Abstract
Objectives
This study aimed to assess the safety and efficacy of stereotactic radiofrequency ablation (SRFA) in patients with hepatocellular adenomas (HCA).
Methods
Retrospective analyses of all patients referred for SRFA treatment at our institution between January 2010 and October 2020 revealed 14 patients (10 women; mean age 34.4 [range, 17–73 years]) with 38 HCAs treated through 18 ablation sessions. Ablations were considered successful if a safety margin >5 mm was achieved. Demographic, interventional, and outcome data were collected and analyzed. Primary and secondary technical efficacy rates were assessed based on follow-up images consisting of contrast-enhanced CT or MR scans.
Results
The mean tumor size was 22 mm (range, 7–75 mm). Overall, 37/38 (97.4%) tumors were successfully ablated at the initial SRFA (primary efficacy rate of 97.4%). The median follow-up duration was 49.6 months. No deaths or adenoma-related complications (hemorrhage or malignant transformation) were observed. Disease-free survival rates at 1, 3, and 5 years from the date of the first SRFA were 100%, 85.8%, and 85.8%, respectively. Two patients developed new distant tumors retreated with consecutive re-ablation. No major complications occurred during any of the 18 ablation sessions.
Conclusions
Percutaneous thermal ablation is efficient in the treatment of HCAs and may thus be considered a valid first-line treatment option. In addition, SRFA allows for an effective, minimally invasive treatment of large and multiple hepatic tumors within one session.
Introduction
Hepatocellular adenoma (HCA) is a rare, benign epithelial tumor of the liver, predominantly arising in young/middle-aged women taking oral contraceptives or other steroid medications [Citation1,Citation2]. HCAs rarely occur amongst children and men, with a reported male-to-female ratio of 1:9 [Citation3]. However, with an overall incidence of 0.3–4 per 100,000 [Citation4], HCA is still the second most common benign tumor of hepatocellular origin after focal nodular hyperplasia (FNH) [Citation5]. HCAs can appear as singular or multiple tumors and can vary considerably in size. Although benign in contrast to FNHs, HCAs often require treatment because of an increased risk of rupture with subsequent hemorrhage or malignant transformation. In fact, the overall frequency of hemorrhage in patients with HCAs was reported as 27.2% per patient and 15.8% per adenoma [Citation6]. Surgical resection [Citation7–9] is currently considered the first choice in the management of HCAs. However, other less invasive treatment options, such as transarterial embolization [Citation10] or percutaneous thermal ablation [Citation4,Citation11], have recently been proposed.
Image-guided percutaneous ablation methods, such as radiofrequency ablation (RFA) or microwave ablation (MWA), have proven to be valid treatment options for a variety of malignant liver tumors. Combination with sophisticated guidance tools, such as stereotaxy, has allowed for local curative treatment in an increasing number of patients even with multiple [Citation12] or very large [Citation13] liver tumors.
Stereotactic radiofrequency ablation (SRFA) is a multiple-needle approach involving 3 D treatment planning and precise stereotactic needle placement, combined with intraprocedural image fusion of pre- and post-interventional CT scans for verification of treatment success. Several publications have demonstrated that SRFA is a safe and effective treatment option for primary and secondary malignant liver tumors of different origins [Citation14–20]. The aim of this retrospective analysis was to assess the safety and efficacy of SRFA in patients with HCAs.
Material & methods
Patients
This study was approved by the Institutional Review Board of Innsbruck (study number: AN4357) and written informed consent was obtained from all patients. Retrospective analysis of prospectively collected data from all the consecutive patients referred to SRFA at the Department of Radiology between January 2010 and October 2020 identified 14 patients with 38 pathologically proven HCAs. None of the patients had to be excluded from this analysis.
The treatment plan and decision to perform SRFA as a first-line therapeutic approach were established by consensus at a multidisciplinary tumor board attended by hepatologists, oncologists, liver surgeons, radiation therapists, and interventional radiologists. Treatment choices were based on tumor characteristics, liver function, anatomical considerations, and the general condition of the respective patient. In addition, 4 out of 14 (28.5%) patients specifically requested to undergo treatment by SRFA.
