Abstract
Objectives
To compare the safety and efficacy of step-by-step debulking Microwave Ablation (MWA) with Transarterial Chemoembolization (TACE) monotherapy for huge (≥10 cm in diameter) unresectable hepatocellular carcinoma (HCC) after TACE refractoriness.
Methods
This is a multi-center retrospective study carried out on 599 patients with huge unresectable HCC who received TACE as first-line therapy at five hospitals from January 2009 to December 2018. A total of 103 patients with TACE refractoriness were divided into two cohorts: monthly step-by-step debulking MWA (n = 52) or continued TACE (n = 51). Overall survival (OS) and progression-free survival (PFS) after refractory TACE were evaluated. Residual liver and tumor volume were recorded for the MWA group.
Results
Median follow-up period was 24.3 months and median OS and PFS were significantly longer in the MWA group than in the TACE group (OS 21.0 vs. 11.7 months, PFS 6.1 vs. 3.0 months, both p < 0.001). The one-, two-, and three-year OS rates in the MWA and TACE groups were 73.1%, 46.6%, and 37.2% versus 43.1%, 15.5%, and 2.9%, respectively. Furthermore, the 0.5-, 1-, and 2-year PFS rates in the MWA and TACE groups were 51.9%, 36.5%, and 25.0% versus 27.5%, 11.8%, and 0, respectively. Multivariate analyses confirmed that switching to debulking MWA treatment was an independent favorable prognostic factor for PFS and OS. In the MWA group, the average additions of residual liver volume/total liver volume were 7.7% ± 6.7%, 7.2% ± 10.2%, and 10.1% ± 8.8% after the first, second, and third MWA procedure.
Conclusion
Step-by-step debulking MWA can significantly improve long-term OS and PFS in patients with huge unresectable HCCs compared with repeated TACE after TACE refractoriness.
The debulking MWA therapy provides significantly longer OS and PFS than continued TACE for patients with huge unresectable HCCs after TACE-refractory, especially with complete tumor ablation.
The most common complications were fever (48.1%) and pain (46.2%) in the MWA group. Two major complications (abdominal infection) were recorded in the MWA group, which recovered after symptomatic treatment.
During the course of repeated MWAs, liver hyperplasia appeared mainly after the second MWA procedure and the average maximum increased RLV/TLV rate was 16.3%±12.7%.
Key Point
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most frequent cause of cancer-related death worldwide [Citation1,Citation2]. Many patients with HCC are diagnosed at an advanced stage of the disease with tumor size > 10 cm due to ignorance of routine HCC screening in China [Citation3,Citation4].
According to BCLC staging system, surgical resection is the only curative treatment for huge HCC [Citation5–7]. However, only a minority of patients are candidates for curative resection due to advanced tumor stage, poor hepatic reserve, or other clinical factors and comorbidities [Citation6,Citation8]. Therefore, transarterial chemoembolization (TACE) is considered the first choice for huge unresectable HCCs. A meta-analysis reported longer patient survival with TACE compared with systemic chemotherapy or best supportive care [Citation9], as well as in patients with huge HCCs [Citation7,Citation10]. However, even after repeated TACEs, tumor complete necrosis rate is still low (10%–20%) [Citation11–13]. Meanwhile, repeated TACE could gradually lead to deterioration of liver function and side effects, which impedes further treatment [Citation14]. TACE refractoriness is mentioned in several treatment guidelines, including the American Association for the Study of Liver Diseases (AASLD), the Japan Society of Hepatology (JSH), and the European Association for the Study of the Liver (EASL) [Citation5,Citation15,Citation16]. Patients with TACE refractoriness should be managed individually.
Sorafenib is recommended for HCC patients with TACE refractoriness, but its use is severely limited by the high adverse event rate and cost [Citation17,Citation18]. Although many patients develop TACE refractoriness, in practice they still receive continued TACE treatment, and the timing of treatment modification is still controversial [Citation19]. Microwave ablation (MWA) has been proven to be a safe and effective treatment modality for local tumor control in HCC patients with many advantages, including rapid heating rate, high intra-tumor temperature, and large ablation zone [Citation20]. Previous studies demonstrated that TACE combined with MWA could improve survival in HCC patients compared with TACE alone [Citation5,Citation6]. Traditionally, MWA ablation is carried out as an alternative treatment to surgical resection for managing HCCs <5 cm at their largest diameter. Meanwhile, repeated thermal ablations of huge HCCs have been increasingly performed at many centers with good results [Citation21,Citation22]. Furthermore, few recent studies have compared the efficacy of step-by-step debulking MWA versus continued TACE in patients with huge unresectable HCC refractory to TACE. The purpose of this multi-center retrospective study was to compare the efficacy and safety of step-by-step debulking MWA with continued TACE for patients with huge unresectable HCC refractory to TACE.
