Abstract
Aim: Pleural dissemination of pseudomyxoma peritonei (PMP) is an extremely rare diagnosis, for which no standard therapy is available.
Methods: We describe the successful treatment of a 67-year-old male diagnosed with left-sided intrapleural dissemination of PMP (low-grade appendiceal mucinous neoplasm), 2 years after treatment of abdominal PMP with cytoreductive surgery (CRS) and hyperthermic intra-peritoneal chemotherapy. Treatment consisted of extended pleural decortication (ePD) and oxaliplatin-based hyperthermic intrathoracic chemotherapy (HITHOC). The patient is doing well without complications or signs of recurrence, 26 months after thoracic surgery.
Conclusion: ePD in combination with HITHOC is a valuable treatment for thoracic PMP.
Introduction
Pseudomyxoma peritonei (PMP) is a rare disease, with an incidence of 0.3%, caused by dissemination of mucinous tumor cells in the peritoneal cavity, originating from a ruptured appendix [Citation1]. Abundance of these mucinous tumor cells cause a gelatinous mass which can result in organ compression and eventually death. Pleural dissemination of these mucinous tumor cells is extremely rare and is mostly diagnosed during the follow-up of a previously diagnosed peritoneal PMP [Citation2]. No standardized treatment for pleural PMP has been defined. Similar to peritoneal PMP, treatment is based on cytoreductive surgery (CRS) and hyperthermic intrathoracic chemotherapy (HITHOC) [Citation2–4]. We describe a case of delayed pleural PMP successfully treated with extended pleural decortication (ePD) and HITHOC.
Case description
A 67-year-old male was diagnosed with peritoneal PMP originating from a low-grade appendiceal mucinous neoplasm. CRS in February 2017 consisted of a total peritonectomy, splenectomy, omentectomy, cholecystectomy, an ileocaecal resection and a glissonectomy of the liver. The peritoneal lining was stripped from both sides of the diaphragms without macroscopic evidence for perforations. CRS was followed by intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC). The peritoneal cavity was perfused with 3800 cc glucose 5% at 41 °C for 30 min with addition of 900 mg oxaliplatin (460 mg/m2) and simultaneous intravenous treatment with 5-fluorouracil (5-FU) and leucovorin.
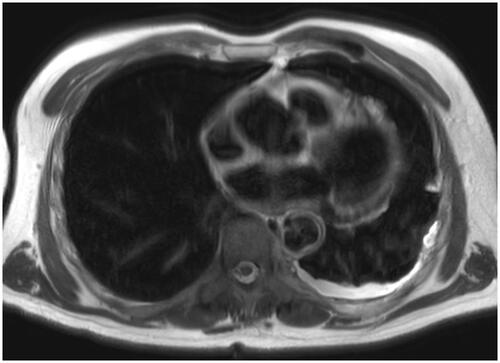
Postoperative recovery was uneventful. During follow-up, carcinogenic embryonic antigen (CEA) levels dropped to 1.8 μg/L. The patient remained disease free for 33 months, after which CEA levels rose to 5.9 μg/L. Magnetic resonance imaging (MRI) showed no abdominal recurrence but revealed diffuse T2-weighted hyperintense foci in the left pleural cavity, extending to the left fissure and pericardium ().
Figure 1. Axial MRI scan showing diffuse T2-weighted hyperintense tissue in the left pleural cavity (white intrapleural rim), extending to the left fissure and pericardium, being highly suspicious for pleural extension of previous peritoneal PMP.
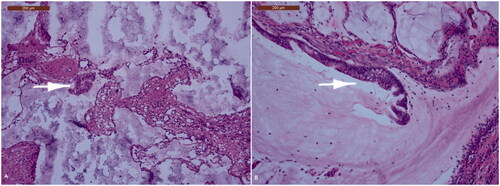
A uniportal left thoracoscopy visualized infiltration of the diaphragm with diffuse dissemination on the left parietal and visceral pleura mainly affecting the posterior basal segment and oblique fissure. Pleural biopsy and talc pleurodesis were performed. Histopathological investigation confirmed pleural dissemination of the previous PMP by microscopic identification of fibrous tissue with acellular mucinous pools, and zones of mucinous epithelium with nuclear atypia (). Tumor spread to other regions was excluded by whole body MRI.
