Queensbury, NY) was determined by the tumor size and shape. The treatment protocol of IRE settings in our study is similar to the protocol standardization of IRE for hepatic malignancies described by Ruarus et al. [Citation13]. The active tip length was opened at 2.0 cm. The electrodes were placed using ultrasound and unenhanced CT guidance for parallel position. Ninety pulses with maximal voltage of 1500 Volt/cm were applied per electrode pair until current rise. The mean current rise was 11.9 ± 4.7 (3–18) ().
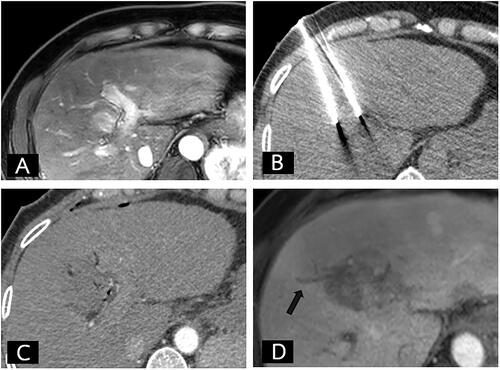
Figure 2. Pre-operative, intra-operative, and post-operative imaging of IRE group. (A) Fifty-one-year-old man with a small hepatocellular carcinoma at liver segment IV. (A) The arterial phase MRI of liver showed a 25-mm arterial enhancing nodule less than 5 mm from the bile duct. (B) The axial non-contrast enhanced CT during the IRE procedure revealed the proper position of the two IRE electrodes. (C) The immediate post-operative contrast-enhanced CT showing the ablated area of the tumor. (D) A 1-month follow-up MRI revealed complete IRE ablation without residual tumor but with adjacent focal bile duct dilatation (arrow).
RFA procedure
All RFA procedures were performed under local anesthesia and IV sedation by six interventional radiologists who had at least 2 years of experience. The expandable RF electrode (Leveen Electrode, Boston Scientific, Marlborough, MA) was inserted under ultrasonography or non-enhanced CT guidance. Tissue impedance was monitored by the generator.
Follow-up protocol
All patients underwent follow-up CT scan or MRI 4–6 weeks after IRE and RFA procedures, and then every 6 months. Data on complication rates, residual tumor rates, and local recurrence tumor rates in both groups were collected. In accordance with the Society of Intervention Radiology (SIR) Guidelines, all complications were categorized as major or minor. Major complications were defined as those that required an increased level of care, prolonged hospitalization, resulted in permanent adverse sequelae or death [Citation14]. Signs of biliary complications such as stricture, leakage, or duct dilatation were recorded [Citation15]. Segmental or lobar liver atrophy was considered to be clinically significant. Baseline laboratory workups were done 1 d before the treatment. A laboratory follow-up evaluation was done 1 month after treatment.
Terminology
Peribiliary location was defined as a distance between the tumor and the primary or secondary bile duct of less than 5 mm. Complete ablation was defined as an ablation margin covering the entire tumor visualized by contrast-enhanced imaging at 1 month showing the ablated area covering the targeted tumor, with a tumor-free margin of 0.5–1 cm. The residual tumor rate was defined as nodular or irregular enhancement adjacent to the ablation zone. LTP was defined as a new viable tumor abutting the ablated area during follow-up imaging with prior evidence of complete ablation [Citation16].
Statistical analysis
The Student’s t-test and Fisher’s exact test were used to compare demographic data and the tumor characteristics of each group. Comparison of complication rates, residual and recurrent rates in both groups were performed using the Chi-square test. The Kaplan–Meier curve was used for LTP, and differences were tested using the log-rank test. Predictive values were calculated using generalized estimating equations. A p value less than .05 with two-tailed analysis was considered statistically significant. SPSS statistical software version 21 (SPSS Inc., Chicago, IL) was used for the statistical evaluation.