Materials
Patients
The medical records of 599 consecutive patients diagnosed as having huge unresectable HCC who received TACE as primary treatment at five different hospitals between January 2009 and December 2018 were retrospectively reviewed. This retrospective study followed the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Sen Yat-sen University Cancer Center(No.B2020-103). Patients deemed refractory to TACE procedures were further identified from the TACE patient pool. The definition of TACE refractoriness is based on the 2014 JSH Consensus Guidelines (15) (). Radiological response and tumor-marked response to TACE were evaluated one month after TACE. In our study, continuous AFP elevation was defined as an increase of >20% from baseline before each TACE within two months [Citation23].
Table 1. Definition of TACE refractoriness [Citation16].
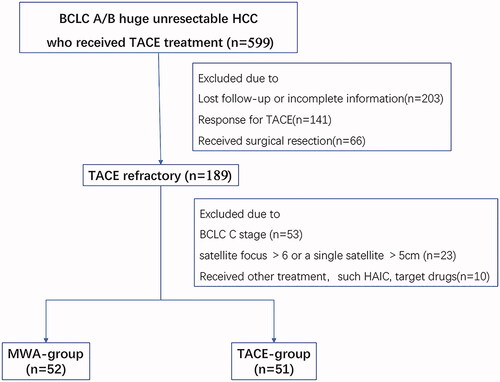
TACE-refractory patients who switched to step-by-step debulking MWA treatment (MWA group) or who continued TACE (TACE group) despite being TACE-refractory were further reviewed. The inclusion criteria were as follows: (1) diagnosis of HCC by liver biopsy, or clinical diagnosis, according to the American Association for the Study of Liver Diseases (AASLD) criteria, (2) diameter of the main lesion ≥10 cm with number of satellite lesions ≤3 and size of satellite lesions ≤5 cm at initial TACE, (3) unresectable HCC within stage A or B BCLC after TACE refractoriness [Citation16], (4) Child-Pugh class A or B, (5) Eastern Cooperative Oncology Group Performance Status of 0 to 1. Exclusion criteria included: (1) patients who underwent other treatment approaches (sorafenib, systemic chemotherapy); (2) patients with stage C BCLC or patients with >6 satellite lesions or a single satellite lesion >5cm after TACE refractoriness; (3) patients with secondary malignancy. And they were randomly assigned to two treatment groups. As a result, 189 patients were deemed TACE-refractory after two to three TACE procedures. Out of these patients, 86 were excluded as shown in . The remaining 103 patients were assigned to the TACE group (n = 51) or MWA group (n = 52), and among them 90 patients from Sun Yat-sen University Cancer Center, 3 patients from Shenzhen people's hospital, 4 patients from Henan Cancer Hospital, 5 patients from Hunan Cancer Hospital and 1 patient from the Second Affiliated Hospital of Nanchang University.
Treatment
Tace procedure
With the patient under local anesthesia, a 5 F French catheter (Yashiro type; Terumo Corporation, Tokyo, Japan) was introduced into the abdominal aorta via the femoral artery using the Seldinger technique. An arteriogram was conducted using fluoroscopy to guide the catheter into the celiac and superior mesenteric arteries. A microcatheter (Renegade Hi Flo; Boston Scientific Corporation, Boston MA, USA) was super-selectively inserted into the feeding arteries. A mixed solution of pirarubicin (80 mg/m2, Shenzhen Main Luck Pharmaceuticals Inc., China), lobaplatin (50 mg/m2, Hainan Changan International Pharmaceutical Co., Ltd., China), and iodized oil (Lipiodol, Guerebet, Villepinte, France) was injected until a vascular cast was observed. Gelfoam sponge embolization was performed following iodized oil embolization. The interval between repeated TACEs was three to four weeks.
MWA procedure
For the patients with huge HCC, due to the influence of ribs, lung and iodine, ultrasound was difficult to display lesions at the top of the liver. So, MWA were performed under CT-guidance routinely. Routine disinfection and local anesthesia were applied around the puncture point, and 16-gauge microwave antennas (FORSEA; Qinghai Microwave Electronic Institute, Nanjing, China) were inserted into the tumor under CT guidance (Brilliance CT Big Bore; PHILIPS). Due to the advantages of low anesthesia risk and rapid recovery rate, intravenous anesthesia (propofol; AstraZeneca S.p.A., Italy) were performed during the entire MWA procedure by anesthesiologists with monitoring of vital signs.
