?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To evaluate the safety and efficacy of microwave ablation (MWA) versus repeat surgery for treating metastatic lymph nodes (MLNs) in papillary thyroid carcinoma (PTC).
Methods
Between July 2017 and October 2020, 67 patients were enrolled in this retrospective study. 19 and 48 patients underwent MWA and repeat surgery, respectively. The primary and secondary endpoints were recurrence-free survival and complication rates, respectively. The largest diameter, volume and volume reduction ratio (VRR) were analyzed before and after MWA. The effects of different ablation powers on the largest diameter, volume and VRR were investigated. Pre and posttreatment variables (e.g., baseline characteristics, serum thyroglobulin [Tg] levels, hospitalization time, treatment costs, recurrence-free survival and complication rates) were compared between groups.
Results
The largest diameter and volume postablation at each follow-up were smaller than the preablation levels (p < 0.05), except at the 1-month follow-up (p > 0.05). The largest diameter, volume, and VRR among the different ablation powers were not significantly different (p > 0.05). The mean serum Tg levels and biochemical remission rates were not significantly different between the groups (p > 0.05). Compared to reoperation, MWA had a shorter hospitalization time and lower treatment cost (p < 0.001). Total and minor complications were higher in the reoperation group (p < 0.05), but major complications were comparable (p > 0.05). The recurrence-free survival rate between groups was not significantly different (p = 0.401). The 1- and 3-year recurrence-free survival rates were comparable between the groups.
Conclusions
MWA may be a safe and effective alternative to repeat surgery for treating MLNs of PTC in select patients.
Introduction
Thyroid cancer is the most common form of endocrine tumor [Citation1]. Papillary thyroid carcinoma (PTC) accounts for approximately 85% of all cases of thyroid cancer [Citation2,Citation3]. PTC has a favorable prognosis with low mortality rates [Citation4] and a good response to conventional treatments, including thyroidectomy, radioiodine ablation (RAI), and thyrotropin (TSH) suppression therapy [Citation5,Citation6]. However, recurrence and metastasis rates as high as 20–30% postsurgery have been reported [Citation7–9]. Repeat surgery and RAI are the mainstream treatments for metastatic lymph nodes (MLNs) of PTC in postoperative patients [Citation8,Citation10,Citation11]. However, distortion of the neck structures secondary to tissue fibrosis and scar formation makes reoperation challenging and increases complication rates [Citation12]. Furthermore, small MLNs may be difficult to identify without ultrasound guidance, thereby limiting the effectiveness of reoperation. Repeat surgery is the preferred treatment for MLNs of PTC in postoperative patients. However, given the associated risk, the American Thyroid Association guidelines [Citation13] recommend active surveillance (AS) as an option for small MLNs. Several studies [Citation13–15] support managing small MLNs with AS. However, the number/volume of MLNs may increase dramatically during the follow-up period. Furthermore, living with an untreated tumor can cause considerable anxiety for patients. Therefore, they could benefit from a minimally invasive treatment [Citation16].
Percutaneous thermal ablation [e.g., radiofrequency ablation (RFA), laser ablation (LA), and microwave ablation (MWA)] has recently been widely used in disease treatments. Previous studies have demonstrated RFA [Citation17–20] and LA [Citation3,Citation12,Citation21,Citation22] as safe and effective therapeutic modalities for MLNs of PTC. Compared to other types of ablations, MWA can achieve a larger ablation area in a shorter time [Citation23–25]. In the past few decades, it has been widely used in treating benign and malignant tumors of the liver [Citation26], kidney [Citation27,Citation28], lung [Citation29], and thyroid [Citation25,Citation30–33]. In particular, MWA for benign [Citation34–37] and malignant [Citation38–41] thyroid nodules have shown promising results and have become an acceptable treatment modality for thyroid nodules. We previously analyzed the efficacy of MWA for treating thyroid papillary microcarcinoma and obtained satisfactory results [Citation33]. A few studies [Citation11,Citation24,Citation30,Citation38,Citation42] have explored the efficacy and safety of MWA for treating MLNs of PTC; however, no studies have compared MWA and repeat surgery. Therefore, we conducted a retrospective study to investigate the safety and efficacy of MWA versus repeat surgery for treating MLNs of PTC.
