Abstract
Objective
To investigate the tolerance and efficacy of HIFU ablation for uterine fibroids with a non-perfused volume ratio (NPVR) ≥ 90%.
Methods
A prospective cohort study of 2411 patients from 20 clinical centers was available. Contrast-enhanced MRI was used to assess the non-perfused volume ratio (NPVR). The International Society of Interventional Radiotherapy (SIR) complication grading system was used as the tolerance index. Uterine Fibroids-related Symptoms-Quality of Life (UFS-QoL) was used to evaluate the efficacy.
Results
A total of 1352 patients underwent USgHIFU ablation treatment enrolled, NPVR was median 91.9% (IQR, 81.4%,100.0%). There was 761 case (56.3%) in the NPVR ≥ 90% group in which 17.5% case experienced SIR-B abdominal pain, 591 cases (43.7%) in NPVR < 90% group in which 9.3% case had SIR-B abdominal pain. There were statistically differences in the improvement degree of UFS at 12 months among the four subgroups (NPVR < 70%, 70%–80%, 80%–90%, 90%–100%) (all p < 0.05).
Conclusions
Patients with NPVR ≥ 90% had a higher incidence of SIR-B lower abdominal pain. NPVR was positively correlated with the degree of symptom relief at 12 months, and NPVR ≥ 90% was more likely to obtain better clinical symptom relief.
1. Introduction
Uterine fibroids are benign lesions or neoplasms of the uterus. Approximately 50% of women with fibroids experience symptoms which may include menorrhagia that may result in anemia, bulk symptoms with bladder, bowel dysfunction, abdominal protrusion, dysmenorrhea, and infertility [Citation1,Citation2]. There are many therapeutic strategies for uterine fibroids, such as hysterectomy, myomectomy, laparoscopic myomectomy, and uterine artery embolization (UAE) therapy [Citation2,Citation3]. However, hysterectomy and myomectomy approaches are associated with a high rate of significant complications and take weeks to recover [Citation4]. There were also reports of prenatal uterine rupture after laparoscopic myomectomy [Citation5], and major complication rates in uterine artery embolization were estimated to range from 1% to 17% [Citation6].
High intensity focused ultrasound (HIFU) ablation of uterine fibroids was an alternative treatment to preserve the uterus as many studies had confirmed that HIFU ablation was safe and effective, and could improve the quality of life of patients [Citation7]. In recent years, high-intensity focused ultrasound (HIFU) has been used in the noninvasive ablation of uterine fibroids, which was a uterus-preserving therapy that proved to be safe and effective, having a potentially positive impact on patients, and highly accepted by patients in the treatment of uterine fibroids [Citation8–10]. However, HIFU technology is still going through a developing phase and is not popularized in many countries. With the progress of HIFU technology, HIFU strives to improve NPVR on the premise of safety. The non-perfused volume ratio (NPVR) of 80% and 90% have been clinically verified [Citation11,Citation12]. A prospective study from 20 centers reported an NPVR of 87% [Citation13], and NPVR up to 90% was technically feasible. However, it is not only the clinical efficacy but also the patient tolerability that should be paid attention to. Therefore, the data of patients from a prospective study cohort were extracted, and the tolerance of patients with uterine fibroids of NPVR ≥ 90% were investigated, aiming to provide the basis for the popularized application of HIFU technology in outpatient and day ward.
2. Materials and methods
2.1. Patients
From March 2011 to December 2013, the patients with uterine fibroids were enrolled in 20 clinical centers in China for a prospective cohort study of HIFU and surgery (including myomectomy and hysterectomy). Patient data was selected from the Multicenter Research Information System of Therapy of Uterine Fibroids (www.hifuctr.com) (Software copyright certificate number: 2011SR094656). This multicentre research was approved by the Chinese Ethics committee of Registering Clinical Trials (IRB approval number: ChiECRCT-2011034). Each patient signed informed consent before inclusion in this prospective study. Written informed consent was obtained from each patient before every procedure.