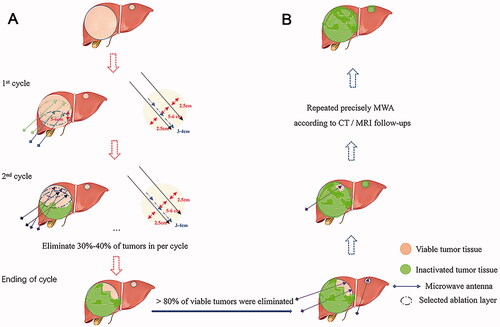
The MWA procedure for huge HCC can be divided into two stages: the heavy ablation stage and residual tumor ablation stage (). Stage 1 (the heavy ablation stage, ): In our study, we strictly abide by the ‘2-2-2’ principle: 2 microwave antennas, 2 chosen CT-layers, and 2 ablation cycles. In a single cycle, 2 microwave antennas were used to conduct overlapping ablations in six sites (three sites for each antenna, and 3 cm to 4 cm between two sites in one antenna) on a single CT-layer. At least 2 CT-layers should be selected to fulfill an ablation cycle. The distances between two antennas and two chosen CT-layers were 4 to 5 cm and 5 to 6 cm, respectively. The ablation parameters were set at 60–70 W/8–10 min at every ablation site. In stage 1, elimination of more than 80% of viable tumors was required. Stage 2 (residual tumor ablation stage, ): after stage 1, residual tumor might be found in the peripheral area of the tumor on MRI image. Repeated ablations were used for residual tumor and daughter foci to achieve the entire elimination of viable tumors. The number of repeated MWAs and the interval were mainly determined through post-treatment assessment and repeated MWAs were suggested when the tumor was found incompletely ablated or a new lesion emerged.
Follow-up
A three-phase helical CT or MRI scan was carried out post-operatively every month for the first three months, then three to six months after CR was achieved. Liver function, blood coagulation profile, and serum alpha-fetoprotein (AFP) were tested monthly. Follow-up was censored on 30 April 2020.
Overall survival (OS) was calculated from the date when TACE refractoriness was diagnosed until either the last follow-up or death. Progression-free survival (PFS) was calculated from the date when TACE refractoriness was diagnosed until either the last follow-up or tumor progression. Local progression-free survival (LPFS) and distant progression-free survival (DPFS) were calculated from the date of refractory TACE until intrahepatic metastases or extrahepatic metastases appeared. The radiological response to MWA and TACE directly after judgment as TACE-refractory was assessed using the modified RECIST criteria (mRECIST). Complications were assessed based on the CTCAE 5.0 and by standardization of terminology and reporting criteria on image-guided tumor ablation. Major complications were defined as events that resulted in substantial morbidity and disability, which led to the requirement for more invasive therapy and/or substantially lengthened hospitalization.
IQQA-liver workstation (EDDA Technology, Princeton, NJ, USA) was used to predict residual liver volume according to the CT/MRI enhance imaging data of the patients seen at our hospital before and after each treatment. First, the patient's image data were uploaded to the system, the images were manually adjusted, and the thin layers were accumulated to finally depict the overall shape of the liver. Then, total liver volume (TLV), tumor volume (TV), and residual liver volume RLV were calculated.
Statistical analysis
Continuous variables are expressed as the mean ± SD and compared between the two groups using t-tests. Categorical variables were compared using Chi-square or Fisher’s exact test (two-tailed), as applicable. Differences in survival were assessed for significance using the log-rank test. Survival rates were calculated using the Kaplan–Meier method. Univariate and multivariate analyses were carried out using the Cox proportional hazards regression model. All variables with p < 0.10 evaluated through univariate analysis were subjected to multivariate analysis. All analyses were performed using the SPSS software package (version 13; SPSS, Inc., Chicago, IL, USA). P values < 0.05 were considered statistically significant.
Results
Demographic characteristics of patients in the study
Patient characteristics and demographics are described in . There was no significant difference in the baseline demographic data between the MWA and TACE groups before TACE-refractory. Tumor diameter in the TACE group was slightly larger than that of the MWA group. TACE was performed 131 times in the MWA group (52 patients, mean 2.24, range 2–3) and 114 times in the TACE group (51 patients, mean 2.52, range 2–3) before TACE refractoriness (p = 0.03). After TACE refractoriness, 51 patients in the TACE group underwent 104 TACE procedures before tumor progression (median: 2, range: 1–6), whereas 52 patients in the MWA group underwent 145 MWA procedures (median: 3, range: 1–7).