Due to the multiprobe approach with overlapping ablation areas in SRFA, the tumor size is not a limiting or an exclusion factor [Citation13], and multiple tumors can be treated simultaneously within one session [Citation12]. However, contraindications for SRFA at our institution include: 1) a platelet count of fewer than 50,000 cells/mm3 or a prothrombin time ratio <50% (prothrombin time with international normalized ratio, G1.7), 2) a history of bile duct surgery with the presence of a biliodigestive anastomosis (elevated risk for bacterial infection of the coagulation zone), and 3) close vicinity (<10 mm) to the central biliary structures.
Stereotactic radiofrequency ablation
A detailed description of the technique for multiprobe SRFA has already been reported previously [Citation14,Citation21,Citation22]. Briefly, the intervention is performed in a dedicated intervention room with a sliding gantry CT (SOMATOM Sensation Open, Siemens Inc.) under general anesthesia with deep muscle relaxation. Respiratory triggering (mandatory for an exact stereotactic registration) is achieved by temporarily disconnecting the endotracheal tube. After obtaining a dual-phase contrast-enhanced planning CT (SOMATOM Sensation Open, Siemens Inc.) having 3 mm slice thickness with the patient already immobilized on the CT table (vacuum mattress), all data is transferred to the optical-based 3 D navigation system (S8, Medtronic Inc.). Subsequently, the interventionalist plans multiple trajectories with the software of the 3 D navigation system on multiplanar reformatted images in order to cover the entire tumor volume, including an appropriate peritumoral safety margin. For tumors, not visible in the planning CT, pre-interventional CT/MRI/PET/SPECT data are fused with the intraprocedural CT dataset [Citation23]. After registration, accuracy check, and sterile draping, the Atlas aiming device (Interventional Systems Inc.) is adjusted using the 3 D navigation system and 15 G/17.2 cm coaxial needles (Bard Inc.) are sequentially advanced through the locked aiming device to the preplanned target point. Correct needle placement is verified with a non-enhanced CT scan superimposed onto the planning CT with a possibility of manual re-adjustment, and a 16 G biopsy sample is collected through the coaxial needles. According to the manufacturer’s instructions, up to three Cool-tip RF-electrodes (Medtronic Inc.) are simultaneously introduced through the coaxial needles for serial tumor ablation with an ablation time of 16 min or until impedance rise (the “roll-off” effect) is obtained. Needle track cauterization is performed while repositioning each probe, and at the final probe removal, to prevent bleeding and potential tumor seeding. A dual-phase contrast-enhanced CT scan is then acquired to assess possible complications (e.g., bleeding, pneumothorax) and the ablation result. As a final and crucial step, the post-interventional CT scan is superimposed onto the pre-interventional CT scan to assess the peritumoral safety margins in 3 D. In case of incomplete ablation (i.e., residual tumor or lack of sufficient safety margin), the intervention may be continued in the same session by the stereotactic placement of additional coaxial needles and subsequent ablation (). To decrease the risk of damage to surrounding organs (bowel, stomach, spleen, lung), the “no-touch” or “partial touch” technique that was previously described by Schullian et al. [Citation24]. is applied.
There is no evidence in the literature regarding the appropriate safety margin needed for the ablation of HCAs. However, due to their similarity to hepatocellular carcinomas (HCCs), a safety margin of >5 mm (as recommended for HCCs [Citation25]) is considered sufficient.
Follow-up
Follow-up consisted of contrast-enhanced CT- or MR scans. CT-scans were obtained following injection of 1.5 ml/kg contrast media (Iopromid 370 mg J/ml [Ultravist 370; Bayer]; injection rate 4 ml/sec) by using a standardized protocol including a non-enhanced, late arterial, and late venous phase of the upper abdomen, as well as a portal venous phase of the whole abdomen, with a 3 mm slice thickness. The standard protocol for contrast-enhanced MR-scans at our institution includes: 1) axial T1w VIBE-DIXON (in-phase and out-of-phase); 2) post-contrast axial T1w VIBE-DIXON in arterial (10–20 s to 25–35 s), portal venous (30–45 s to 90 s), delayed (>2 min to 4–5 min), and hepatobiliary delayed phase (>5 min to 10 min) after injection of 0.1 ml/kg contrast media (Gadoxetate disodium 0.25 mmol/mL [Primovist; Bayer]); 3) axial/coronal T2w HASTE; 4) axial T2w fat-saturated; and 5) axial DWI (b50/400/1000).