Materials and methods
This retrospective study was approved by the Institutional Review Board (QYFY WZLL 26772) of the Affiliated Hospital of Qingdao University (Qingdao, China). The requirement of informed consent for inclusion was waived owing to the retrospective nature of the study. All patients provided written informed consent for treatment before MWA or surgery.
Patients
Medical records of 121 patients with PTC-associated MLNs, treated with MWA or repeat surgery at our hospital (July 2017 to October 2020), were reviewed. Repeat surgery was initially recommended for all patients. MWA was proposed in cases of surgical ineligibility (e.g., high surgical risk, poor medical condition, or patient refusal). All selected patients met the following inclusion criteria [Citation1]: total thyroidectomy and, subsequently, at least one neck dissection [Citation2]; MLNs were from PTC, confirmed with fine-needle aspiration (FNA) or washout thyroglobulin (Tg) concentration in FNA (FNA-Tg) [Citation3]; no distant metastasis [Citation4]; follow-up data and data on serum Tg levels were available; and [Citation5] follow-up lasted at least 12 months.
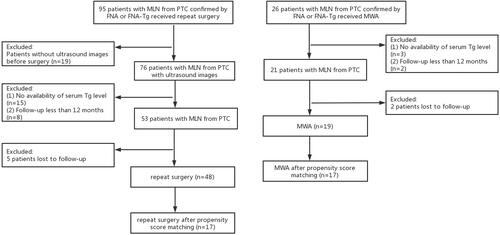
Based on the aforementioned criteria, 67 patients were enrolled. The enrollment process is summarized in . All patients received TSH suppression therapy after treatment to reduce the risk of recurrence.
All patients underwent pretreatment laboratory examinations (e.g., complete blood count, coagulation test, thyroid function test, serum calcium, Tg and TgAb) and imaging examinations [e.g., ultrasound, chest CT and positron emission tomography (PET)]. Even if one sonographic feature (i.e., loss of the fatty hilum, rounded shape, calcifications, cystic change, hyperechogenicity, or peripheral vascularity) was detected with ultrasound, the cervical lymph node was suspected of being malignant. FNA was thereafter conducted to obtain a definitive diagnosis.
Each lesion was carefully evaluated using ultrasound to record its location, size, and vascularity. Its volume was calculated by using the equation, V = π abc/6, where V is the volume, a is the largest diameter, and b and c are the two perpendicular diameters.
Data on patient demographics, tumor characteristics, and treatment characteristics (e.g., hospitalization time and treatment costs) were also collected. The cost of MWA included pretreatment examinations, operation, local anesthesia, ablation needle, hospital stay, nursing care, and posttreatment examinations. Reoperation costs included preoperative examinations, operation, general anesthesia, hospital stay, nursing, postoperative medications, and examinations.
MWA procedure
MWA was performed by a radiologist with at least 5 years of experience. During the procedure, blood pressure, heart rate, and oxygen saturation were closely monitored. Conventional ultrasound and contrast-enhanced ultrasound (CEUS) were conducted using real-time ultrasound systems (LOGIQ Healthcare and Philips Healthcare) equipped with linear probes (ML6-15, 9 L, and L12-5). An MWA system (EC0-100A1; Yigao Co. Ltd., Nanjing, China) and a 16 G needle were used for the MWA procedure.