Eligibility criteria for patients [Citation13]: (1) Premenopausal women, who have given birth and had no recent plan for further pregnancy. (2) Imaging-confirmed diagnosis of uterine fibroids had any of the following indications for hysterectomy: (a) enlarged uterus (uterine volume equal to or greater than that at 10 weeks gestation); (b) menorrhagia and/or secondary anemia; (c) pelvic pain, urinary frequency, or constipation. (3) For patients with multiple fibroids, no more than three fibroids with minimal diameters of 2 cm based on abdominal ultrasound present. (4) Fibroids are clearly imaged by abdominal ultrasound. (5) For patients with abdominal surgical scars, the width of image blurring due to acoustic attenuation had to be < 10 mm.
Exclusion criteria [Citation13]: (1) Patients with uterine adenomyosis. (2) Previous myomectomy. (3) Concurrent pregnancy. (4) Pedunculated subserous or submucosal fibroids. (5) Any single fibroid >10 cm maximum diameter. (6) Acute pelvic inflammation or uncontrolled systemic disease. (7) Patients were unable to communicate with physicians or were unwilling to sign informed consent. Patients were provided with written information describing the potential risks and benefits, including the potential impact on fertility and the risk of recurrence of symptoms.
2.2. HIFU ablation procedure
A single session of HIFU ablation was performed by using the Focused Ultrasound Tumor Therapeutic System (Model-JC/JC200, Chongqing Haifu Medical Technology Co., Ltd, Chongqing, China). Equipment parameters used in this study: the frequency of the transducer was 0.8 MHz; the physical focal region was 1.5 mm × 1.5 mm × 10 mm; and the therapeutic power was 300–400 W. Standardized clinical program was for physician training and clinical treatment. Patients were placed in the prone position on the HIFU treatment bed with the anterior abdominal wall fully contacting with degassed water. Ultrasound-guided localization of acoustic pathway was employed to avoid intestinal therapy. If the patient had burning pain on the abdominal skin or lumbosacral, hip, lower limb pain, stop sonication and replace the focal area to continue treatment; the fentanyl (1 μg/kg) – midazolam (0.02 mg/kg) scheme was used for sedation and analgesia. Fentanyl and midazolam were added every 30–40 min. The maximum dose of midazolam was 0.15 mg/kg body weight. All patients maintained awake or shallow sleep and their respiration, oxygen saturation, heart rate and blood pressure were monitored during procedure. The treatment power, continuous ultrasonic sonication (emission) time and cooling time were adjusted according to the patient’s tolerance, and the ablation results were evaluated in real time according to the gray increase of the target area displayed by monitoring ultrasound imaging. When the gray enhancement area was covered the treatment area, the ultrasound ablation was completed. Dose control standard: total dose ≤ 750KJ, treatment time ≤ 3 h. After prone position for 2 h, the patients accompanied by family members discharged home or back to the ward to rest.
2.3. MRI evaluation
All patients received pelvic MRI scans with a 3.0 T MRI system before operation (GE Medical system, Milwaukee, WI, USA). The three-dimensional diameters of dominant uterine fibroids and uterus were measured based on T2W images. Enhanced MR scans were performed again within 1 week after the treatment and used to measure the diameters of the non-perfused volume (NPV). The fibroid volume and NPV were calculated by using the following equation: V = 0.5233 × D1 × D2 × D3 (longitudinal (D1), anteroposterior (D2), and transverse (D3) [Citation14]. Non-perfused volume ratio (NPVR) was the ratio of the volume of the non-perfusion area in the post-ablation to the volume of the fibroids. The signal intensity of the uterine fibroids was classified into 3 types according to the pretreatment T2WI: hypointense, isointense or hyperintense [Citation15].