Table 2. Demographic and characteristics of study participants with unresectable huge hepatocellular carcinoma.
Survival after being identified as TACE-refractory
The median follow-up period of the entire cohort was 24.3 months (interquartile range, 18.5–41.3 months). Tumor progression occurred in 78.8% and 100% the patients in the MWA and TACE groups, respectively. Median PFS for the MWA and TACE groups was 6.1 vs. 3.0 months, respectively (p < 0.001). The 0.5-, 1-, and 2-year PFS rates in the MWA and TACE groups were 51.9%, 36.5%, and 25.0% versus 27.5%, 11.8%, and 0, respectively (). The paths of tumor progression are described as follows: 49 cases of intrahepatic metastases (27/52.3% in the TACE group, 22/42.3% in the MWA group), 20 cases of extrahepatic metastases (11/21.6% in the TACE group, 9/17.3% in the MWA group), 8 cases of synchronous intrahepatic and extrahepatic metastasis (5/9.8% in the TACE group, 3/5.8% in the MWA group), and 15 cases of vascular invasion (8/15.7% in the TACE group, 7/13.5% in the MWA group). The MWA group had longer LPFS and DPFS (LPFS: 7.6 months vs. 3.5 months, p < 0.001; DPFS: 31.0 months vs. 6.9 months, p < 0.001; ) than the TACE group.
Figure 3. Survival curves for huge unresectable HCC patients who underwent repeated TACE monotherapy and step-by-step debulking MWA therapy. Graph shows Kaplan–Meier estimation of overall survival (A) and progression-free survival (B) for the two groups. Graphs show cumulative incidence of (C) local progression-free survival and (D) distanct progression-free survival for the two groups. HCC: hepatocellular carcinoma; TACE: transarterial chemoembolization; MWA: microwave ablation.
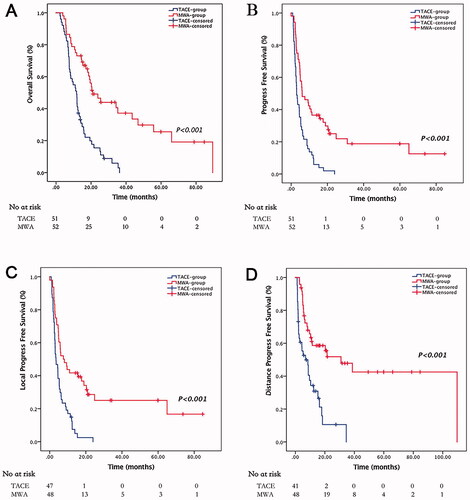
At the end of the study, 18 patients in the MWA group and 3 patients in the TACE group were still alive. Median OS was significantly longer in the patients who received MWA than in the patients who received TACE (21.0 vs. 11.7 months, p < 0.001, ). The one-, two-, and three-year OS rates in the MWA and TACE groups were 73.1%, 46.6%, and 37.2% versus 43.1%, 15.5%, and 2.9%, respectively ().
Univariate and multivariate analyses were performed to examine OS and PFS in the cohort (). Univariate analyses identified that the following factors – BCLC stage A, AST < 40U/L, tumor diameter < 15cm, and step-by-step debulking MWA – significantly contributed to prolonged PFS. In multivariate analysis, tumor diameter < 15 cm, Child-Pugh A after TACE- refractory, and step-by-step debulking MWA were independent prognostic factors for PFS. Regarding OS, Child-Pugh A at baseline, AST < 40U/L, and step-by-step debulking MWA were independent prognostic factors.
Table 3. Prognostic factors predicting overall survival and progression-free survival in patients with the unresectable huge hepatocellular carcinoma after TACE-refractory.
