Abstract
Introduction
This study aimed to investigate and compare the therapeutic efficacy and safety of ultrasound-guided radiofrequency ablation (RFA), between primary hyperparathyroidism (PHPT) and secondary hyperparathyroidism (SHPT) patients, with or without previous parathyroidectomy (PTX).
Subjects and Methods
A total of 21 patients (7 PHPT, 14 SHPT) underwent RFA for hyperparathyroidism (HPT) at Kaohsiung Chang Gung Memorial Hospital, Taiwan. Five of the 14 SHPT patients had previously received PTX. The laboratory data, volume change of each parathyroid nodule, symptomatic scores, and complications were analyzed and compared between all groups at 1 and 7 days, and at 1, 3, 6, and 12 months after RFA.
Results
After RFA, the volume reduction ratio (VRR) for all patients at the last follow-up was 93.76%, and clinical symptoms significantly improved. At 12 months, all PHPT patients achieved successful treatment of intact PTH (iPTH). In SHPT patients, the mean iPTH value significantly decreased 1-day post-RFA, subsequently exhibiting a transient rebound which proceeded to decrease, with 57.1% reaching successful treatment standards. SHPT patients with PTX showed a lower complication score, shorter ablation time, higher iPTH baseline and outcomes, and lower VRR, compared to patients without PTX. The serum calcium level significantly decreased to normal range in 85.7% of all patients at 12 months. Severe hypocalcemia occurred in 23.8% at 1 week, and all were corrected with calcium supplements.
Conclusions
RFA demonstrates a therapeutic efficacy similar to PTX. It can thus be considered an effective alternative treatment for PHPT, SHPT, or post-PTX patients who are unsuitable for another PTX.
1. Introduction
Hyperparathyroidism (HPT) is a clinical condition in which levels of parathyroid hormone (PTH) in the blood are elevated, and it can be further categorized into primary hyperparathyroidism (PHPT) and secondary hyperparathyroidism (SHPT) due to different mechanisms [Citation1]. PHPT results from one or more of the parathyroid glands secreting excessive PTH, primarily caused by a benign solitary parathyroid adenoma, with a minority of cases caused by multiple adenomas or parathyroid carcinoma [Citation2]. SHPT commonly occurs as a severe complication in end-stage renal disease (ESRD) patients, when excessive PTH secretion is stimulated by long-term low calcium levels and high phosphorus levels [Citation3].
In PHPT patients, surgical parathyroidectomy (PTX) is the standard treatment when symptomatic [Citation1]. As for patients with SHPT, current first-line therapeutic approaches include phosphate intake control and vitamin D supplementation, along with pharmacotherapy including administration of calcimimetic agents [Citation4]. In patients with severe SHPT who have failed to respond to pharmacotherapy, PTX is subsequently recommended [Citation3]. However, some patients may refuse surgery due to mortality and morbidity concerns, cosmetic concerns, or are unsuitable candidates for surgery due to ectopic or thoracic parathyroid glands, or recurrent HPT after PTX. Furthermore, patients who have undergone surgical PTX may encounter recurrent HPT, with a recurrence rate of up to 10-70% in SHPT patients and 5–10% in PHPT patients [Citation5–7] and may be considered unsuitable for repeat surgery due to scarring, adhesion, and a destruction of tissue. In such patients, ultrasound (US)-guided ablation therapies have been suggested as alternative treatments to surgery due to their precision and minimal invasiveness [Citation8–16].
Several percutaneous ablation methods, including ethanol injection [Citation15,Citation17], acetic acid injection [Citation18], laser ablation [Citation19–21], high-intensity focused ultrasound (HIFU) treatment [Citation22,Citation23], microwave ablation (MWA) [Citation8–10,Citation24,Citation25], and radiofrequency ablation (RFA) [Citation11–14,Citation16,Citation26,Citation27] are available to treat patients with HPT. US-guided MWA has been previously reported as a safe and efficient technique in recurrent and persistent SHPT after PTX [Citation28]. Meanwhile, RFA has been reported to effectively reduce the size of hyperplastic parathyroid glands and normalize the biochemical parameters in both PHPT and SHPT patients [Citation11–14,Citation26,Citation27], although the therapeutic effectiveness of RFA in patients with previous PTX remains unknown.
US-guided RFA is considered a relatively new therapy for HPT patients, aside from PTX. This study aimed to investigate and compare the therapeutic efficacy and safety of US-guided RFA between PHPT and SHPT patients, with or without previous surgical PTX.