Newly detected tumors distant to the ablation zone were defined as "distant new tumors." Primary technical efficacy was defined by the absence of residual tumors at the first follow-up CT- or MR- scans after the intervention. Conversely, the secondary technical efficacy was defined as successful re-ablation of the residual tumor at the first follow-up. The follow-up duration was calculated from the date of the first SRFA to the most recent follow-up imaging. Disease-free survival was calculated from the date of the first SRFA to the date of appearance of distant new tumor(s) (i.e., event), or the most recent follow-up visit registered in our hospital information system (i.e., censoring). All images were evaluated by the consensus of two board certified abdominal radiologists (BR, PES) with more than 10 years of experience.
Statistical analysis
The distribution (parametric/non-parametric) of all possible variables was assessed using histograms and verified with the Kolmogorov–Smirnov Test. Data were expressed as mean ± SD, or median and range, as appropriate. Survival data were analyzed using the Kaplan–Meier method. All statistical analyses were performed using the SPSS Version 27 (SPSS Inc.).
Results
Patient characteristics
Fourteen patients (10 females/4 males) with a mean age of 34.4 years (range, 17–73), having 38 hepatocellular adenomas were included in this analysis. Among these patients, nine were treated for a solitary tumor, while the remaining were treated for 2, 3, 4, 5, and 15 tumors, respectively; four patients (28.6%) had nonalcoholic steatohepatitis (NASH) and three (21.4%) had glycogen storage disease type I (GSD I) as the main risk factor for developing hepatocellular adenoma(s). All the patients underwent a biopsy for at least one of the treated adenomas. Only 2 patients (14.3%) underwent biopsy before SRFA, whereas the remaining 12 (85.7%) underwent histologic confirmation during SRFA due to unclear MR findings and hepatocellular carcinoma could not be ruled out (5 [35.7%]), or to confirm the diagnosis of a strongly suspected adenoma (7 [50%]). The histological subtypes included unclassified (9 [64.3%]), inflammatory (2 [14.3%]), β-Catenin activated (2 [14.3%]), and HNF-1α inactivated (1 [7.1%]) HCA. Twelve patients underwent a single ablation session, while the remaining two patients underwent three ablation sessions each, for 5 and 15 adenomas, respectively (). During a median follow-up of 49.6 months, none of the patients in this study succumbed to the disease (i.e., event), and no instances of malignant transformation or adenoma-related hemorrhages were observed. Two patients developed distant new tumors (14.2%), who were successfully retreated with SRFA. The disease-free survival rates at 1, 3, and 5 years from the date of the first SRFA were 100%, 85.8%, and 85.8%, respectively.
Table 1. A detailed description of SRFA sessions in HCA patients.
On average, 2.1 tumors were treated per session (range, 1–8) with a median number of 7.5 coaxial needles per session (range, 3–19), and an ablation time of 35 min (range, 18–66 min). In 3 out of 15 (20%) ablation sessions, the planning CT scans were fused with pre-interventional MR scans due to insufficient visibility of the tumors in the contrast-enhanced CT scans. No major complication occurred in any of the 18 ablation sessions. The median length of hospital stay was 4 days (range, 2–9) per patient and session. A detailed description of the 18 ablation sessions is presented in .
Tumor characteristics
Thirty-eight hepatocellular adenomas with a mean tumor size of 22 mm (7–75 mm) were treated. Two (5.3%) tumors had direct proximity to an extrahepatic organ, 14 (36.8%) were subcapsular in location, and 4 (10.5%) were adjacent to a major intrahepatic vessel. Overall, 24 tumors were located in the right liver lobe, 12 in the left liver lobe, and two adenomas were located at the transition of the right/left liver lobes. To decrease the risk of damage to extrahepatic organs during the ablation of both tumors with direct proximity to an extrahepatic organ the “no-touch” or “partial touch” technique was adopted [Citation24]. The initial SRFA successfully ablated 37/38 (97.4%) tumors, resulting in a primary technical efficacy rate of 97.4%. The patient with a residual tumor after the first SRFA was not retreated.