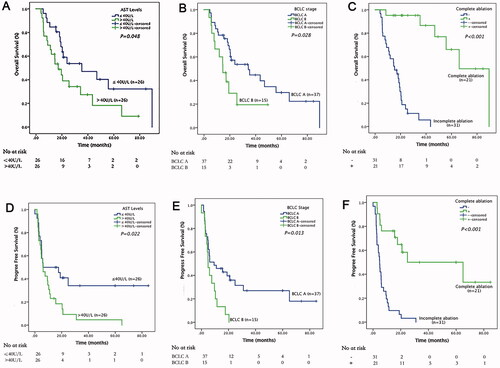
In the MWA group, 21 (40.4%) patients reached tumor complete ablation () and the median of the number of MWA procedures was 3 (25–75% interquartile range two to five times). Patients with complete ablation had significant longer OS and PFS than those with incomplete ablation (OS: 66.0 vs. 15.3 months; PFS 62.0 vs. 4.8 months, both p < 0.001, ). The one-, two-, and three-year cumulative OS rates for patients with and without complete tumor ablation in MWA group were 95.2%, 95.2%, and 86.6% versus 58.1%, 15.0%, and 5.6% (p < 0.001), whereas the 0.5-, 1-, and 2-year cumulative PFS rates were 81.0%, 81.0%, and 58.4% versus 32.2%, 9.7%, and 3.2% (p < 0.001), respectively . Meanwhile, patients with stage A BCLC and AST ≤40 U/L (OS: 34.3 vs. 14.6 months, p = 0.028; PFS: 9.33 vs. 4.90 months, p = 0.013) may also gain longer survival benefits from step-by-step debulking MWA treatment. (BCLC A/B: OS 34.3 vs. 14.6 months, p = 0.028; PFS 9.33 vs. 4.90 months, p = 0.013. AST ≤40/>40U/L: OS 35.3 vs. 17.1 months, p = 0.048; PFS 6.07 vs. 5.93 months, p = 0.022.)
Figure 4. A case of complete tumor ablation selected from our study. The patient with huge unresectable HCC achieved complete ablation after receiving step-by-step debulking MWA therapy post-TACE-refractory. (A) CT scan of the huge unresectable HCC prior to treatment. (B) After three TACE procedures, the tumor remained unchanged with unsatisfied deposition of iodized oil, which was deemed as TACE-refractory. (C,D) Heavy ablation stage: patients underwent three cycles of heavy ablation. (E,F) MRI scans showed elimination of >80% active tumor tissues. (G,H) Residual tumor ablation stage: patients underwent five rounds of targeted MWA according to MRI results. (I-L). MRI scan showed the huge HCC was completely eliminated by our step-by-step debulking MWA. HCC: hepatocellular carcinoma; TACE: transarterial chemoembolization; MWA: microwave ablation; CT: computed tomography; MRI: magnetic resonance imaging.
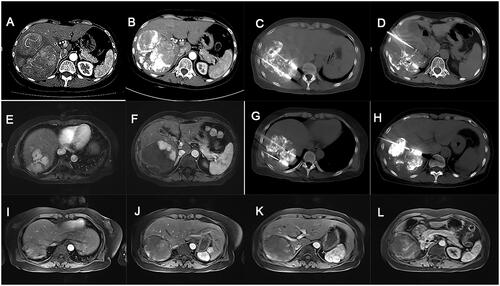
Figure 5. Survival curves for huge unresectable HCC patients who underwent step-by-step debulking MWA after TACE-refractory. Graph shows Kaplan–Meier estimation of overall survival for (A) AST ≤ 40U/L/>40U/L, (B) BCLC A/B stage and (C) complete ablation +/− groups. Graphs show Kaplan–Meier estimation of progression-free survival for (D) AST ≤ 40U/L/>40U/L, (E) BCLC A/B stage, and (F) complete ablation +/− groups. HCC: hepatocellular carcinoma; MWA: microwave ablation; TACE: transarterial chemoembolization; AST: aspartate transaminase; BCLC: the Barcelona Clinic Liver Canc.
Liver function, liver volume after step-by-step MWA
There were no significant differences in the median Child-Pugh scores at one and three months between the two groups. Further analysis showed no significant differences in serum albumin levels and PT level between the two groups at one month and three months since treatment began, whereas the serum bilirubin level was significantly higher in the TACE group than in the MWA group at three months (median: 14.1 vs. 9.9 μmol/L, p = 0.006).