2. Materials and methods
2.1. Patients
This retrospective study enrolled patients with PHPT and SHPT. From April 2019 to December 2020, a total of 21 patients (7 with PHPT, 14 with SHPT) underwent RFA as treatment for HPT at the Kaohsiung Chang Gung Memorial Hospital Medical Center in Taiwan. Five of the 14 SHPT patients had received PTX previously.
The baseline data of all patients prior to RFA were collected from past medical records, including clinical characteristics, operation history, sonographic parameters of nodules, and laboratory results. All patients gave their informed consent to the treatment prior to each procedure. The protocol of the present retrospective study was reviewed and approved by the Research Ethics Committee of Chang Gung Memorial Hospital (IRB no. 202200239B0) and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. Patient data were deidentified in this retrospective study and signed informed consent was therefore waived.
2.2. Definition of hyperparathyroidism
The diagnostic criteria for PHPT included an intact PTH (iPTH) level above the upper limit of the normal range (18.5-88 pg/mL). The diagnostic criteria for SHPT included an iPTH level >300 pg/mL, according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative [Citation3] in which iPTH levels in end-stage renal disease (ESRD) patients should be 150–300 pg/mL.
2.3. Inclusion and exclusion criteria
The inclusion criteria for performing RFA were as follows: (1) iPTH >88 pg/ml in PHPT; (2) iPTH >300 pg/ml after pharmacotherapy or PTX in SHPT; (3) positive CT results or Single Proton Emission Computerized Tomography scan (SPECT) results; (4) detectable hyperplastic parathyroid glands in US examination; and (5) refusal or ineligibility for surgery.
The exclusion criteria were: (1) parathyroid carcinomas; (2) unapproachable parathyroid nodule due to other thyroid lesions; (3) abnormal laryngoscopy findings of vocal cord movement; (4) abnormal results of coagulation function tests; (5) severe cardiopulmonary dysfunction; and (6) patient refusal of RFA. To exclude parathyroid carcinomas, clinical presentation, laboratory results, suspicious US image features, and pathology reports were taken into consideration for diagnosis [Citation29].
All patients in this study underwent SPECT to detect ectopic parathyroid glands in the mediastinum and to rule out supernumerary glands.
2.4. Pre-ablation assessment
The nodular number, size, and volume were recorded on the day of ablation prior to the RFA procedure. Three orthogonal diameters of the parathyroid glands (the largest diameter and two other perpendicular diameters) were measured by sonography. The volume of the parathyroid glands was calculated using the following equation: V = πabc/6 (V: volume; a: the largest diameter; b and c: the other two perpendicular diameters).
Serum levels of iPTH and calcium were obtained within 24 h prior to the ablation. The HPT-related symptomatic score was recorded by patients through a questionnaire concerning five clinical symptoms: restless legs, arthralgia, ostealgia, calcinosis cutis, and cutaneous pruritus. For each symptom recorded, we allocated one point, therefore the symptomatic scores ranged from 0 to 5.
2.5. Ablation technique
RFA was performed in both inpatient and outpatient settings. The ablation procedure was performed by a radiologist with over 10 years of experience in US-guided procedures. All patients received a single RFA session. Following US examination guidelines, we chose the electrode tip size according to the size of the nodules and nearby critical structures. An internally cooled electrode (18 gauge, with 5 mm or 7 mm active tip) with an RF generator (VIVA, STARmed and M2004, RF Medical) was used.
Prior to ablation, a mixed solution of 2% lidocaine hydrochloride, Sodium Bicarbonate (jusomin) and epinephrine was used as local anesthesia and injected around the puncture site and the hyperplastic parathyroid glands. To prevent thermal injury of nearby structures, Dextrose 5% in water was injected around the hyperplastic parathyroid glands for hydrodissection and to establish an isolating liquid zone between the parathyroid glands and nearby structures.
The trans-isthmic approach was performed, with the electrode passing through the thyroid parenchyma and into the parathyroid gland, while carefully observing the vessels along the route. An electrode was inserted into the deepest and most distant part of the parathyroid nodule. The nodule was ablated with the moving shot technique [Citation30], by sliding the electrode tip back and forth, bottom to top. When all visual fields of the nodule had transformed to transient hyperechoic zones, termination of the procedure was indicated. Patients were referred to the otolaryngology department after ablation for a flexible fiberoptic laryngoscopy evaluation to rule out the possibility of vocal cord paralysis.