Discussion
In this study, we demonstrated that SRFA is a safe and effective treatment option for large and multifocal HCAs. Hemorrhage and malignant transformation are two major potential complications of HCAs. While the risk for hemorrhage is significantly high at 27.2% per patient and 15.8% per tumor [Citation6], the reported risk for malignant transformation is relatively low at 4.2% [Citation26]; although the probability is 10 times more in men than in women [Citation27]. Risk factors for hemorrhage include increasing tumor size and recent (within six months) hormonal usage [Citation28]. In our study, we did not observe mortality, instances of malignant transformation, or adenoma-related hemorrhages during a median follow-up of 49.6 months. These findings are in line with the findings of similar studies and case reports on the thermal ablation of HCAs [Citation11,Citation29–32]. Histological subtypes may play an important role in the risk of malignant transformation/hemorrhage. HCA can be classified into inflammatory, HNF-1α inactivated, β-Catenin activated, and unclassified. The inflammatory and β-Catenin activated types have a higher risk of hemorrhage and malignant transformation, respectively. In contrast, the HNF1-α inactivated type is reported to have a lower risk for both complications [Citation33,Citation34]. However, the limited number of (or missing in the case of HNF1-α) histological subtypes in our study precludes us from analyzing their influence on patient outcomes or complication risks.
In 2016, the European Association for the Study of the Liver (EASL) proposed a guideline for the management of benign liver tumors including HCA [Citation35]. They recommended resection or curative treatment for all HCAs diagnosed in men, regardless of the size. HCAs <5 cm in women, on the other hand, could be managed conservatively (discontinuation of oral contraceptives; lifestyle changes). All HCAs should be re-assessed with contrast-enhanced MRI after 6 months. HCAs persistently greater than 5 cm, or exhibiting an increase in diameter of more than 20% (as per the RECIST criteria in solid lesions [Citation36]) should be considered for curative treatment. The recommended first-line therapy for larger tumors (>5 cm) is resection, aiming to remove the whole tumor to reduce the risk of malignant transformation/hemorrhage. Furthermore, non-surgical modalities, such as arterial embolization or thermal ablation, should only be pursued as an alternative to resection in poor surgical candidates. However, all recommendations regarding the curative management of HCAs do not exceed a level of evidence superior to II-3 (i.e., multiple time series, dramatic uncontrolled experiments) [Citation35]. Thus, further prospective studies evaluating and comparing curative (including the latest technical advances in non-surgical approaches) and conservative management of HCAs would be desirable in order to define standardized guidelines containing a high level of evidence and strong recommendations.
The primary technical efficacy rate in our study was 97.4%, which is consistent with 88–100% efficacy rates in previous reports of thermal ablation for HCAs [Citation4,Citation11,Citation29]. Van Vledder et al. [Citation37] on the other hand reported a remarkably lower primary technical efficacy rate of 57.8% using conventional RFA. The authors attributed this rate to the relatively high number (>50%) of tumors >3 cm. Although in our study only 26.3% of the tumors treated were >3 cm, tumor size per se does not represent a limiting factor for SRFA. Therefore, we are confident that the rate of primary technical efficacy in patients with HCA would minimally change even in patients with a high number of large tumors; the reason being the nature of the technique for SRFA. In contrast to conventional US- or CT-guided percutaneous thermal ablation techniques, SRFA uses coaxial needles as guidance for consecutive serial tumor ablation with up to three RFA-probes. Thus, multiple (even more than 30) trajectories may be planned, with the possibility of achieving very large coagulation zones owing to multiple overlapping ablation areas. Moreover, this computer-assisted multiple-needle approach technique facilitates simultaneous treatment of multiple tumors in both the liver lobes [Citation12], such as in patients with liver adenomatosis (). Another advantage of using coaxial needles is the ability to combine bioptic verification with treatment using one (or the same) planning path, without additional risks. Differentiation of HCA from HCC can be difficult via imaging, as observed in our study, where the histological diagnosis of HCA was confirmed during SRFA in 12 out of 14 (85.7%) patients. The above-mentioned advantages of SRFA over conventional single-probe, US or CT-guided thermal ablation should definitely be considered important and crucial while considering treatment options for HCAs.