2.4. Patient tolerance and clinical symptom evaluation
Complications after HIFU treatment were recorded and graded by using the guidelines of the Society of Interventional Radiology (SIR) where SIR-A and SIR-B were considered to be minor while the major complication included SIR-C to SIR-F [Citation16]. The lower abdominal pain of SIR-B was taken as the tolerance index. The complication of SIR-A needed no therapy and no consequences; SIR-B needed nominal therapy and no consequence, but only overnight admission for observation [Citation16].
Uterus Fibroids-related Symptoms-Quality of Life (UFS-QoL) was used to assess the changes in symptoms and quality of life for 6 and 12 months after HIFU compared with those before HIFU. UFS-QoL was quantified into two subscales, UFS and QoL, which were converted into 0–100 points. Higher QoL scores represent better outcomes and higher UFS scores represent worse symptoms [Citation17].
2.5. NPVr and grouping
In this study, the different NPVRs were divided into NPVR <90% and NPVR ≥90% two groups. The NPVR <90% group was segmented and divided into three subgroups: <70%, 70%–80% and 80%–90%.
2.6. Statistical analysis
All statistical analyses were performed by using SPSS version 22.0 software (SPSS IBM, Chicago, IL, USA). Normal distribution measurement data were expressed as mean ± standard deviation (SD), and non-normal distribution measurement data were expressed as median (P25, P75). Count data were expressed in frequency and percentage. The comparison of measurement data between two groups used an independent Mann-Whitney U test; the comparison of measurement data between groups used independent Kruskal-Wallis H, count data utilized the Chi-Square test. Logistic regression analysis was further used to analyze statistically significant variables. p < 0.05 indicated that the difference was statistically significant.
3. Results
3.1. Patients
A total of 1352 patients were included in the analysis. The patient's age was 42.0 years (IQR, 38.0 years, 45.0 years), uterine volume was 258.6 cm3(IQR, 186.4 cm3, 360.3 cm3), and the main myoma volume was 61.3 cm3(IQR, 48.2 cm3, 137.5 cm3). UFS baseline was 15.6 (IQR, 9.4, 28.1) and QoL baseline was 75.0 (IQR, 62.9, 85.3).
3.2. HIFU ablation results
The average power of HIFU ablation was 400.0 W (IQR, 400.0 W, 400.0 W); sonication time was 1041.5 s (IQR, 667.0 s, 1548.5s); the total dose was 415.0 kJ (IQR, 265.3 kJ, 621.3 kJ); NPVR was 91.9% (IQR, 81.4%, 100.0%). Most patients had lower abdominal pain after the HIFU procedure, the incidence is 62.3%, of which 654 cases were mild, tolerable, and did not need analgesic treatment, classified as SIR-A; 188 cases needed symptomatic pain relief, classified as SIR-B, in which 181 took non-steroidal anti-inflammatory drugs orally, 7 cases of intramuscularly injected tramadol or dolantin, all having relieved within 24 h. Other adverse reactions included: the incidence of vaginal discharge reached 16.2%, lumbar and back (sacrum) pain 11.2%, vaginal bloody secretions 10.4%, vaginal bleeding 6.7%, lower limb numbness and pain 2.6%, nausea and vomiting 1.6%, anal bulge 0.8%, lower limb weakness 0.7%, urinary retention 0.7%, inguinal pain 0.3%, fever 0.3%, superficial second degree burns 0.2%.
3.3. Patient tolerance evaluation
A total of 761 cases (56.3%) obtained NPVR ≥ 90% of uterine fibroids, as NPVR ≥ 90% group with NPVR was median 100.0% (94.3%,100.0%), and NPVR < 90% group included 591 cases (43.7%) with NPVR was median 79.4% (70.3%,85.2%) (). The incidence of SIR-B lower abdominal pain was 17.5% in the NPVR ≥ 90% group, which was higher than the NPVR < 90% group of 9.3%, and the difference was significant (p = 0.000). The subgroups of NPVR < 70%, 70% −80% and 80%–90%, were all lower than the NPVR ≥ 90% group when compared in pairs, (all p < 0.05) (). The results of binary logistic regression analysis showed that the NPVR < 90% was associated with less SIR-B lower abdominal pain compared with NPVR ≥ 90% (p < 0.05) ().