The patient was placed supine with the neck extended. After administering local anesthesia with 2% lidocaine, a mixture of lidocaine and normal saline was injected around the MLN to prevent thermal damage to adjacent vital structures. Under ultrasound guidance, a 16 G needle was subsequently inserted into the target lesion. Power outputs of 20, 25 or 30 W were used, depending on the size and location of the metastasis. The moving-shot technique was used for large lesions, whereas the fixed ablation technique was used for small lesions. The needle was slowly withdrawn when the lesion was completely covered by a hyperechoic area. Track ablation was used to avoid the risk of tumor cell seeding. After MWA, the extent of ablation was evaluated with CEUS. It was ‘complete’ if no enhancement was detected on CEUS; otherwise, additional ablation was administered (Supplementary Figure S1).
Reoperation procedure
Cervical lymph node dissection was performed under general anesthesia by a single surgeon with at least 10 years of experience in thyroid surgery. The extent of neck dissection was determined, based on the site of recurrence. Lateral cervical neck dissection was performed for recurrence in the lateral compartment. Central cervical neck dissection was used for recurrence in the central compartment.
Follow-up
Patients were followed-up with ultrasonography and routine measurement of serum Tg levels. Ultrasonography was conducted in the reoperation group every 3–6 months and in the MWA group at 1, 3, 6, 9 and 12 months postprocedure, and every 6 months thereafter. The tumor diameter was measured thrice, and the volume reduction ratio was calculated as follows:
If the ablation area remained visible for at least 1 year or recurrence was suspected, FNA was conducted to make a definitive diagnosis. If the serum Tg level continued to increase or remained elevated, chest CT and PET were conducted to exclude distant metastasis.
Study endpoints
The primary endpoint was recurrence-free survival. Recurrence was defined as [Citation1] persistent ablation zones, confirmed with FNA or FNA-Tg, as MLN of PTC after MWA and [Citation2] recurrence in situ or a new cervical compartment after MWA or repeat surgery.
The secondary endpoint was the complication rate. Complications were major [e.g., permanent hypocalcemia and permanent recurrent laryngeal nerve (RLN) injury] or minor (e.g., transient hoarseness, transient hypocalcemia, pain, fever, skin burns, and neck hematoma). We considered hoarseness lasting >6 months posttreatment as a permanent RLN injury if direct laryngoscopy confirmed vocal cord dysfunction. We considered hoarseness lasting <6 months as transient. Hypocalcemia was similarly classified as transient (recovery within 6 months) or permanent (lasting >6 months and requiring treatment).
Statistical analyses
Statistical analyses were conducted using SPSS statistical software (version 26.0, IBM Corp., Armonk, NY, USA). Continuous data are presented as the mean ± the standard deviation and were analyzed using the t-test, Wilcoxon signed-rank test, or Mann–Whitney U-test. Categorical data are expressed as frequencies (percentages) and were analyzed using the χ2 test or Fisher’s exact test. Different power outputs among groups were compared using analysis of variance or the rank-sum test. Kaplan–Meier curves and log-rank tests were used to compare the recurrence-free survival rates between the two groups. Statistical significance was set at p < 0.05.
To limit selection bias arising from lack of randomization, 1:1 propensity score matching (PSM) was conducted between the two groups by using the nearest-neighbor-matching method, based on the following variables: age at the time of MWA or repeat surgery; sex; number, location, and the largest diameter of MLNs; follow-up time; RAI before MWA or repeat surgery; and interval from diagnosis to treatment. Match tolerance was set at 0.05. 34 patients (n = 17 per group) were subsequently included in the final analysis.
Results
Baseline characteristics of the patients
Of the 121 patients who underwent MWA or repeat surgery between 2017 and 2020, 7 patients were lost to follow-up and 47 patients were excluded because of incomplete information. Of those remaining, 19 patients and 48 patients were enrolled in the MWA group and reoperation group, respectively. Before PSM, the two groups were not significantly different, except in the number of previous operations [1.47 ± 0.84 (MWA) vs. 1.04 ± 0.20 (reoperation); p = 0.039)]. Regarding the number of treated MLNs at level VI, no significant difference existed between the groups [22.9% (MWA) vs. 27.0% (reoperation); p = 0.630]. In the reoperation group, the number of surgically proven MLNs of PTC (3.79 ± 3.57) was greater than the number of lesions (2.10 ± 1.04, p = 0.001) with a high probability of being preoperative tumors on ultrasound. Surgical pathology revealed 0.69 ± 1.31 MLNs and 3.10 ± 3.24 MLNs in the central and lateral compartments, respectively. After PSM, 17 patients in each group had comparable baseline characteristics ().