Figure 1. Case of a 35-year-old woman with a β-Catenin positive HCA in liver segment V/VI referred for SRFA. (A) Pre-interventional CT scan in the late arterial phase revealed a 42 mm enhancing nodule in the subcapsular region (white arrows). (B) Maximum intensity projection (MIP) of the non-enhanced control CT-scan with the placement of seven coaxial needles. (C) Post-interventional CT scan in the late arterial phase with coagulation zone (white arrowheads) and visible residual vital tumor tissue with contrast enhancement (white arrow). (D) MIP of non-enhanced CT scan of the immediate re-ablation of the residual tumor using two coaxial needles in the same ablation session. (E) Final post-interventional CT-scan in portal venous phase with correspondingly large coagulation zone (white dashed circle). (F) MRI in the hepatobiliary phase with a shrinking coagulation zone was performed at a one-year follow-up (white dashed circle).
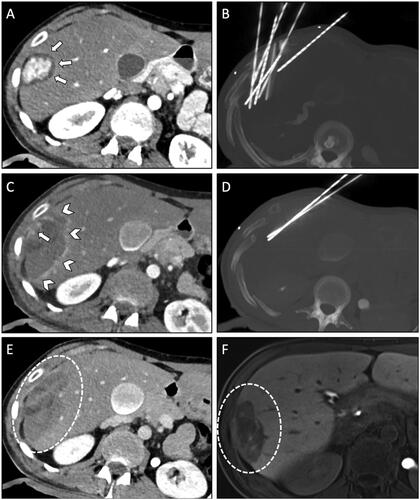
Figure 2. The second ablation session (of three) with simultaneous ablation of six HCAs in a 29-year-old woman with hepatocellular adenomatosis (β-Catenin positive) referred for SRFA. (A) Screenshot of the navigation system with a 3D reconstruction of the planning CT scan with nine planned trajectories. (B) MIP of the non-enhanced control CT-scan depicting all nine coaxial needles in place. (C,E,G,I,K) Pre-interventional CT scan in the late arterial phase shows a total of six HCAs (white arrows). Note the coagulation zone of a previous ablation, 22 months earlier (red arrow in E). (D,F,H,J,L) Post-interventional CT-scan in portal venous phase with corresponding coagulation zones (white arrowheads).
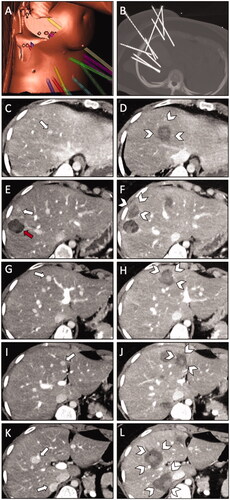
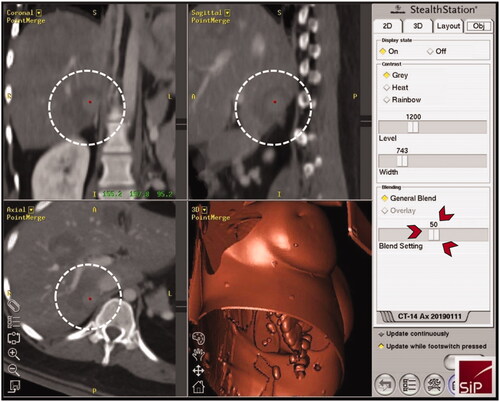
Figure 3. Three-dimensional views of the intraprocedural image fusion (HCA of ; white dashed circles) of the contrast-enhanced planning and final CT-scan using the rigid-registration tool implemented in the navigation system; possibility to switch between images using the blending function (red arrowheads) with immediate intraprocedural verification of treatment success (complete necrosis including a sufficient safety margin) as an integral part of the SRFA procedure.