Figure 1. Enhanced MR images of uterine fibroids with different ablation rates (NPVR) after HIFU treatment (a) For anterior uterine fibroids, no perfusion volume (NPV) was 48.1 cm3, and NPVR was 62.3%. (b) Anterior uterine fibroids, with NPV of 69.1 cm3 and NPVR of 72.0%; (c) Anterior uterine fibroids, with NPV of 90.2 cm3 and NPVR of 88.9%; (d) Anterior uterine fibroids, NPV 69.2 cm3, NPVR 99.6%.
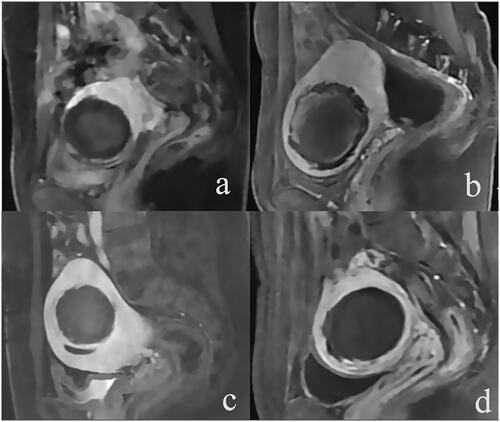
Table 1. Comparison of SIR-B lower abdominal pain in patients with different NPVR groups.
Table 2. Logistics regression analysis of NPVR and SIR-B lower abdominal pain.
3.4. UFS-QoL comparison of patients in different NPVR groups
During the 6 months and 12 months follow-ups, UFS values of patients in different NPVR groups decreased, and QoL values increased. During the 12 – month follow-up, the difference between the UFS value and baseline was statistically significant (p < 0.05) (). NPVR was significantly correlated with UFS at 12 months of improvement (p = 0.005). NPVR ≥ 90% was more likely to obtain stable remission of clinical symptoms ().
Table 3. UFS-QoL comparison of patients in different NPVR groups.
Table 4. Correlation analysis of 12 – month UFS improvement and NPVR.
4. Discussion
NPVR is an effective indicator of short-term efficacy; in the early stage of HIFU technology application, the average NPV reached 36.4%, which could alleviate the clinical symptoms, but the reintervention rate reached 66.7% [Citation18,Citation19]. Regression analysis showed that NPVR reached 60%, and the cumulative clinical recurrence rate of two years after operation was about 10%, which was equivalent to that of myomectomy [Citation20,Citation21]. Two other studies indicated that when NPVR was higher than 70%, the 2-year clinical cumulative recurrence rates after HIFU were lower when compared to myomectomy [Citation22,Citation23]. Thus, the technical requirements for operation training were set to NPVR 70%. As was reported by Park et al. [Citation24] that it was safe when immediate NPVR reached at least 80% in HIFU ablation of uterine fibroids. Recent studies have shown that NPVR ≥ 90% could be used as a standard for HIFU ablation of uterine fibroids [Citation25]. However, obtaining higher NPVR in clinical practice is the consistent pursuit of physicians and patients, it deserves further study whether the continuous improvement of NPVR standards can achieve better clinical outcomes under the premise that the treatment results have reached safety and effectiveness.