Table 1. Baseline characteristics of patients.
MWA response
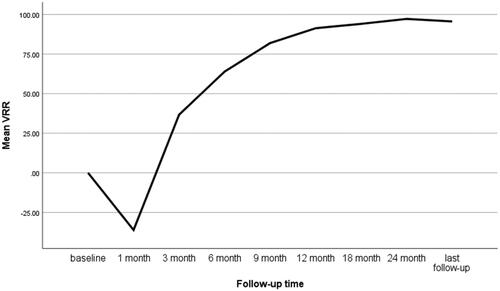
After ablation, no MLN was enhanced on CEUS, indicating a technical success rate of 100%. The largest diameter and volume at the 1-month follow-up were greater than those at pretreatment, but the differences were not significant (p > 0.05). At the last follow-up, the largest diameter decreased from 1.17 ± 0.08 cm to 0.19 ± 0.07 cm (p < 0.001) and the volume reduced from 494.92 ± 775.08 mm3 to 35.83 ± 143.21 mm3 (p < 0.001). The VRR at the last follow-up was 95.57 ± 10.07% (, ). Complete disappearance rates were 5.7%, 14.3%, 34.3%, 42.9% and 74.3% at the 3-month, 6-month, 9-month, 1-year and final follow-ups, respectively. Residual ablation zones were confirmed as having no malignant cells and with undetectable FNA-Tg.
Table 2. Changes in mean largest diameter, volume and VRR in the MWA group at each follow-up.
With regard to complications, only one (5.3%) patient had hypocalcemia and recovered within 1 month (). During follow-up, MLNs that were distinguishable from the ablation zones were detected in three (15.8%) patients; one patient underwent a second MWA session, one patient underwent RAI, and one patient opted for AS only (Supplementary Table S3).
Table 3. Comparisons of the MWA and reoperation groups.
Comparison of different ablation powers in the MWA group
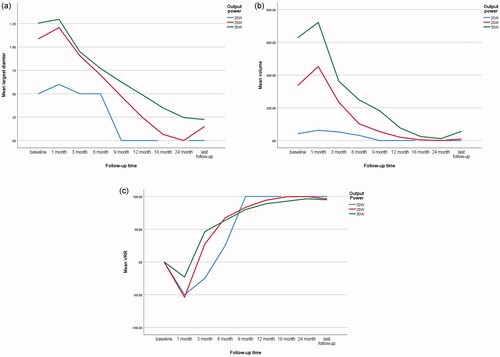
Among the 35 MLNs, the power output used was 20 W in 1 MLN, 25 W in 14 MLNs, and 30 W in 20 MLNs (Supplementary Table S2). During follow-up, the largest diameter and volume gradually decreased but were not significantly different among the three groups (p = 0.090 and p = 0.178, respectively). The VRR at the last follow-up was nearly 100% without any significant difference (p = 0.885) ().
Reoperation response
With regard to complications, 9 (18.8%) of 48 patients had hypocalcemia: 6 patients had transient hypocalcemia and 3 patients had permanent hypocalcemia. Three (6.25%) patients had transient hoarseness, including 1 patient with hypocalcemia; 1 (2.1%) patient with hypocalcemia experienced pain in the left shoulder and back; 9 (18.75%) patients complained of incision pain; 1 (2.1%) patient developed a fever on the first day postsurgery (). 14 (29.2%) patients experienced a recurrence, one of whom underwent RFA, while the others underwent AS only (Supplementary Table S3).