In our study, no major complications occurred in any of the 18 ablation sessions. Previous studies on thermal ablation of HCAs reported either no major complications [Citation11,Citation29] or low rates of complications of 4.5% [Citation4] and 11% [Citation37], respectively. A systematic review [Citation38] of transarterial embolization as an acute (bleeding tumor) or elective treatment option for HCAs revealed an overall major complication rate of 5.3%. However, when comparing these results with that of surgical resection (applied by most institutions in the curative management of HCAs), the major complication rate after percutaneous ablation, and particularly after SRFA, is significantly lower. In fact, reported major complication rates after surgical resection of HCAs to range from 9.7 to 15% [Citation7,Citation8,Citation39]. The lack of complications in our study group may be explained by a young patient population (mean age, 34.4 years) with relatively fewer/no comorbidities. A retrospective study [Citation40] assessing the frequency of major complications after SRFA of liver malignancies through 1,235 ablation sessions over a period of 15 years demonstrated higher complication rates, with a reported overall major complication rate of 7.4%, decreasing significantly from 11.5% before January 2011 to 6% thereafter, probably attributable to the learning curve.
Similar results to those described in this analysis using SRFA may be achievable with (multiprobe) stereotactic MWA [Citation41], as both techniques allow the preservation of large amounts of healthy liver tissue while achieving complete destruction of the tumor(s). This is particularly important in multifocal diseases involving both liver lobes. The possibility of preserving more healthy liver tissue with these techniques is, therefore, another advantage of thermal ablation over surgical resection that should not be ignored. A recent review [Citation42] comparing different surgical approaches in patients with colorectal liver metastases highlighted the advantages of sparing healthy liver tissue. Tumor removal without the unnecessary sacrifice of functional parenchyma is associated with a lower surgical burden, fewer postoperative complications, better outcomes, and higher feasibility of future treatments (e.g., resection, RFA).
Major limitations of our study lie in its retrospective design and single-center bias. Moreover, we acknowledge that the number of patients included in this analysis was relatively less, and we lacked a comparative arm due to the exclusive use of a stereotactic approach for RFA at our institution. The comparison of our results with those of similar studies is hampered by the rare use of stereotactic approaches for liver ablations other than in our own institution. Nevertheless, the results are encouraging, and we believe that this analysis will help in generating further interest in the technique.
This analysis demonstrates that percutaneous thermal ablation can achieve high technical efficacy rates, with very few to no major complications in the treatment of HCAs. Therefore, thermal ablation may be considered a valid first-line treatment option for this tumor entity if the entire tumor can be sufficiently covered by the coagulation zone including a safety margin > 5 mm. Moreover, due to its multi-needle approach with 3 D planning, precise needle/probe placement, and intraoperative verification of the result by means of image fusion (), SRFA represents a reliable minimally invasive method, even for the treatment of large and multiple tumors, within one session.
Disclosure statement
Reto Bale is a consultant for Siemens, Medtronic, and Interventional Systems. The other authors report no potential conflict of interest.
References
- Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB 2005;7(3):780–196.
- Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979;242(7):644–648.
- Shanbhogue AK, Prasad SR, Takahashi N, et al. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology 2011;258(3):673–693.
- Mironov O, Jaberi A, Beecroft R, et al. Retrospective Single-Arm cohort study of patients with hepatocellular adenomas treated with percutaneous thermal ablation. Cardiovasc Intervent Radiol 2018;41(6):935–941.
- Renzulli M, Clemente A, Tovoli F, et al. Hepatocellular adenoma: an unsolved diagnostic enigma. World J Gastroenterol 2019;25(20):2442–2449.
- van Aalten SM, de Man RA, IJzermans JN, et al. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg 2012;99(7):911–916.
- Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 2009;137(5):1698–1705.
- Kammula US, Buell JF, Labow DM, et al. Surgical management of benign tumors of the liver. Int J Gastrointest Cancer 2001;30(3):141–146.
- Fodor M, Primavesi F, Braunwarth E, et al. Indications for liver surgery in benign tumours. Eur Surg 2018;50(3):125–131.
- Deodhar A, Brody LA, Covey AM, et al. Bland embolization in the treatment of hepatic adenomas: preliminary experience. J Vasc Interv Radiol 2011;22(6):795–799; quiz 800.
- Rhim H, Lim HK, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular adenoma: initial experience in 10 patients. J Gastroenterol Hepatol 2008;23(8pt2):e422–7.
- Schullian P, Putzer D, Eberle G, et al. Simultaneous stereotactic radiofrequency ablation of multiple (≥ 4) liver tumors: Feasibility, safety, and efficacy. J Vasc Interv Radiol 2020;31(6):943–952.