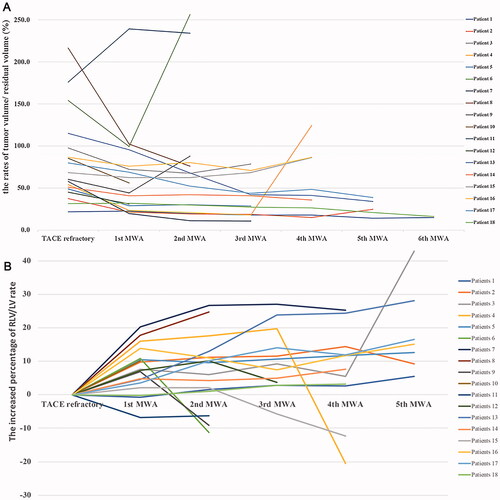
Liver and tumor volume were analyzed in 18 patients who had complete imaging data during follow-up, to assess the changes in residual liver volume after MWA procedures. The changes in residual liver and tumor volume are shown in . Step-by-step MWA did not decrease the residual volume of normal liver, whereas intrahepatic tumor progression correlated with reduced residual liver volume/total liver volume (RLV/TLV) rates. During repeated MWAs, liver hyperplasia appeared after the second MWA procedure, and the average maximum increased RLV/TLV rate was 16.3% ± 12.7%. Patients with complete tumor ablation underwent the most obvious liver hyperplasia with an added RLV/TLV rate of 28.0%, and the average addition of RLV/TLV was 7.7% ± 6.7%, 7.2% ± 10.2%, and 10.1% ± 8.8% after the first, second, and third MWA procedure ().
Figure 6. Changes in residual liver and lesion volume of 18 patients in MWA group. (A) Increased percentage of residual liver volume/liver volume rate during MWA procedure compared with TACE-refractory (%). (B) The changes of tumor volume/residual liver volume during step-by-step debulking MWA duration(%). MWA: microwave ablation; TACE: transarterial chemoembolization.
Safety
Three major complications were encountered in our study, but all of them were fully overcome after symptomatic treatment. Two patients in the MWA group suffered from abdominal Escherichia coli infection and recovered with antibiotic therapy, whereas one patient in TACE group experienced bone marrow suppression. There were no complications such as bleeding, abscess, infarction, and thermal injury. No permanent adverse sequelae or treatment-related deaths were observed. The most common Grade 1 to 2 adverse events were abdominal pain and fever in both groups. Details of the major and minor complication rates are provided in .
Table 4. Complications in patients with the unresectable huge hepatocellular carcinoma in both groups.
Discussion
According to our study results, step-by-step debulking MWA therapy is associated with a significantly higher long-term OS and PFS than continuous, repeated TACE for huge HCC after TACE-refractory.
Sorafenib is recommended for HCC patients with TACE refractoriness after they are considered as TACE-refractory. However, because of its low efficiency and high incidence of adverse reactions [Citation18], it has not been widely used in practice yet in China. Many patients with unresectable huge HCC underwent continuous, repeated TACE treatment despite being refractory to TACE. The reported one-, three-, and five-year OS rates for patients with huge HCC were 33%–68%, 13%–34.7%, and 9.7%–23.1% with repeated TACE therapy [Citation23–26]. Our study has a similar one-year OS rate, but a lower three-year OS rate compared with earlier studies. This is mainly because all patients in our study were refractory to TACE with more advanced tumor and poorer response for additional repeated TACEs. Due to their vascular invasive character [Citation27–29], huge HCCs exhibit a relatively high arteriovenous shunt rate (60%), which obviously has an impact on the deposition of embolic agent. Meanwhile, huge HCCs usually have multiple feeding arteries and complete embolization of all tumor-feeders is difficult; furthermore, TACE has a limited therapeutic effect on huge HCCs. The complete necrosis rate of TACE monotherapy in huge HCC is less than 38% [Citation30] and, as tumor size increases, the incidence of microvascular invasion and intrahepatic metastasis increases [Citation31]. After TACE, tumor cells surviving hypoxic stress and toxicity of chemotherapeutics can change to sarcomatous or mixed phenotypes that are usually TACE-resistant [Citation32]. They also produce vascular endothelial growth factors and promote tumor progression [Citation33]. Moreover, attenuation of the hepatic circulation due to arteritis by TACE could lower the rate of deep penetration of embolic materials into the tumor vessels during the following TACE and exaggerate the blood supply not only from extrahepatic vessels but also from the portal veins to tumors [Citation25,Citation34].