2.6. Follow-up evaluation
Complications were assessed immediately after the RFA procedure, recording three main complications: hoarseness, neck pain, and hemorrhage. For each complication recorded, we allocated one point, therefore the complication scores ranged from 0 to 3.
The follow-up serum iPTH and calcium levels were taken at 1 and 7 days, as well as at 1, 3, 6, and 12 months post-RFA. The follow-up sonographic evaluations for volume and volume reduction ratio (VRR) were evaluated at 1, 3, 6, and 12 months post-RFA. The VRR was calculated by the following equation: volume reduction ratio (%) = initial volume (ml) – final volume (ml) × 100/initial volume. The standard terminology of the Society of Interventional Radiology (SIR) was referenced when assessing major and minor complications [Citation31].
Successful treatment was defined as: (1) iPTH <88 pg/mL in PHPT; (2) iPTH <300 pg/mL in SHPT; (3) serum calcium level within 7.9–9.9 mg/dL. Clinically, serum calcium ≤7.5 mg/dL is considered as severe hypocalcemia which requires immediate treatment [Citation32].
2.7. Analyses and statistics
Statistical data analyses were performed using SPSS software (SPSS for Windows 26.0, SPSS, Inc. Chicago, IL, USA). All continuous variables were presented as the mean ± standard deviation (SD). Mann–Whitney U Test, Kruskal–Wallis Test, and Wilcoxon Test were performed for comparison of continuous variables data between groups and subgroups. Standard Chi-Square and Fisher’s exact test were used for comparison of categorical data between groups and subgroups. Statistical significance was defined as point p value < 0.05.
3. Results
The baseline clinical characteristics of the patients are summarized in , and treatment results are shown in and Citation3.
Table 1. Patient characteristics and treatment details.
Table 2. Outcomes based on nodule volume change and clinical results.
3.1. PHPT
3.1.1. Clinical and volume changes
According to the follow-up questionnaire, there was a clear improvement in HPT-related symptoms after the RFA procedure, with the symptomatic score reducing from 2.33 ± 1.56 at baseline to 0.33 ± 0.73 at 1 month. Only 1 (1/7, 14.2%) patient suffered from transient hoarseness after ablation and recovered without sequela within 1 month. No other complications occurred in other PHPT patients.
The nodular volume at baseline and at each follow-up for the 7 hyperplastic parathyroid glands treated are presented in . The VRR at the last follow-up was 95.05% ± 3.89%, while the nodular volume reduced over time ().
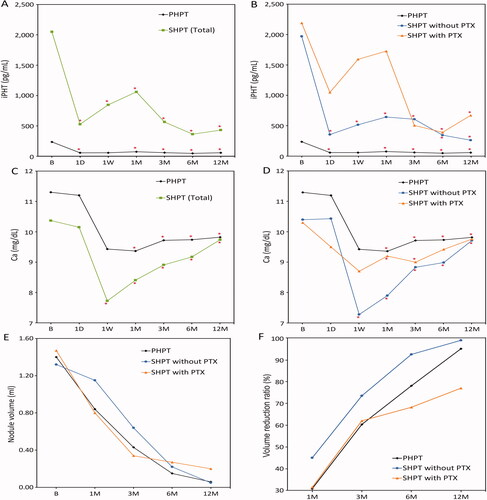
Figure 1. Changes in iPTH, serum calcium levels, nodule volume, and volume reduction ratio (VRR) of the three groups before and after hyperplastic parathyroid gland radiofrequency ablation (RFA) at each follow-up. The iPTH in PHPT and SHPT both showed successful treatment responses compared with baseline (A) even though both SHPT subgroups showed a transient rebound (B). The calcium level in PHPT and SHPT both decreased at follow-up duration (C), but SHPT groups experienced transient hypocalcemia, especially in the SHPT without PTX subgroup (D). Nodule volumes in all three groups significantly decreased over time after RFA (E). The VRR in PHPT and SHPT without PTX were both over 95% and significantly higher than SHPT with PTX (F). *p < 0.05 versus baseline.
3.1.2. Laboratory results
In PHPT patients, the baseline and respective follow-up laboratory results of iPTH and calcium levels are presented in and . The mean value of iPTH before RFA was 233.96 ± 108.92 pg/mL, which markedly decreased to 55.02 ± 53.37 pg/mL within 24 h after RFA (p < 0.05). A slight rebound of iPTH, with a mean value of 72.52 ± 54.11 pg/mL, was noted at 1 month after RFA, without statistical significance (p = 0.655) compared to the value at 1 week. After the transient rebound, the mean level of iPTH proceeded to decrease to 57.57 ± 16.16 pg/mL, 45.15 ± 10.65 pg/mL, and 55.35 ± 19.11 pg/mL respectively at 3, 6, and 12 months after RFA, all showing significant decreases of the iPTH level compared to baseline (p < 0.05). All PHPT patients demonstrated iPTH values within the normal range (<88 pg/mL) at the end of follow-up.