This study was the first to explore whether NPVR is necessary to reach 90%–100% and whether patients had good tolerance from two aspects of UFS-QoL and SIR-B lower abdominal pain. Studies showed that UFS was very important in the evaluation of uterine fibroids [Citation13,Citation26]. The lower abdominal pain of SIR-B in the postoperative SIR complication grading system was used as the tolerance index, and UFS-QoL was used to quantify the symptoms of uterine fibroids and the quality of life-related to health. Chen et al. [Citation13] reported that UFS-QoL and adverse reactions were important evaluation indexes for comparing HIFU treatment results with other surgical procedures. Better UFS-QoL improvement and reducing adverse reactions couldn’t only reduce the pain of patients, but also be particularly critical in the development of HIFU. The results showed that when NPVR was 90%–100%, there was no significant difference in the improvement of UFS after 6 months, QoL after 6 months and QoL after 12 months compared with the results of other groups, respectively. (p > 0.05). The UFS improvement at 12 months was positively correlated with NPVR (p = 0.005). Therefore, to obtain a long-term stable clinical effect, better NPVR is necessary.
HIFU treatment is an in vitro noninvasive tumor treatment technology, the principle is irreversible damage to tissue cells caused by the physical factors of ultrasound. Lower abdominal pain is the most common intraoperative response in patients with uterine fibroids treated by HIFU. Most patients still have pain response after surgery, especially in patients with a previous history of lower abdominal surgery [Citation26]. Reducing the incidence and degree of postoperative pain and reducing the use of analgesics were important parts of comfortable care and rapid rehabilitation, which were of great significance to the popularization and application of HIFU technology in the clinic and day ward. The incidence of lower abdominal pain in this group was 62.3%. SIR-A lower abdominal pain didn't need to be treated, and it was often only a mild discomfort symptom of lower abdominal pain similar to menses. SIR-B lower abdominal pain affected the activity and sleep of patients, thus increased care and observation being needed for patients. Most patients could be relieved by oral administration of non-steroidal anti-inflammatory drugs. Special individual patients needed to be given an intramuscular injection of anesthetic analgesics, by which pain could generally be relieved and could disappear in 24 h. In this study, SIR-B lower abdominal pain was analyzed. Compared with the group with 90%–100% NPVR, other groups showed a negative correlation with SIR-B lower abdominal pain (p < 0.05). In the course of clinical treatment, it seemed that the adverse reactions were more obvious when patients got higher NPVR, and more patients' pain will continue until after surgery. While NPVR was improved, it was the closer normal tissue around uterine fibroids that were more prone to thermal injury and causes pain [Citation26]. There were many factors limiting NPVR, including equipment, physicians and patients, and there were different treatment plans according to the purpose of treatment. Further improvement of NPVR after reaching the effective ablation standard needs to assess patient tolerance and clinical benefits, and it is not recommended to blindly improve NPVR. With the progress of technology and the accumulation of physicians' experience, when the NPVR is increased to 90% in patients with tolerance, attention should be paid to the occurrence of SIR-B lower abdominal pain and some preventive measures should be taken. When the ablation range is increased, attention should be paid to controlling the speed of energy delivery, stopping sonication in time, and using cold (4 °C) saline to wash the bladder to accelerate local heat dissipation
The limitations of the study was that the patients were from 20 clinical centers, and each hospital had different characteristics, such as specialized hospitals, general hospitals and teaching hospitals. Patient source and education level might affect the patient's tolerance to pain and the evaluation of pain. Physician experience was also quite different. Whether it has an impact on postoperative pain results still needs further study. The increased NPVR values at each stage and the pathological changes and damage of surrounding tissues are also worthy of further study [Citation18,Citation26].