Unlike with other sizes of HCC, it is tough to treat huge unresectable HCC, which needed a long-term treatment with multiple methods and individualized regiments. Our study suggested that step-by-step debulking MWA is an effective local treatment which can obviously improve survival in patients with huge unresectable HCC after refractory to TACE. Moreover, although all patients with huge HCCs enrolled in our study were unresectable, the MWA group had a similar one-year OS rate with surgical resection, as reported by Fang et al. (78.5%) and Wei et al. (78.1%) [Citation26,Citation35]. The advantages of MWA and our tumor ablation strategy may account for this. Compared with other ablative methods, MWA allowed for higher and faster temperature peaks and appeared less sensitive to the heat-sink effect and could produce a larger ablation zone; therefore, MWA can effectively inactivate solid tumors [Citation36]. Compared with the conventional MWA procedure, we used a more efficient method to ablate tumors by performing multi-antenna overlapping ablation. More than 80% of viable tumors were eliminated in the heavy ablation stage (stage 1). The pre-clinical study showed that higher-power and faster heating protocols may potentially mitigate peri-ablational inflammation and distant tumor growth. It is not possible to completely inactivate a tumor with a single MWA procedure due to large tumor volume. Residual lesions should be precisely ablated according to the enhancing MRI/CT imaging. Effectively eliminating lesion and reducing tumor load within a short period of time may help delay tumor metastasis, and the MWA group has an obviously longer LPFS and DPFS than the TACE group (LPFS: 7.6 months vs. 3.5 months; DPFS: 31.0 months vs. 6.9 months, both p < 0.001). The reasons why at least two cycles should be performed in the heavy ablation stage were to ensure the enough elimination of active tumor and to avoid tumor lysis syndrome resulting from extensive ablation during a single procedure. As in several other studies [Citation37,Citation38], patients with complete tumor ablation have a significantly longer OS and PFS than those who underwent incomplete ablation. Furthermore, complete tumor ablation is of vital importance to achieve a curative effect. After step-by-step debulking MWA, 21 out of 52 (40.4%) patients achieved complete ablation. Our study also found that patients with early tumor stage (stage A BCLC) and good liver function (AST ≤ 40U/L) benefit more from MWA after deemed TACE-refractory for huge HCCs.
Most patients with huge HCCs in China have a long history of chronic hepatitis B cirrhosis, which may worsen liver function reserve, leading to a low resection rate [Citation19,Citation35]. Protecting the residual liver function is very important for huge HCCs. Similar to our study, several studies have reported that liver function is an independent prognostic factor for survival of hepatocellular carcinoma [Citation36–38]. Child-Pugh A was a significant independent prognostic factor for PFS in the multivariate analysis of our study, whereas Child-Pugh A and AST < 40U/L were significant independent prognostic factors for OS. Furthermore, as Tadaaki et al. reported, repeated TACE may lead to deterioration of liver function [Citation24]. An increase in Child-Pugh scores was noticed at three months and six months after TACE-refractory, and the repeated TACE group had an obviously lower albumin level and longer PT. Although median Child-Pugh scores at one and three months showed no significant differences between the two groups in our study, the serum bilirubin level was significant higher in the TACE group (median 14.1 μmol/L, 25–75% interquartile range 10.9–31.1 μmol/L) than in the MWA group (median 9.9 μmol/L, 25–75% interquartile range 7.0–14.0 μmol/L p = 0.006) at three months, which implied that the step-by-step MWA procedures did not lead to liver function impairment. In addition, during the measurement of liver volume in 19 patients, it was determined that step-by-step MWA did not lead to the reduction of residual liver volume, whereas intrahepatic tumor progression is the main reason for loss of residual liver volume. Conversely, as tumor size decreased after effective MWA ablations, liver hyperplasia appeared during follow-up, especially in patients achieving complete tumor ablation. As Vanessa et al. found in a swine liver model, thermal ablation could upregulate Ki67-positive cells and HSC-like cells inside the ablation lesion, which most likely reflects tissue repair and liver regeneration [Citation39]. MWA therapy did not significantly increase the risk of complications compared with TACE monotherapy after TACE-refractory. In addition, no permanent adverse sequelae or treatment-related deaths were observed, and the most common complications were fever (48.1% vs. 64.7%, p = 0.089) and pain (46.2% vs. 17.6%, p = 0.002) in both groups.
Although the current study was based on a large sample population, it had several potential limitations. First, this was a retrospective study, which made it vulnerable to several potential biases. Second, the HBV rate in China was higher than the published rates from Western countries and Japan and, therefore external validation from independent study groups is needed. Third, the follow-up treatment modalities were different in some patients, depending on the severity of liver function impairment. Fourth, the observation time for liver function index was not long enough, and liver volume was not determined in all patients. Despite these limitations, step-by-step debulking MWA therapy is worthy of application in patients with huge unresectable HCCs after TACE-refractory. Furthermore, this study was the first-time step-by-step debulking MWA was reported in the literature to date for the treatment of patients with huge HCCs after TACE-refractory.