- Schullian P, Johnston EW, Putzer D, et al. Safety and efficacy of stereotactic radiofrequency ablation for very large (≥8 cm) primary and metastatic liver tumors. Sci Rep 2020;10(1):1618.
- Laimer G, Schullian P, Bale R. Stereotactic thermal ablation of liver tumors: 3D planning, multiple needle approach, and intraprocedural image fusion are the key to Success-A narrative review. Biology 2021;10(7):644.
- Bale R, Schullian P, Eberle G, et al. Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology 2019;70(3):840–850.
- Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35(5):1074–1082.
- Bale R, Schullian P, Schmuth M, et al. Stereotactic radiofrequency ablation for metastatic melanoma to the liver. Cardiovasc Intervent Radiol 2016;39(8):1128–1135.
- Schullian P, Johnston E, Laimer G, et al. Stereotactic radiofrequency ablation of breast cancer liver metastases: short- and long-term results with predicting factors for survival. Cardiovasc Intervent Radiol 2021;44(8):1184–1193.
- Schullian P, Laimer G, Putzer D, et al. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol 2020;46(8):1503–1509.
- Schullian P, Johnston EW, Putzer D, et al. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur J Surg Oncol 2021;47(4):866–873.
- Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol 2010;75(1):32–36.
- Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Intervent Radiol 2011;34(4):852–856.
- Schullian P, Johnston E, Laimer G, et al. Thermal ablation of CT 'invisible' liver tumors using MRI fusion: a case control study. Int J Hyperthermia 2020;37(1):564–572.
- Schullian P, Laimer G, Johnston E, et al. Technical efficacy and local recurrence after stereotactic radiofrequency ablation of 2653 liver tumors: a 15-year single-center experience with evaluation of prognostic factors. Int J Hyperthermia 2022;39(1):421–430.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. J Vasc Interv Radiol 2014;25(11):1691–1705.e4.
- Stoot JH, Coelen RJ, De Jong MC, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB 2010;12(8):509–522.
- Farges O, Ferreira N, Dokmak S, et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut 2011;60(1):85–89.
- Deneve JL, Pawlik TM, Cunningham S, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 2009;16(3):640–648.
- Smolock AR, Cristescu MM, Potretzke TA, et al. Microwave ablation for the treatment of hepatic adenomas. J Vasc Interv Radiol 2016;27(2):244–249.
- Ahn SY, Park SY, Kweon YO, et al. Successful treatment of multiple hepatocellular adenomas with percutaneous radiofrequency ablation. World J Gastroenterol 2013;19(42):7480–7486.
- Kim TY, Kim BS, Hyun CL, et al. [Hepatocellular adenoma treated with radiofrequency ablation in young male]. Korean J Gastroenterol 2011;57(6):384–387.
- Rocourt DV, Shiels WE, Hammond S, et al. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg 2006;41(6):1149–1152.
- Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007;46(3):740–748.
- Blanc JF, Frulio N, Chiche L, et al. Hepatocellular adenoma management: call for shared guidelines and multidisciplinary approach. Clin Res Hepatol Gastroenterol 2015;39(2):180–187.
- EASL clinical practice guidelines on the management of benign liver tumours. J Hepatol 2016;65(2):386–398.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–247.
- van Vledder MG, van Aalten SM, Terkivatan T, et al. Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J Vasc Interv Radiol 2011;22(6):787–793.
- van Rosmalen BV, Coelen RJS, Bieze M, et al. Systematic review of transarterial embolization for hepatocellular adenomas. Br J Surg 2017;104(7):823–835.
- Cho SW, Marsh JW, Steel J, et al. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol 2008;15(10):2795–2803.
- Schullian P, Johnston E, Laimer G, et al. Frequency and risk factors for major complications after stereotactic radiofrequency ablation of liver tumors in 1235 ablation sessions: a 15-year experience. Eur Radiol 2021;31(5):3042–3052.
- Tinguely P, Frehner L, Lachenmayer A, et al. Stereotactic image-guided microwave ablation for malignant liver Tumors-A multivariable accuracy and efficacy analysis. Front Oncol 2020;10:842.
- Alvarez FA, Sanchez Claria R, Oggero S, et al. Parenchymal-sparing liver surgery in patients with colorectal carcinoma liver metastases. World J Gastrointest Surg 2016;8(6):407–423.