Table 3. Laboratory outcomes.
The mean serum calcium level was 11.3 ± 0.94 mg/dL before RFA, which dropped significantly to 9.43 ± 0.43 mg/dL (p < 0.05) at 1 week and stayed within the normocalcemia range with a mean value of 9.82 ± 0.77 mg/dL (p < 0.05) until 12 months after RFA. No patient presented with severe hypocalcemia. Only 1 (14.2%) patient presented with recurrent hypercalcemia at the 12-month follow-up.
3.2. SHPT
3.2.1. Clinical and volume changes
Among the SHPT group, the follow-up questionnaire revealed HPT-related symptom improvements after RFA. The mean symptomatic score was 2.79 ± 1.63 pre-RFA, which improved significantly to 0.43 ± 0.85 at 1 month. After the RFA procedure, two patients complained of transient hoarseness, while five patients complained of neck pain. All of whom recovered without sequela within 1 month.
A total of 35 hyperplastic parathyroid glands in 14 SHPT patients were treated with RFA, with the nodular volume outcomes presented in . The mean baseline volume of the hyperplastic parathyroid glands was 1.35 ± 1.7 ml, while the mean volume at last follow-up was 0.09 ± 0.09 ml. These results demonstrate that the overall nodular volume reduced significantly after RFA treatment over time (p < 0.05). The VRR of 93.44% ± 10.59% at the last follow-up showed the significant reduction.
Among SHPT patients without previous PTX, 29 nodules were treated in nine patients. The mean volumes at baseline and the last follow-up were 1.32 ± 1.69 ml and 0.05 ± 0.06 (p < 0.05), respectively. The VRR reached 98.93% ± 1.51% at the last follow-up.
Among SHPT patients with previous PTX, six nodules were treated in five patients. The mean volumes at baseline and the last follow-up were 1.47 ± 1.90 ml and 0.20 ± 0.08 ml, respectively. The VRR was 76.95% ± 7.00% at the last follow-up.
3.2.2. Laboratory results
In SHPT patients, the baseline and respective follow-up laboratory results of iPTH and calcium levels are presented in and . In all SHPT patients, the baseline iPTH (2050.04 ± 1189.05) markedly decreased at 1 day (526.10 ± 580.45 pg/mL, p < 0.05) after RFA. A rebound in the iPTH level was noted at 1 week (845.80 ± 1314.60 pg/mL) and peaked at 1 month (1059.08 ± 1345.31 pg/ml). After the transient rebound, the iPTH level proceeded to decrease to 562.65 ± 429.05 pg/mL, 361.63 ± 313.30 pg/mL, and 429.48 ± 499.70 pg/mL at 3, 6, and 12 months after RFA, respectively. Of all SHPT patients, 8/14 patients (57.1%) had iPTH values less than 300 pg/mL, which is within the standard for successful treatment, according to a previous report.
In SHPT patients without previous PTX, the baseline mean iPTH value was 1971.4 ± 812.24 pg/mL, which markedly decreased 1 day after RFA to 352.14 ± 170.31 pg/mL (p < 0.05). A rebound of the iPTH level at 1 week was noted, with a mean value of 513.41 ± 325.88 pg/mL, which proceeded to rise to 641.31 ± 331.44 pg/ml at 1 month after RFA. The level then decreased to 605.3 ± 417.9 pg/mL, 342.16 ± 187.99 pg/mL, and 258.76 ± 169.34 pg/mL at 3, 6, and 12 months after RFA, respectively. In SHPT patients without previous PTX, 3/5 patients (60.0%) had iPTH values less than 300 pg/mL.
In SHPT patients with previous PTX, the baseline mean value of iPTH was 2191.58 ± 1799.05 pg/mL, which decreased to 1047.97 ± 1091.94 pg/mL 1 day after RFA (p < 0.05). A rebound of the iPTH level was noted, with a mean value of 1593.68 ± 2356.32 pg/mL at 1 week, and increased to 1727.52 ± 2080.81 pg/ml at 1 month after RFA. It then proceeded to decrease to 502.94 ± 486.45 pg/mL, 388.9 ± 464.04 pg/mL, and 668.5 ± 721.97 pg/mL at 3, 6, and 12 months after RFA, respectively. In SHPT patients with previous PTX, 5/9 patients (55.6%) had iPTH values less than 300 pg/mL.