Figure 2. The pleura is transformed into a fibrous tissue containing irregular mucinous pools. These may contain slightly atypical cilindrical epithelium (white arrow), either free-floating in the mucin (A) or still attached to the fibrous wall of the pool (B).
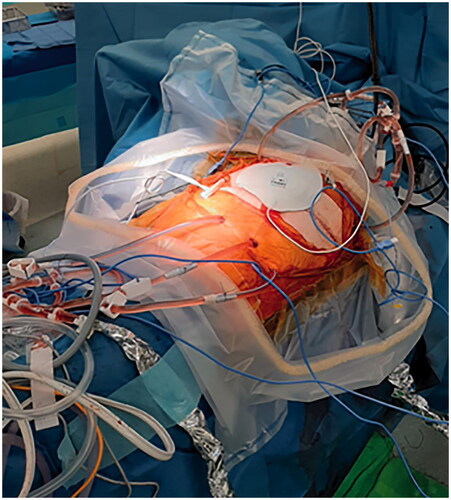
After a reassuring pre-operative work-up (FEV1 = 81%, FVC = 92%, VO2 max = 1507 mL/min), treatment by ePD with hilar lymphadenectomy and HITHOC was performed. A standard posterolateral thoracotomy was performed followed by complete parietal pleurectomy. Visceral pleurectomy was performed by careful manual dissection with the aid of scissors. No major bleeding was observed intra-operatively, apart from a classic oozing from the thoracic wall following the extended pleurectomy. In order to obtain a complete macroscopic resection, CRS was extended by the removal of the left hemidiaphragm revealing an extensive tumor load with diaphragmatic muscle invasion. The left hemidiaphragm was reconstructed with a 2 mm thick Gore-Tex® mesh. The pericardium and right pleura were left intact. Exploration of the upper abdomen did not reveal peritoneal recurrence of PMP. In accordance with the previously applied HIPEC-protocol, 1 h prior to the start of the HITHOC, intra-venous chemotherapy was administered, containing 734,6 mg (400 mg/m2) of 5-FU and 37 mg (20 mg/m2) of leucovorin. After hemostasis, 2 inflow (Ch24) and 3 outflow cannulas (Ch28) were positioned as well as 2 temperature probes (). The chest was temporarily closed except for a 3 cm incision to allow thoracoscopic evaluation of the pleural cavity. The target temperature, within the closed chest, for the HITHOC procedure was reached within 5 min. The left pleural cavity was perfused for 30 min at 41 °C with 4700 cc of Dianeal and oxaliplatin (460 mg/m2). Oxaliplatin was selected as chemotherapeutic agent, based on the successful results of the previous HIPEC procedure. The same dose of the previous HIPEC procedure was used during the HITHOC procedure consisting of 460 mg/m2. During HITHOC the lung was not ventilated in order to minimize changes in circulating volume. The patient remained hemodynamically stable and no major serum electrolyte imbalances or glucose changes occurred. No additional preemptive measures for nephroprotection were taken apart from maintaining a standard positive fluid balance as normally performed during ePD for mesothelioma.
Figure 3. Intra-operative image of HITHOC using 2 inflow (Ch24) and 3 outflow cannulas (Ch28). The left pleural cavity was perfused for 30 min at 41 °C using 4700 cc of Dianeal and oxaliplatin (460 mg/m2).
Most hemostasis was performed prior to the intrathoracic perfusion, but after HITHOC treatment, additional hemostasis of the thoracic wall as well as rigorous aerostasis of the left lung was performed. The latter is a technique that we recently developed in which the larger defects in the lung parenchyma are sutured, while the inflated lung is covered with Neoveil and Tisseel. This technique is used in order to prevent prolonged air leak following pleural decortication. Three chest drains were left behind at a negative pressure of −15 cmH20. Post-operative chest X-ray confirmed adequate expansion of the left lung and a correct position of the reconstructed left hemidiaphragm. Postoperatively, the patient was transferred to the intensive care unit (ICU) and remained intubated for 6 h. Antibiotic prophylaxis (cefazolin, metronidazole and levofloxacin) was continued for 5 d, based on our institutional mesothelioma protocol.