Results
A total of 42 patients with 44 peribiliary liver tumors were included in this study; 13 tumors were treated by IRE and 31 tumors by RFA. Two patients received both RFA and IRE; but for different tumors. Twenty-six patients (61.9%) were male. The mean age was 67.5 ± 10.4 years (range 44–92) and there was no significant difference between both groups (p=.49). In the IRE group, all patients had hepatocellular carcinoma (n = 11; 100%) while in the RFA group 29 (93.5%) patients had hepatocellular carcinoma and two (6.7%) had metastatic colon cancer. All patients in the IRE group and 93.5% in the RFA group were Child-Pugh class A. The mean duration of follow-up was 25.2 ± 15.9 months in the IRE group and 22.2 ± 20.2 months in the RFA group (p=.31).
Tumor characteristics
The mean tumor size was 17.2 ± 5.2 mm in the IRE group (n = 13) and 18.4 ± 7.0 mm in the RFA group (n = 31) (p=.56). The tumor locations were predominately in hepatic segment V in the IRE group (n = 5; 38.5%) and in hepatic segment IV in the RFA group (n = 14; 45.2%) (p=.24). The mean distance between the tumor and the bile duct was significantly shorter in the IRE group (0.2 ± 0.9 mm) than in the RFA group (1.5 ± 1.9 mm) (p=.04). In the IRE group, 46.2% of tumors were near the primary bile duct, while 83.9% of tumors in the RFA group were near the secondary bile ducts (p=.06).
Technical success and outcomes
The technical success rate and complete ablation rate were 100% in both groups. The size of the ablation zone (mean ± SD) in the IRE group (38.2 ± 10.8 mm) was significantly larger than in the RFA group (25.6 ± 6.0 mm) (p= <.001). In the IRE group, there were two (15.4%) LTPs. The mean time to recurrence was 12.6 ± 7.3 months. In the RFA group, there were nine (29.0%) LTPs and the mean time to recurrence was 16.4 ± 12.5 months. The LTP rate was 7.7% (IRE) and 21.5% (RFA) at 1 year, 23.1% (IRE) and 32.7% (RFA) at 2 years and 23.1% (IRE) and 44.0% (RFA) at 3 years (p=.289) ().
Complications
There were no procedure-related deaths. Complications occurred in three (23.1%) tumors in the IRE group and 13 (41.9%) tumors in the RFA group (p= .314) (). Major complications were found in two (15.4%) patients in the IRE group, including one vascular injury and one bile duct injury causing segmental lobar liver atrophy. Major complications occurred in five (16.1%) patients in the RFA group including segmental and lobar liver atrophy caused by bile duct and/or vascular injury. Clinically significant segmental or lobar liver atrophy were detected in one (7.7%) IRE patient and in five (16.1%) RFA patients. Nevertheless, these were asymptomatic and no further management was needed ().
Figure 4. Biliary complication with clinically significant liver atrophy after the RFA procedure. (A) Seventy-six-year-old woman with a small hepatocellular carcinoma. (A) Contrast-enhanced CT of the liver in the portal phase showed an 11-mm washout nodule abutting the bile duct (white arrow). (B) Realtime sonography was performed to confirm the position of the RFA electrode. (C) A 1-month follow-up contrast-enhanced CT of the liver shows complete ablation with segmental bile duct dilatation (arrow). (D) A 5-month follow-up revealed lobar atrophy of the whole left lobe of liver (arrowhead) representing a major bile duct and vascular pedicle injury.
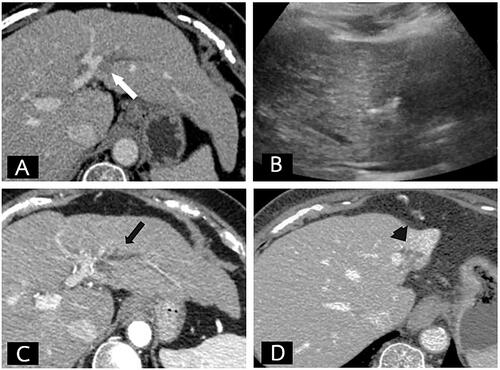
Table 2. Outcomes and complications of the procedure.