The mean serum calcium level of all SHPT patients was 10.36 mg/dL before RFA, which decreased to the lowest level of 7.71 mg/dL at 1 week after RFA. Severe hypocalcemia (<7.5 mg/dL) occurred in 5 (23.8%) patients (4 without PTX; 1 with PTX), all of whom were corrected with calcium supplements. The mean calcium level gradually increased to 9.66 mg/dL over the 12-month follow-up. However, a total of 4 (28.5%) SHPT patients (2 without PTX, 2 with PTX) presented with recurrent hypercalcemia at the 12-month follow-up.
3.3. Group comparison
3.3.1. PHPT vs. total SHPT
The nodular volume and VRR showed no significant differences between the PHPT and all SHPT patients (). There was no significant difference in the complications scores between the groups ().
The mean iPTH level at all follow-up times between PHPT and all SHPT patients () showed a significant difference (p < 0.01), except at 1-week post-RFA (p = 0.069), with SHPT patients having a significantly higher iPTH level at all times. The calcium level between PHPT and all SHPT patients showed a significant difference (p < 0.05) pre-RFA, at 1 day, and 1-week post-RFA, with PHPT patients showing a significantly higher calcium level. There was no significant difference in calcium level from the first month after RFA.
3.3.2. SHPT with vs. without PTX
The nodular size showed no significant difference between the subgroups of SHPT patients with and without PTX. However, the VRR between these two subgroups showed a statistical difference (p = 0.036). The mean iPTH and calcium levels at all follow-up times also showed no significant difference between the two subgroups.
4. Discussion
To the best of our knowledge, the current study is the first to evaluate and compare the clinical efficacy and safety of RFA for three different scenarios of benign parathyroid lesions (PHPT, SHPT with PTX, and SHPT without PTX). The results of the current study reveal a significant efficacy among both PHPT and SHPT patients, with or without previous PTX. The mean iPTH level and calcium level in all three groups primarily exhibited a significant decrease at the 12-month follow-up compared to those of baseline. Although a significant transient rebound in the iPTH level was noted after RFA in SHPT patients, all patient levels subsequently decreased, with a majority of patients reaching a successful treatment range at the 12-month follow-up. While previous studies have reported results regarding the efficacy of RFA in HPT, our findings demonstrate that RFA can significantly reduce iPTH and calcium levels in patients with PHPT and SHPT, with or without PTX.
For patients with PHPT, our study reveals a significant nodule volume reduction, and successful biochemical remission to normal was reported in all (100%) patients (). Previous studies have reported on limited numbers of patients, with successful iPTH remission rates reaching 73%, at best, in PHPT patients [Citation13,Citation27,Citation33]. For patients with SHPT, our results show the successful treatment of iPTH in 57% of patients. Past studies have reported variable outcomes, with a therapeutic success rate of 37.5% after 6 months [Citation33], and 44.1% at 1 year [Citation27]. Meanwhile, studies related to open surgery have reported 76–95% success rates for persistent and recurrent SHPT [Citation34,Citation35], and 97–98% in PHPT [Citation36]. However, RFA has a shorter procedure time, fewer complications, and a wider range of indications as compared to surgery. Therefore, RFA may be considered a viable alternative treatment option to achieve reliable outcomes for HPT.
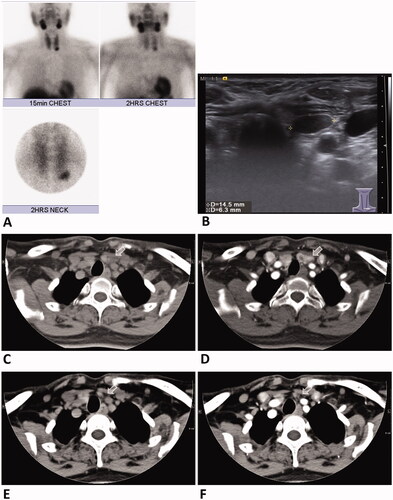
Figure 2. A 54-year-old female with primary hyperparathyroidism. Technetium (99mTC)-sestamibi scan (A) and US examination (B) revealed a hyperfunctional left lower parathyroid adenoma measuring 14.5 mm × 6.3 mm. CT scan of the parathyroid adenoma (arrow) without (C) and with contrast enhancement before RFA (D). CT scan without (E) and with contrast enhancement 1 year after RFA (F) showed total regression (arrow) of the left inferior parathyroid adenoma. Serum PTH and calcium levels were decreased and remained in the normal range at the 1-year follow-up. RFA: radiofrequency ablation; PTH: parathyroid hormone; US: ultrasound.