Conclusion
This study revealed that step-by-step debulking MWA therapy provides significantly better long-term survival for patients with huge unresectable HCCs after TACE-refractory, with no increase in mortality or morbidity, compared with TACE monotherapy.
Disclosure statement
None of the authors have conflict of interest statement
Additional information
Funding
References
- Akinyemiju T, Abera S, Ahmed M, Global Burden of Disease Liver Cancer C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691.
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Zhou L, Rui JA, Wang SB, et al. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol. 2011;37(6):521–525.
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255.
- Lo GH. Updated management of hepatocellular carcinoma. Hepatology. 2011;54(3):1113.
- Hwang S, Lee Y-J, Kim K-H, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015;19(7):1281–1290.
- Shrager B, Jibara GA, Tabrizian P, et al. Resection of large hepatocellular carcinoma (≥ 10 cm): a unique Western perspective. J Surg Oncol. 2013;107(2):111–117.
- Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62(5):1131–1140.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442.
- Xue T, Le F, Chen R, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32(3):64.
- Min YW, Lee JH, Gwak GY, et al. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014;29(5):1043–1048.
- Lencioni R. Chemoembolization for hepatocellular CarcinomaSeminars. Oncology. 2012;39(4):503–509.
- Farinati F, Giacomin A, Vanin V, et al. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol. 2012;57(1):221–222.
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212–220.
- European Association For The Study Of The L, European Organisation For R, Treatment Of C EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Kudo M, Matsui O, Izumi N, Liver Cancer Study Group of Japan, et al. JSH Consensus-Based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3-4):458–468.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.
- Brunocilla PR, Brunello F, Carucci P, et al. Sorafenib in hepatocellular carcinoma: prospective study on adverse events, quality of life, and related feasibility under daily conditions. Med Oncol. 2013;30(1):345.
- Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67(1):173–183.
- Ahmed M, Solbiati L, Brace CL, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Lau WY, Leung TW, Yu SC, et al. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237(2):171–179.
- Ke S, Gao J, Kong J, et al. Repeated radiofrequency ablation combined with ablated lesion elimination and transarterial chemoembolization improves the outcome of solitary huge hepatocellular carcinomas 10 cm or larger. Medicine. 2016;95(16):e3393.
- Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330–341.
- Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262.
- Miyayama S, Kikuchi Y, Yoshida M, et al. Outcomes of conventional transarterial chemoembolization for hepatocellular carcinoma ≥ 10 cm. Hepatol Res. 2019;49(7):787–798.
- Wei CY, Chen PC, Chau GY, et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med. 2020;8(5):238–238.
- Kido C, Sasaki T, Kaneko M. Angiography of primary liver cancer. Am J Roentgenol Radium Ther Nucl Med. 1971;113(1):70–81.
- Sugano S, Miyoshi K, Suzuki T, et al. Intrahepatic arteriovenous shunting due to hepatocellular carcinoma and cirrhosis, and its change by transcatheter arterial embolization. Am J Gastroenterol. 1994;89:184–188.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(6):1330–1335.
- Hidaka T, Anai H, Sakaguchi H, et al. Efficacy of combined bland embolization and chemoembolization for huge (≥ 10 cm) hepatocellular carcinoma. Minim Invasive Ther Allied Technol. 2021;30(4):221–228.
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–1092.
- Zen C, Zen Y, Mitry RR, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17(8):943–954.
- Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529.
- Miyayama S, Matsui O, Zen Y, et al. Portal blood supply to locally progressed hepatocellular carcinoma after transcatheter arterial chemoembolization: Observation on CT during arterial portography. Hepatol Res. 2011;41(9):853–866.
- Fang Q, Xie QS, Chen JM, et al. Long-term outcomes after hepatectomy of huge hepatocellular carcinoma: a single-center experience in China. Hepatobiliary Pancreat Dis Int. 2019;18(6):532–537.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Zhao J, Wu J, He M, et al. Comparison of transcatheter arterial chemoembolization combined with radiofrequency ablation or microwave ablation for the treatment of unresectable hepatocellular carcinoma: a systemic review and meta-analysis. Int J Hyperthermia. 2020;37(1):624–633.
- Zheng L, Li HL, Guo CY, et al. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19(2):237–246.
- Stadlbauer V, Lang-Olip I, Leber B, et al. Immunohistochemical and radiological characterization of wound healing in porcine liver after radiofrequency ablation. Histol Histopathol. 2016;31(1):115–129.