On postoperative day (POD) 4, the patient developed atrial fibrillation which was successfully treated by pharmacological reconversion using amiodarone, one day later the patient was discharged from the ICU. The last chest drain was removed on POD 10. Hospital-acquired pneumonia was suspected on POD11 based on consolidation of the left lower lobe visualized using chest computed tomography (CT), leukocytosis (19.600/microL, normal range: 4000–10.000/microL) and an elevated CRP (148 mg/L, normal range: 0–5 mg/L). This was successfully treated using piperacillin-tazobactam. On POD 20 the patient was discharged from the hospital. Further recovery was uneventful. Final pathology confirmed intrapleural pseudomyxoma and 12 negative lymph nodes. Last follow-up was 26 months after ePD and HITHOC. The patient was symptom-free, thoraco-abdominal MRI did not show recurrence of pleural or peritoneal PMP and CEA levels dropped to 1.0 μg/L.
Approval for study reporting was granted by the Institutional Ethics Review Board (University Hospitals Leuven). The patient signed an informed consent for the report of his case.
Discussion
We reported a rare case of pleural PMP, successfully treated with ePD and HITHOC, presenting 33 months after abdominal CRS and HIPEC for peritoneal PMP.
A pleural presentation of PMP can present synchronously with peritoneal PMP or can present in a delayed fashion, months or even years after abdominal treatment [Citation2, Citation3, Citation5]. The intrathoracic dissemination can be explained by several mechanisms. Iatrogenic dissemination can occur after diaphragmatic perforation during peritoneal stripping of the diaphragm. Other suggested mechanisms of dissemination are congenital pleuroperitoneal communications, direct diaphragmatic invasion or lymphovascular invasion. In the minority of cases, the pleural extensions are considered idiopathic [Citation2, Citation3, Citation5–8].
The rare incidence of this disease is illustrated in a large retrospective study in which only 6 out of 827 patients with peritoneal PMP presented with delayed pleural PMP [Citation2]. Time to recurrence ranged from 12.1 to 135 months after initial surgical intervention. The reported overall 5-year survival was 80% with no pleural recurrence after successful CRS.
In a smaller series [Citation4], 5 cases of pleural PMP were described. A median time to pleural dissemination of 28 months (12–66 months) was reported. All included patients were treated with thoracic CRS and HITHOC. All patients had a history of a diaphragmatic peritoneal stripping that resulted in a diaphragmatic perforation with a need for reconstruction in two patients. One pleural recurrence 10 months after thoracic CRS and HITHOC was reported. The other patients were alive 4.6–47.4 months after treatment without evidence for recurrence.
In our case, we opted for ePD instead of the more radical extrapleural pneumonectomy (EPP). Our choice for ePD was mainly based upon two large meta-analyses [Citation9, Citation10] that compared outcome and adverse events in patients who underwent ePD vs. EPP for malignant pleural mesothelioma. EPP leads to a significantly higher morbidity and short-term mortality compared to patients treated by ePD. Those results were confirmed by a large retrospective trial [Citation11] reporting a longer survival and lower early mortality rates in patients following ePD compared to EPP.
HITHOC following CRS has already been described in the treatment of multiple malignant intrathoracic diseases like malignant mesothelioma, thymoma with pleural dissemination (PROSPERO database; CRD42020208242) or abdominal malignancies with dissemination to the thoracic cavity. Overall, most reports describe HITHOC as a feasible and safe option with low morbidity rates.
A recent systematic review on the use of HITHOC for malignant pleural mesothelioma reported seven high-quality studies comparing CRS with or without HITHOC. It was concluded that overall survival was higher for patients receiving additional HITHOC (11–75 months) compared to patients receiving CRS alone (5–36 months) [Citation12]. Future guidelines on multimodality treatment of MPM should therefore consider to discuss and/or include HITHOC as a type of ‘adjuvant’ treatment after debulking surgery [Citation13, Citation14].