There were statistically significant differences in complication rates by ablation technique and the adjacent bile duct distance with a hazard ratio of 9.71 (95% CI = 1.27, 76.92; p=.032) in the IRE group and 0.63 (95%CI = 0.40, 0.996; p=.048) in the RFA group. All other parameters, including order of bile duct, tumor size, and ablation zone, were not statistically significantly different (). There was a median increase in serum bilirubin of 0.14 mg/dL (range, −0.52 to 0.43) in the IRE group and 0.05 mg/dL in the RFA group (−0.7 to 2 mg/dL) (p=.44). There was a median increase in alkaline phosphatase of 9.0 U/mL (range −55 to 44) in the IRE group and 9.0 U/mL (range −55 to 297 mg/dL) in the RFA group (p=.93).
Table 3. Multivariate analysis of predictive factors for biliary complications.
Vascular complications were found in one patient from each group. In the IRE group, one patient had active bleeding after removing the electrode that was detected by contrast-enhanced CT scan. Embolization was not performed because there was no active contrast extravasation during angiography. The patient did not continue to bleed and was discharged 2 d after admission. One patient in the RFA group showed hepatic infarction of segment II on follow-up CT scan, represent a major hepatic vascular injury.
Discussion
RFA is considered a promising alternative to standard surgery for the treatment of small liver cancer. However, RFA can cause mechanical and thermal injuries to the bile ducts and major vessels. Moreover, the heat-sink effect may contribute to local tumor recurrence [Citation17]. IRE has a major role in peribiliary tumors based on its mechanism of action. Non-thermal ablation using high-current electrical pulses leads to nanopore formation and apoptotic cell death. Thus, the adjacent organ damage and heat sink effect are thought to be less than thermal ablation procedures [Citation18]. However, one study reported a risk of biliary injury after high-intensity IRE causing local heating and inducing bile duct injury [Citation11].
We observed that use of RFA is a risk factor for biliary complications (HR 9.71, p=.032). This confirms the hypothesis that thermal effects can cause biliary complications. However, we also observed biliary complications in the IRE group (15.4%), consistent with a study by Faroja et al. [Citation11]. This could be the result of direct thermal injury to the area surrounding the electrodes (about 5 mm). In a study by Distelmaier et al. [Citation12] biliary complications occurred in five of 23 (22%) tumors that developed mild to moderate cholestasis 2–6 weeks after the IRE procedure. However, no further treatment was required in that study, nor in ours. Ridouani et al. [Citation19] reported that liver tumors treated with IRE involute more rapidly than tumors treated with MWA, mainly due to the faster reparative process following apoptosis. Additional research is needed to look at changes in outcomes across different periods of follow-up to determine the importance of bile duct dilatation from secondary edema. We observed that proximity to the bile duct carries an increased risk of biliary complication (HR 0.63, p=.048). This insight is valuable for interventional radiologists that perform liver ablation procedures in peribiliary locations.
Intraluminal IRE, a novel technique to treat intraluminal bile duct tumors, has been reported to be safe in recent studies [Citation20–22]. However, most of these studies were conducted in normal swine tissue with different levels of energy that are used in human liver IRE. More human studies are needed to confirm the safety of intraluminal IRE.
The heat-sink effect, influenced by large vessels adjacent to the peribiliary tumor, may lead to an increased risk of LTP [Citation5]. However, we did not find a significant difference in tumor control efficacy between groups (p=.461). Van Tilborg et al. [Citation23] also reported a local tumor recurrence rate of 26.7% in perivascular and peribiliary unresectable colorectal liver metastasis (CRLM) treated with RFA. Thamtorawat et al. [Citation24] also reported that RFA ablation at high-risk locations adjacent to large vessels showed no statistically significant difference in efficacy. Moreover, Kang et al. [Citation7] found no significant difference in long-term outcomes of perivascular tumor. Furthermore, Freeman et al. [Citation25] compared IRE to RFA in HCC patients and reported no statistically significant differences in local recurrence-free survival (LRFS) between the two groups. This may be explained by improved technical precision, sufficient current to tumor, and more operator experience.