Comparison of the MWA and reoperation groups
The reoperation group had longer hospitalization times and higher treatment costs than the MWA group (p < 0.001). The total and minor complications were lower in the MWA group than in the reoperation group (p < 0.05). The major complications, hypocalcemia (transient and permanent), transient hoarseness, and pain were comparable between the groups (p > 0.05) ().
No significant differences in major complications, hypocalcemia (transient and permanent), transient hoarseness, and pain existed between the groups in the central and lateral cervical compartments (p > 0.05). The reoperation group had more total and minor complications in the lateral compartment than did the MWA group (p < 0.05). After PSM, all results remained similar between the two groups (Supplementary Table S4).
Posttreatment follow-up of the serum Tg level
The negative conversion rates of TgAb after treatment among patients who had pretreatment TgAb levels >100 IU/mL were not significantly different between the two groups (p > 0.999). No significant differences existed in the mean pre and posttreatment serum Tg levels in all patients and in patients with serum Tg levels ≥1 ng/mL between the groups (p > 0.05). No significant difference existed in the mean decrease in serum Tg levels (p = 0.232). The MWA group had a higher negative conversion rate of serum Tg levels than did the reoperation group, but without a significant difference (p = 0.438) ().
Table 4. Serum Tg level changes after MWA or repeat surgery.
Comparison of recurrence between the MWA and reoperation groups
The mean follow-up times for the MWA and reoperation groups were 22.74 ± 12.10 months and 26.00 ± 12.94 months, respectively (Supplementary Table S5). Recurrence was not significantly different between the groups (15.8% vs. 29.2%, p = 0.411) (). However, the time lag for recurrence was longer in the MWA group (mean time, 12 months; range, 9–15 months) than in the reoperation group (mean time, 8.57 months; range, 1–32 months) (Supplementary Table S3). Kaplan–Meier survival curves revealed an insignificant difference in the overall recurrence-free survival rates between the groups (p = 0.303). No significant differences existed between the MWA and reoperation groups in the 1-year (89.5% vs. 77.1%, p = 0.321) and 3-year (84.2% vs. 70.8%, p = 0.356) recurrence-free survival rates ().
Figure 4. Kaplan–Meier recurrence-free survival curves of microwave ablation (MWA; red) and reoperation (blue) groups (a) before and (b) after propensity score matching.
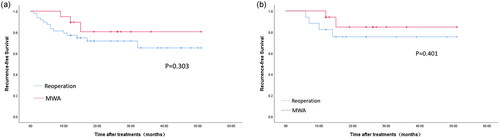
After PSM, the recurrence-free survival curves remained similar between the two groups (p = 0.401). The 1-year and 3-year recurrence-free survival rates were 94.1% and 88.2%, respectively, for the MWA group and 82.4% and 76.5%, respectively, for the reoperation group. No significant differences existed in the 1-year and 3-year recurrence-free survival rates (p = 0.601 and p = 0.656, respectively) (). No distant metastasis occurred during follow-up.
Discussion
The goal of this study was to evaluate the safety and efficacy of MWA in the treatment of MLNs in PTC compared to those of repeat surgery. We found that MWA is an effective treatment for MLNs of PTC because all lesions in the MWA group were successfully ablated in a single procedure. Thus, the technical success rate was 100%. We believe that applying CEUS is critical for successful treatment using MWA [Citation43]. In the present study, the largest diameter and volume of MLNs at 1-month postablation were slightly larger than the preablation levels, which may be related to the larger extent of ablation. However, a gradual decrease occurred during subsequent follow-ups. The VRR was nearly 100% at the last follow-up, which is consistent with the findings of Teng et al. [Citation42]. Furthermore, the VRR in our study was slightly higher than, but comparable to, previous results of 76.9–95.1% obtained with RFA [Citation17–20].