In parallel, the addition of HIPEC in the treatment of abdominal PMP resulted in 5- and 10-year survival rates of over 75% in low-grade disease, compared to 5% in patients undergoing CRS alone [Citation2, Citation15–17]. Therefore, current reports on HITHOC seem promising in terms of oncological effectiveness [Citation12,Citation13,Citation18–20], and we decided that HITHOC could have a similar added benefit in the thoracic cavity when combined with CRS in this unique case of pseudomyxoma.
Different HITHOC/HIPEC protocols for pleural PMP have been proposed by various authors. Mitomycin C (MMC) based protocols with a perfusion time of 90 min at 41.5–42 °C have been reported by several centers [Citation2, Citation4, Citation6, Citation8]. One center used a similar protocol with 30–60 min of MMC perfusion at 42 °C [Citation21]. Another center reported the combination of MMC and doxorubicin as a secondary agent [Citation22]. In our case, we applied an oxaliplatin-based HITHOC/HIPEC protocol. Since there were no signs of abdominal recurrence, we opted for the same chemotherapy regimen in HITHOC as initially administered during HIPEC, consisting of oxaliplatin/5-FU/leucovorin, also known as FOLFOX4, which has demonstrated promising results in patients with abdominal PMP [Citation22, Citation23].
There is great variability in cytotoxic agents used for HITHOC/HIPEC procedures, but most authors suggest using MMC or oxaliplatin-based regimens [Citation24, Citation25].
A recent systematic review on hyperthermic chemotherapy for peritoneal metastases in colorectal cancer studied the differences between oxaliplatin- and MMC-based chemotherapy. This study showed superiority of neither MMC nor oxaliplatin as a cytotoxic agent for hyperthermic chemotherapy in terms of disease-free interval and overall survival [Citation24]. Nevertheless, analyzing the pharmacokinetics of heated oxaliplatin, it has been shown that due to its lower systemic absorption, higher intra-peritoneal concentrations can be attained, and less systemic toxicity is seen [Citation26–30]. Another advantage of oxaliplatin is the reduced duration of perfusion ranging from 30 to 45 min, which seems favorable compared to the 90 min for MMC [Citation26–30].
To prevent pleural dissemination of PMP some authors recommended the use of hyperthermic intrathoraco-abdominal chemotherapy (HITAC) if the diaphragmatic barrier is breached during initial surgical resection [Citation6, Citation22]. Combining HITHOC with HIPEC had almost no added morbidity due to the minimal systemic absorption of chemotherapeutic drugs in the pleural cavity [Citation22].
Although HITHOC/HIPEC-related adverse events are rare, close monitoring of the patient is required due to reported post-intervention hemodynamic instability, systemic hyperthermia, exacerbation of coronary artery disease, pulmonary edema, acute lung injury and acute kidney failure [Citation2, Citation21]. Goal-directed fluid therapy based upon cardiac output is a proposed strategy to maintain hemodynamic stability and normovolemia and as such prevent peri-and post-operative complications [Citation21].
In conclusion, pleural dissemination of PMP is a rare entity, and only a few cases have been described so far [Citation3, Citation4, Citation6–8, Citation31]. Our case describes the successful treatment of a 67-year-old male patient diagnosed with pleural dissemination of a PMP 33 months after initial treatment of abdominal PMP with CRS and HIPEC. Twenty-six months after ePD and HITHOC there was no evidence for pleural or peritoneal recurrence of PMP.
Ethical approval
Written informed consent for publication has been obtained from the patient, with approval from the Ethics Committee Research UZ/KU Leuven.
Acknowledgments
The authors acknowledge the Department of Thoracic Surgery and recognize the efforts of all our colleagues involved in the multidisciplinary approach of this case.
Disclosure statement
Laurens Ceulemans holds a named chair at the KU Leuven sponsored by Medtronic, a post-doctoral fellowship from the University Hospitals Leuven (KOOR) and project funding from FWO (G090922N).
All coauthors report there are no competing interests to declare.
References
- Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperth. 2017;33(5):511–519.
- Doyle MP, Villanueva CI, Davies MP, et al. Cytoreductive surgery and heated intrathoracic chemotherapy for thoracic extension of pseudomyxoma peritonei. Integr Cancer Sci Therap. 2016;3(4):504–508.