Our study has limitations. First, our sample size was small and not randomly selected, which may have introduced bias and limited our ability to arrive at definitive conclusions. Second, differing levels of operator experience with ablation in peribiliary tumor might have affected the outcome. Third, there is no standard protocol to perform IRE in this specific location. Randomized, prospective studies with larger sample sizes are needed to confirm our results.
In conclusion, both IRE and RFA may be used effectively in peribiliary tumor ablation. However, RFA may have a higher risk of significant bile duct injury. Therefore, in the hands of an experienced operator, IRE may be the preferred modality for the ablative treatment of peribiliary tumors.
Acknowledgments
The authors thank Mark Simmerman, PhD and Assist. Prof. Dr. Chulaluk Komoltri for their useful advice in the preparation of this article.
Disclosure statement
The authors report that they have no competing interests to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):751–424.
- Virani S, Bilheem S, Chansaard W, et al. National and subnational Population-Based incidence of cancer in Thailand: assessing cancers with the highest burdens. Cancers (Basel). 2017;9(12):108.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Hu H, Chi JC, Liu R, et al. Microwave ablation for peribiliary hepatocellular carcinoma: propensity score analyses of long-term outcomes. Int J Hyperthermia. 2021;38(1):191–201.
- McGhana JP, Dodd GD. 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176(1):3–16.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43(5):1101–1108.
- Kang TW, Lim HK, Lee MW, et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270(3):888–899.
- Kalra N, Gupta P, Gorsi U, et al. Irreversible electroporation for unresectable hepatocellular carcinoma: Initial experience. Cardiovasc Intervent Radiol. 2019;42(4):584–590.
- Fang C, Kibriya N, Heaton ND, et al. Safety and efficacy of irreversible electroporation treatment in hepatobiliary and pancreatic tumours: a single-Centre experience. Clin Radiol. 2021;76(8):599–606.
- Hui TC, Kwan J, Pua U. Advanced techniques in the percutaneous ablation of liver tumours. Diagnostics (Basel). 2021;11(4):585.
- Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013;266(2):462–470.
- Distelmaier M, Barabasch A, Heil P, et al. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology. 2017;285(3):1023–1031.
- Ruarus AH, Barabasch A, Catalano O, et al. Irreversible electroporation for hepatic tumors: protocol standardization using the modified delphi technique. J Vasc Interv Radiol. 2020;31(11):1765–1771.e15.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Intervent Radiol. 2014;25(1):112–118.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960.
- Charpentier KP, Wolf F, Noble L, et al. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford). 2011;13(3):168–173.
- Ridouani F, Ghosn M, Cornelis F, et al. Ablation zone involution of liver tumors is faster in patients treated with irreversible electroporation than microwave ablation. Medicina (Kaunas). 2021;57(9):877.
- Lee KW, Lee JM, Choi HS, et al. Novel ablation therapy using endoscopic irreversible electroporation in the bile duct: a pilot animal study. Clin Endosc. 2021;54(3):413–419.
- Li Q, Ren F, Zhang Y, et al. Acute and subacute effects of irreversible electroporation on normal common bile ducts in a rabbit model. J Hepatobiliary Pancreat Sci. 2020;27(10):776–784.
- Ueshima E, Schattner M, Mendelsohn R, et al. Transmural ablation of the normal porcine common bile duct with catheter-directed irreversible electroporation is feasible and does not affect duct patency. Gastrointest Endosc. 2018;87(1):300.e1–e6.
- van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and Lesion-Based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–1446.
- Thamtorawat S, Limsuwarn P, Tongdee T, et al. Incidence of complication and tumor recurrence after radiofrequency ablation in high-risk location of hepatocellular carcinoma patients. J Med Assoc Thai. 2014;97(1):95–100.
- Freeman E, Cheung W, Ferdousi S, et al. Irreversible electroporation versus radiofrequency ablation for hepatocellular carcinoma: a single Centre propensity-matched comparison. Scand J Gastroenterol. 2021;56(8):942–947.