At the last follow-up, 74.3% of the MLNs had completely disappeared, which was consistent with the reports of Zhou et al. (76.2% with MWA) [Citation11] and Mauri et al. (80% with LA) [Citation3]. The complete disappearance rate in our study was higher than the 44.7% rate reported by Cao et al. [Citation10] but lower than the 100% rate reported by Teng et al. [Citation42]. The possible reasons for the discrepancy may be the shorter follow-up period of some patients in our study and the categorization of scar-like lesions as a ‘disappearance’ by Teng et al. Moreover, some MLNs in the Teng study were small and easily absorbed. In our study, 25.7% of the lesions were persistently visible on ultrasound at the last follow-up, which may be related to the large volume of the lesions. Nevertheless, patients without a complete disappearance of lesions showed a good trend and were followed up regularly.
We also analyzed the effects of different ablation powers on PTC-associated MLNs. However, no differences were observed, which may be related to the small sample size. When selecting output power, we considered factors like tumor size and location. To our knowledge, the ideal output power of MWA for MLNs of PTC has not been clearly defined to date. Previous reports using powers of 20 [Citation24,Citation42], 35 [Citation11] and 40 W [Citation11] achieved satisfactory results. We primarily used 25 and 30 W and the results were comparable. Further studies are warranted to test the effects of different output powers on the treatment of MLNs.
We found that, compared to reoperation, MWA had a shorter hospitalization time, lower treatment cost, and fewer complications. These findings may be attributable to the minimal invasiveness of MWA, which is performed under local anesthesia, compared to surgery, which is more invasive and requires general anesthesia, thereby increasing treatment risks and costs.
A previous study [Citation44] reported rates of transient and permanent hypocalcemia after surgery as 27.9% and 0.9%, respectively. In the current study, one patient in the MWA group had hypocalcemia, which may be attributable to thermal damage caused by MWA. Nine patients in the reoperation group had hypocalcemia, which may be because of extensive neck dissection affecting the blood supply to the parathyroid glands or accidental resection of parathyroid glands owing to the difficulty in distinguishing MLNs from the parathyroid glands intraoperatively.
Hoarseness is a severe complication primarily caused by RLN injury. Tissue adhesion secondary to a previous operation causes the lymph nodes to lie adjacent to the RLN, thereby making it prone to injury. In one study [Citation45], the reported incidences of permanent and transient RLN injury after surgery for PTC were 2.3% and 9.8%, respectively. In the current study, no hoarseness occurred in the MWA group, whereas transient hoarseness occurred in three (6.3%) patients in the reoperation group. In the reoperation group, one patient experienced pain in the left shoulder and back, possibly caused by intraoperative stimulation of the brachial plexus; 18.8% of patients complained of incision pain (although it was bearable), owing to the large extent of dissection and surgical incision.
No significant differences in major complications occurred, although total and minor complications were lower in the MWA group. This finding can be attributed to [Citation1] lymph node capsule blocking heat conduction, which, coupled with low output power, caused a higher temperature inside an MLN than outside it [Citation2]; applying hydrodissection protected surrounding structures [Citation3]; continuous injection of normal saline around MLNs prevented the diffusion of heat; and [Citation4] the entire procedure, performed under ultrasound guidance, prevented thermal damage to surrounding structures.
Kim et al. [Citation46] found that the 1-year and 3-year recurrence-free survival rates after RFA and reoperation were comparable. In our study, no significant difference in recurrence existed between the two groups. Moreover, the 1- and 3-year recurrence-free survival rates were similar. However, MWA had a longer average time to recurrence than did repeat surgery, possibly because of the use of CEUS, which guaranteed complete ablation. Cao et al. [Citation10] reported that the 1- and 3-year recurrence-free survival rates after LA were 86.9% and 100%, respectively. Mauri et al. [Citation22] found that the local control rate of LA for treating MLNs was 86.9%. These findings are similar to those of our study.