The current study shows a significant difference between PHPT and SHPT patients in terms of iPTH levels and calcium levels. More specifically, PHPT patients presented with a lower baseline iPTH level due to the nature of the single hyperplastic parathyroid nodule, while SHPT patients presented with a higher baseline iPTH level due to long-term hormone autoregulation. In PHPT patients, by removing the hyperexcretion source of iPTH, the iPTH level can be reduced to within a normal range. However, it may be more difficult to regulate the iPTH level in SHPT patients through removal of the hyperplastic parathyroid nodules. This may be due to the residual influence of long-term low calcium levels and high phosphorus levels in SHPT patients. As such, PHPT patients exhibited a 100% remission rate, as opposed to the 57% rate noted in SHPT patients. Previous studies have presented similar results, with higher remission rates noted in PHPT patients as compared to SHPT patients [Citation13,Citation33].
For patients with SHPT who had previously received PTX, limited studies have been reported [Citation13]. Sormaz et al. reported on a single patient having been treated with RFA, with an iPTH level within the normal range at 6 months. In our study, successful iPTH remission was reported in 60% of patients (3/5), with 1 patient within the recommended level and continuing decrease, and one patient with persistent SHPT. Of note, this is the first study to evaluate the long-term efficacy and safety of RFA treatment in multiple SHPT patients with previous PTX.
Regarding the iPTH level, a higher iPTH level was noted in SHPT patients with previous PTX than those without, although no significant difference was noted throughout all follow-up times. This may be due to the longer onset of SHPT in the patients who had already undergone PTX and encountered recurrent SHPT, or due to a more resilient nature of the SHPT in these patients, potentially resulting from ectopic or unfound parathyroid gland tissues during the PTX procedure contributing to the difference. This may also result from the anatomical change or tissue adhesion after PTX, making the hyperplastic parathyroid nodules harder to approach. Indeed, we found the RFA procedure in such patients more challenging, specifically in terms of identifying the surrounding structures under sonography, stiffness, and adhesion of the adjacent tissues, which may contribute to more untreated or undertreated parathyroid tissues, resulting in the statistical difference of VRR between the SHPT subgroups.
In SHPT patients with previous PTX, RFA presented multiple treatment advantages. Firstly, even in patients with hyperplastic parathyroid nodules situated in dangerous locations (adjacent to the trachea, vessels, or nerves) or surgically inoperable locations (intrathyroidal ectopy), RFA provides a safe and accessible approach to treatment (). Secondly, due to fewer nodules in SHPT patients after the PTX procedure had already removed a certain number of parathyroid glands, the total procedure time is significantly shorter than that for SHPT patients without PTX. Moreover, patients with PTX in this study had a lower complication score than those without PTX. With more hyperplastic parathyroid nodules, the risk of complication is elevated due to the repeated steps required during the RFA procedure, treating multiple nodules in different locations. This could also explain the lower complication score reported in the PHPT group, in which all patients presented with only a single hyperplastic parathyroid nodule.
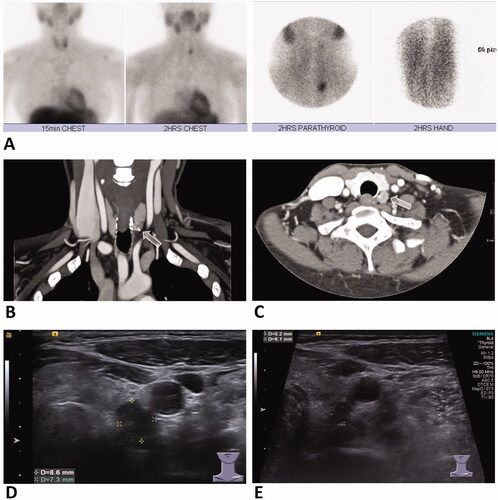
Figure 3. A 41-year-old female with secondary hyperparathyroidism status post parathyroidectomy. Technetium (99mTC)-sestamibi scan (A) revealed a left inferior hyperparathyroid nodule, with high signal also shown on forearm due to parathyroid tissue autotransplantation postparathyroidectomy. Contrast-enhanced CT scan of coronal (B) and axial view (C) revealed a hyperplastic parathyroid nodule in the left inferior neck (arrow). US examination (D) revealed a left inferior parathyroid hyperplastic nodule measuring 8.6 mm × 7.3 mm. US examination follow-up at 4 months after RFA (E) showed significant decrease in size of the nodule measuring 6.1 mm × 5.2 mm. Serum PTH and calcium levels were decreased at 3-month follow-up after treatment. RFA: radiofrequency ablation; PTH: parathyroid hormone; US: ultrasound.