In conclusion, this paper provides a new idea, which can provide a basis for the setting of HIFU ablation standards and draw more attention to patient tolerance and the relationship between UFS-QoL and NPVR in the treatment. Compared with NPVR < 90% group, NPVR ≥ 90% group was more likely to have SIR-B lower abdominal pain. NPVR was positively correlated with the degree of symptom relief at 12 months, and NPVR ≥ 90% was more likely to obtain more clinical symptom relief. This study provides a basis for physicians to better combine the postoperative adverse reactions of patients and with UFS-QoL to further evaluate the treatment plan. After reaching the basic requirement of 70% ≥ NPVR and the standard of effective ablation of NPVR ≥ 80%, the therapeutic effect of increasing NPVR to 90% is a “double-edged sword,” which is related to more SIR-B lower abdominal pain, but the reduction of UFS in the 12-month group is better than the others. In clinical practice, the reduction of UFS is more important for the treatment results than that of SIR-B lower abdominal pain. However, in the treatment of HIFU, blind pursuit of the improvement of the treatment standard of NPVR is not advisable. It is necessary to formulate standardized and personalized clinical programs based on the actual situation of patients, combined with the comprehensive evaluation of postoperative UFS-QoL and adverse events.
Ethics statement
This multicentre research was approved by the Chinese Ethics Committee of Registering Clinical Trials (IRB approval number: ChiECRCT-2011034).
Disclosure statement
The Author(s) declare(s) that there is no conflict of interest.
Additional information
Funding
References
- Stewart E, Cookson C, Gandolfo R, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512.
- Faustino F, Martinho M, Reis J, et al. Update on medical treatment of uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2017;216:61–68.
- Silberzweig JE, Powell DK, Matsumoto AH, et al. Management of uterine fibroids: a focus on uterine-sparing interventional techniques. Radiology. 2016;280(3):675–692.
- Ciebiera M, Łoziński T, Wojtyła C, et al. Complications in modern hysteroscopic myomectomy. Ginekol Pol. 2018;89(7):398–404.
- Abbas AM, Michael A, Ali SS, et al. Spontaneous prelabour recurrent uterine rupture after laparoscopic myomectomy. J Obstet Gynaecol. 2018;38(7):1033–1034.
- Toor SS, Jaberi A, Macdonald DB, et al. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(5):1153–1163.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19(4):724–732.
- Keserci B, Duc NM, Nadarajan C, et al. Volumetric MRI-guided, high-intensity focused ultrasound ablation of uterine leiomyomas: ASEAN preliminary experience. Diagn Interv Radiol. 2020;26(3):207–215.
- Qin J, Chen JY, Zhao WP, et al. Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet. 2012;117(3):273–277.
- Keserci B, Duc NM. Magnetic resonance imaging parameters in predicting the treatment outcome of high-intensity focused ultrasound ablation of uterine fibroids with an immediate nonperfused volume ratio of at least 90%. Acad Radiol. 2018;25(10):1257–1269.
- Yang MJ, Yu RQ, zhi CW, et al. A prediction of NPVR ≥ 80% of ultrasound-guided high-intensity focused ultrasound ablation for uterine fibroids. Front Surg. 2021;8:663128.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG: Int J Obstet Gy. 2018;125(3):354–364.
- Orsini LF, Salardi S, Pilu G, et al. Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology. 1984;153(1):113–116.
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196(2):184.e1.
- Goodwin SC, Bonilla SC, Sacks D, et al. Reporting standards for uterine artery embolization for the treatment of uterine leiomyomata. J Vasc Interv Radiol. 2003;14(9 Pt 2):S467–S476.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269.
- Cheung VYT. High-intensity focused ultrasound therapy. Best Pract Res Clin Obstet Gynaecol. 2018;46:74–83.
- Verpalen IM, Anneveldt KJ, Nijholt IM, et al. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: a systematic review and meta-analysis. Eur J Radiol. 2019;120:108700.
- Fauconnier A, Chapron C, Babaki-Fard K, et al. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(6):1428–1428.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Park MJ, Kim Y s, Rhim H, et al. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging–guided High-Intensity focused US therapy. J Vasc Interv Radiol. 2014;25(2):231–239.
- Kröncke T, David M. MR-Guided focused ultrasound in fibroid treatment – results of the 4th radiological-gynecological expert meeting. Rofo. 2019;191(7):626–629.
- Yin N, Hu L, Xiao ZB, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34(8):1298–1303.