The serum Tg level is a valuable indicator for monitoring recurrence and metastasis in PTC [Citation13,Citation47]. A continuously increasing or elevated serum Tg level often indicates recurrence or distant metastasis. Our study demonstrated that the serum Tg level posttreatment was lower than that at pretreatment in both groups. However, no significant differences existed between the groups in the posttreatment serum Tg levels and biochemical remission rates, which indicated comparable therapeutic efficacies. The combination of serum Tg levels with ultrasound could help improve the diagnostic accuracy of difficult-to-detect small MLNs.
Our study had some limitations. It lacked sufficient power to show clinically meaningful differences because of the small sample size. Selection bias cannot be excluded because of the retrospective nature of the study. Moreover, MWA cannot eliminate occult MLNs. Furthermore, patients who underwent reoperation may have had more MLNs than did patients who underwent MWA, which may have tipped the results in favor of MWA. Finally, the follow-up period was short. Therefore, further large multicenter studies with long-term follow-ups are required to confirm our results.
In conclusion, MWA may be an effective and safe treatment for PTC-associated MLNs. This minimally invasive treatment could prevent trauma associated with reoperation.
Author contributions
Study conception and design: C.Z. and W.H.S.; data acquisition: X.Y.N.; data analysis and/or interpretation: W.Q.T., X.F.W. and R.L.W.; literature research: X.Y.W., M.Z.Z., W.B.J. and D.N.J.; manuscript drafting: W.Q.T.; revising the manuscript critically for important intellectual content: C.Z.; approval of the final version of submitted manuscript: all authors.
Acknowledgment
The authors thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ji-Bin LMD, Kun HMD. Application of ultrasonography in the diagnosis and management of papillary thyroid microcarcinoma. Adv Ultrasound Diagn Ther. 2020;4(4):284.
- Song Q, Gao H, Tian X, et al. Evaluation of ultrasound-guided radiofrequency ablation as a treatment option for papillary thyroid microcarcinoma in the isthmus: a retrospective study. Front Endocrinol. 2020;11:599471.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab. 2013;98(7):E1203.
- Sippel RS, Robbins SE, Poehls JL, et al. A randomized controlled clinical trial: no clear benefit to prophylactic central neck dissection in patients with clinically node negative papillary thyroid cancer. Ann Surg. 2020;272(3):496–503.
- McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381(9871):1046–1057.
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795.
- Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid carcinoma: rationale, techniques, and controversies. Radiology. 2008;249(2):429–444.
- Burman KD. Treatment of recurrent or persistent cervical node metastases in differentiated thyroid cancer: deceptively simple options. J Clin Endocrinol Metab. 2012;97(8):2623–2625.
- Liu FH, Kuo SF, Hsueh C, et al. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. 2015;112(2):149–154.
- Cao XJ, Wei Y, Zhao ZL, et al. Efficacy and safety of microwave ablation for cervical metastatic lymph nodes arising post resection of papillary thyroid carcinoma: a retrospective study. Int J Hyperthermia. 2020;37(1):450–455.
- Zhou W, Chen Y, Zhang L, et al. Percutaneous microwave ablation of metastatic lymph nodes from papillary thyroid carcinoma: preliminary results. World J Surg. 2019;43(4):1029–1037.
- Papini E, Bizzarri G, Bianchini A, et al. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab. 2013;98(1):E92–E97.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Robenshtok E, Fish S, Bach A, et al. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012;97(8):2706–2713.
- Tomoda C, Sugino K, Matsuzu K, et al. Cervical lymph node metastases after thyroidectomy for papillary thyroid carcinoma usually remain stable for years. Thyroid. 2016;26(12):1706–1711.
- Mauri G, Hegedüs L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10(7):144–149.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. Am J Roentgenol. 2011;197(2):W331.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170.
- Spartalis E, Karagiannis SP, Plakopitis N, et al. Percutaneous laser ablation of cervical metastatic lymph nodes in papillary thyroid carcinoma: clinical efficacy and anatomical considerations. Expert Rev Med Devices. 2021;18(1):75–82.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79(1):124–130.