Regarding the transient rebound of iPTH levels after RFA in either PHPT or SHPT, a similar phenomenon has been reported in PHPT patients who underwent PTX [Citation37]. As reported by Tisell et al., we herein found that after RFA of the parathyroid glands, the iPTH level would immediately drop, while the serum calcium level remained elevated. Subsequently, the calcium level would begin decreasing, stimulating the serum PTH concentration to rebound. Hypocalcemia may be expected at approximately 7 to 10 days post-RFA but should reach normocalcaemia at 1 month after treatment. Additionally, Abugassa et al. suggested that a decrease in the serum calcium concentration due to increased calcium deposition in bones is the primary reason for the transient postoperative rebound in PTH secretion [Citation38].
Our study results show a continuous decrease of the iPTH level, after the transient rebound, at 6 to 12 months after the RFA procedure. This may be due to the different mechanisms of cell destruction which occur during and after RFA. During RFA, the tissues of the hyperplastic parathyroid nodules should encounter extreme hyperthermia, resulting in rapid water vaporization, desiccation, carbonization, and ultimately leading to cell death [Citation39]. Cellular injury, including protein denaturation, coagulative necrosis, and cell membrane collapse also occurs as the result of direct damage. Additionally, indirect delayed cellular injury, including surrounding vascular damage which causes ischemia, lysosomal content released during tumor necrosis, and further immune activation all contribute to the future necrosis and apoptosis of the parathyroid cells [Citation40].
This study has several limitations. First, this is a retrospective study, and uncontrolled bias may thus be introduced. Second, this study enrolled a relatively small number of patients. Third, the 12-month follow-up duration was relatively short, and further investigation is required to more accurately determine long-term efficacy. Fourth, we could not control the pharmacotherapeutic treatments following RFA in the clinical scenario. However, RFA may also be seen as a potential downstaging therapy, making previously uncontrollable persistent SHPT patients easier to manage with pharmacotherapy. Fifth, there is currently no reliable tool to evaluate the viable tissues remaining in the hyperplastic nodules after treatment. There is also the possibility of needle tract seeding due to the strong regenerative power of the parathyroid tissues which we are not able to identify or record.
We herein demonstrate that RFA is a safe and effective therapeutic alternative for patients with PHPT and SHPT, regardless of a previous PTX. Although, the treatment efficacy may indeed vary according to differing nodule characteristics presenting in PHPT and SHPT patients, and in those patients having undergone a PTX procedure. We further hope that this study encourages future investigations to include patients with previous PTX, providing such patients a chance to improve their quality of life.
Acknowledgements
The authors wish to thank all the subjects who participated in this study and the staff of Thyroid Head and Neck Ablation Center of Kaohsiung Chang Gung Memorial Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Fraser WD. Hyperparathyroidism. Lancet. 2009;374(9684):145–158.
- Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993–4004.
- Kidney Disease: Improving Global Outcomes, C.K.D.M.B.D.U.W.G. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney Disease-Mineral and bone disorder (CKD-MBD). 2017. Kidney Int Suppl. 2011;7(1):1–59.
- Cozzolino M, Galassi A, Conte F, et al. Treatment of secondary hyperparathyroidism: the clinical utility of etelcalcetide. Ther Clin Risk Manag. 2017;13:679–689.
- Henry JF. Reoperation for primary hyperparathyroidism: tips and tricks. Langenbecks Arch Surg. 2010;395(2):103–109.
- Stracke S, Keller F, Steinbach G, et al. Long-term outcome after total parathyroidectomy for the management of secondary hyperparathyroidism. Nephron Clin Pract. 2009;111(2):c102–9.