- Han ZY, Dou JP, Cheng ZG, et al. Efficacy and safety of percutaneous ultrasound-guided microwave ablation for cervical metastatic lymph nodes from papillary thyroid carcinoma. Int J Hyperthermia. 2020;37(1):971–975.
- Zu Y, Liu Y, Zhao J, et al. A cohort study of microwave ablation and surgery for low-risk papillary thyroid microcarcinoma. Int J Hyperthermia. 2021;38(1):1548–1557.
- Wang Z, Liu M, Zhang DZ, et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5 cm hepatocellular carcinoma. Hepatology. 2022 DOI:10.1002/hep.32323.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology. 2014;270(3):880–887.
- Krieger JR, Lee FT, McCormick T, et al. Microwave ablation of renal cell carcinoma. J Endourol. 2021;35(S2):S33–S37.
- Ni Y, Ye X, Yang X, et al. Microwave ablation for non-small cell lung cancer with synchronous solitary extracranial metastasis. J Cancer Res Clin Oncol. 2020;146(5):1361–1367.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Korkusuz Y, Mader OM, Kromen W, et al. Cooled microwave ablation of thyroid nodules: initial experience. Eur J Radiol. 2016;85(11):2127–2132.
- Zhao J, Qian L, Liu Y, et al. A long-term retrospective study of ultrasound-guided microwave ablation of thyroid benign solid nodules. Int J Hyperthermia. 2021;38(1):1566–1570.
- Wang X, Niu X, Mu S, et al. Analysis and evaluation of the efficacy of ultrasound-guided microwave ablation for papillary thyroid microcarcinoma. Int J Hyperthermia. 2021;38(1):1476–1485.
- Li S, Yang M, Guo H, et al. Microwave ablation vs traditional thyroidectomy for benign thyroid nodules: a prospective, non-randomized cohort study. Acad Radiol. 2022;29(6):871–879.
- Luo F, Huang L, Gong X, et al. Microwave ablation of benign thyroid nodules: 3-year follow-up outcomes. Head Neck. 2021;43(11):3437–3447.
- Jin H, Fan J, Lu L, et al. A propensity score matching study between microwave ablation and radiofrequency ablation in terms of safety and efficacy for benign thyroid nodules treatment. Front Endocrinol. 2021;12(584972):584972.
- Guo DM, Chen Z, Zhai YX, et al. Comparison of radiofrequency ablation and microwave ablation for benign thyroid nodules: a systematic review and meta-analysis. Clin Endocrinol. 2021;95(1):187–196.
- Cao XJ, Zhao ZL, Wei Y, et al. Microwave ablation for papillary thyroid cancer located in the thyroid isthmus: a preliminary study. Int J Hyperthermia. 2021;38(1):114–119.
- Teng DK, Li WH, Du JR, et al. Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid. 2020;30(12):1752–1758.
- Zhou W, Ni X, Xu S, et al. Ultrasound-guided laser ablation versus microwave ablation for patients with unifocal papillary thyroid microcarcinoma: a retrospective study. Lasers Surg Med. 2020;52(9):855–862.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab . 2020;105(6):dgaa128.
- Teng D, Ding L, Wang Y, et al. Safety and efficiency of ultrasound-guided low power microwave ablation in the treatment of cervical metastatic lymph node from papillary thyroid carcinoma: a mean of 32 months follow-up study. Endocrine. 2018;62(3):648–654.
- Kloth C, Kratzer W, Schmidberger J, et al. Ultrasound 2020 - diagnostics & therapy: on the way to multimodal ultrasound: contrast-enhanced ultrasound (CEUS), microvascular doppler techniques, fusion imaging, sonoelastography, interventional sonography. Rofo. 2021;193(1):23–32.
- Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine. 2014;47(2):537–542.
- Zhang D, Pino A, Caruso E, et al. Neural monitoring in thyroid surgery is here to stay. Gland Surg. 2020;9(1):S43–S46.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Jeon SJ, Kim E, Park JS, et al. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol. 2009;10(2):106–111.