- Zitt E, Rix M, Ureña Torres P, et al. Effectiveness of cinacalcet in patients with recurrent/persistent secondary hyperparathyroidism following parathyroidectomy: results of the ECHO study. Nephrol Dial Transplant. 2011;26(6):1956–1961.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Liu C, Wu B, Huang P, et al. US-Guided percutaneous microwave ablation for primary hyperparathyroidism with parathyroid nodules: Feasibility and safety study. J Vasc Interv Radiol. 2016;27(6):867–875.
- Fan B-Q, He X-W, Chen H-H, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Xu S-y, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54(7):e137-40–e140.
- Kim BS, Eom TI, Kang KH, et al. Radiofrequency ablation of parathyroid adenoma in primary hyperparathyroidism. J Med Ultrason (2001). 2014;41(2):239–243.
- Sormaz IC, Poyanlı A, Açar S, et al. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Korkusuz H, Wolf T, Grunwald F. Feasibility of bipolar radiofrequency ablation in patients with parathyroid adenoma: a first evaluation. Int J Hyperthermia. 2018;34(5):639–643.
- Sung JY, Baek JH, Kim KS, et al. Symptomatic nonfunctioning parathyroid cysts: role of simple aspiration and ethanol ablation. Eur J Radiol. 2013;82(2):316–320.
- Lin W-C, Kan N-N, Chen H-L, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Figari M, Musso CG, Plantalech L, et al. Local ethanol injection for the treatment of deltoid parathyroid cell hyperplasia. Int Urol Nephrol. 2014;46(1):247–249.
- Onoda N, Kurihara S, Sakurai Y, et al. A case of secondary hyperparathyroidism whose high turnover bone improved after the direct injection of acetic acid into the parathyroid glands. Clin Nephrol. 2004;61(1):68–73.
- Jiang T, Chen F, Zhou X, et al. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J endocrinol. 2015. 2015;2015:673604.
- Bennedbaek FN, Karstrup S, Hegedus L. Ultrasound guided laser ablation of a parathyroid adenoma. Br J Radiol. 2001;74(886):905–907.
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol. 2012;199(5):1164–1168.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. High-intensity focussed ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(1):76–80.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Zhuo L, Zhang L, Peng LL, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperthermia. 2019;36(1):29–35.
- Diao Z, Wang L, Li D, et al. Efficacy of microwave ablation for severe secondary hyperparathyroidism in subjects undergoing hemodialysis. Ren Fail. 2017;39(1):140–145.
- Zeng Z, Peng CZ, Liu JB, et al. Efficacy of ultrasound-guided radiofrequency ablation of parathyroid hyperplasia: single session vs. two-session for effect on hypocalcemia. Sci Rep. 2020;10(1):6206.
- Peng C, Zhang Z, Liu J, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation of hyperplastic parathyroid gland for secondary hyperparathyroidism associated with chronic kidney disease. Head Neck. 2017;39(3):564–571.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32(2):180–186.
- Bae JH, Choi HJ, Lee Y, et al. Preoperative predictive factors for parathyroid carcinoma in patients with primary hyperparathyroidism. J Korean Med Sci. 2012;27(8):890–895.
- Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15(6):836–843.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Goldfarb M, Gondek SS, Lim SM, et al. Postoperative hungry bone syndrome in patients with secondary hyperparathyroidism of renal origin. World J Surg. 2012;36(6):1314–1319.
- Ha EJ, Baek JH, Baek SM. Minimally invasive treatment for benign parathyroid lesions: treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1383–1392.
- Karakas E, Müller H-H, Schlosshauer T, et al. Reoperations for primary hyperparathyroidism–improvement of outcome over two decades. Langenbecks Arch Surg. 2013;398(1):99–106.
- Nawrot I, Chudziński W, Ciąćka T, et al. Reoperations for persistent or recurrent primary hyperparathyroidism: results of a retrospective cohort study at a tertiary referral center. Med Sci Monit. 2014;20:1604–1612.
- Singh Ospina NM, Rodriguez-Gutierrez R, Maraka S, et al. Outcomes of parathyroidectomy in patients with primary hyperparathyroidism: a systematic review and meta-analysis. World J Surg. 2016;40(10):2359–2377.
- Tisell L-E, Jansson S, Nilsson B, et al. Transient rise in intact parathyroid hormone concentration after surgery for primary hyperparathyroidism. Br J Surg. 2005;83(5):665–669.
- Abugassa S, Nordenström J, Eriksson S, et al. Skeletal remineralization after surgery for primary and secondary hyperparathyroidism. Surgery. 1990;107(2):128–133.
- Brace C. Thermal tumor ablation in clinical use. IEEE Pulse. 2011;2(5):28–38.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