- Ghosh RK, Somasundaram M, Ravakhah K, et al. Pseudomyxoma peritonei with intrathoracic extension: a rare disease with rarer presentation from low-grade mucinous adenocarcinoma of the appendix. BMJ Case Rep. 2016;2016:bcr2015211076.
- Chua TC, Yan TD, Yap ZL, et al. Thoracic cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy for pseudomyxoma peritonei. J Surg Oncol. 2009;99(5):292–295.
- Geisinger KR, Levine EA, Shen P, et al. Pleuropulmonary involvement in pseudomyxoma peritonei: morphologic assessment and literature review. Am J Clin Pathol. 2007;127(1):135–143.
- Pestieau SR, Esquivel J, Sugarbaker PH. Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Ann Surg Oncol. 2000;7(3):199–203.
- Pestieau SR, Wolk R, Sugarbaker PH. Congenital pleuroperitoneal communication in a patient with pseudomyxoma peritonei. J Surg Oncol. 2000;73(3):174–178.
- Senthil M, Harrison LE. Simultaneous bicavitary hyperthermic chemoperfusion in the management of pseudomyxoma peritonei with synchronous pleural extension. Arch Surg. 2009;144(10):970–972.
- Cao C, Tian D, Park J, et al. A systematic review and Meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer. 2014;83(2):240–245.
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg. 2015;99(2):472–480.
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol. 2014;9(3):390–396.
- Migliore M, Ried M, Molins L, et al. Hyperthermic intrathoracic chemotherapy (HITHOC) should be included in the guidelines for malignant pleural mesothelioma. Ann Transl Med. 2021;9(11):960–960.
- Migliore M, Combellack T, Williams J, et al. Hyperthermic intrathoracic chemotherapy in thoracic surgical oncology: future challenges of an exciting procedure. Futur Oncol. 2021;17(30):3901–3904.
- Dawson A, Kutywayo K, Mohammed SB, et al. Cytoreductive surgery with hyperthermic intrathoracic chemotherapy for malignant pleural mesothelioma: a systematic review. Thorax. 2022;thoraxjnl-2021-218214.
- Alzahrani N, Ferguson JS, Valle SJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: long-term results at St george hospital, Australia. ANZ J Surg. 2016;86(11):937–941.
- Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2009;249(2):243–249.
- Vaira M, Cioppa T, Marco D, et al. Management of pseudomyxoma peritonei by cytoreduction + HIPEC (hyperthermic intraperitoneal chemotherapy): results analysis of a twelve-year experience. In Vivo. 2009;23(4):639–644.
- Zhao ZY, Zhao SS, Ren M, et al. Effect of hyperthermic intrathoracic chemotherapy on the malignant pleural mesothelioma: a systematic review and metaanalysis. Oncotarget. 2017;8(59):100640–100647.
- Järvinen T, Paajanen J, Ilonen I, et al. Hyperthermic intrathoracic chemoperfusion for malignant pleural mesothelioma: systematic review and Meta-analysis. Cancers (Basel). 2021;13(14):3637.
- Zhou H, Wu W, Tang X, et al. Effect of hyperthermic intrathoracic chemotherapy (HITHOC) on the malignant pleural effusion. Medicine (Baltimore). 2017;96(1):e5532.
- Ashraf-Kashani N, Bell J. Haemodynamic changes during hyperthermic intra-thoracic chemotherapy for pseudomyxoma peritonei. Int J Hyperthermia. 2017;33(6):675–678.
- Sugarbaker PH, Chang D, Stuart OA. Hyperthermic intraoperative thoracoabdominal chemotherapy. Gastroenterol Res Pract. 2012;2012:1–7.
- Chen CF, Huang CJ, Kang WY, et al. Experience with adjuvant chemotherapy for pseudomyxoma peritonei secondary to mucinous adenocarcinoma of the appendix with oxaliplatin/fluorouracil/leucovorin (FOLFOX4). World J Surg Oncol. 2008;6:118.
- Wisselink DD, Braakhuis LLF, Gallo G, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019;142:119–129.
- Sgarbura O, Al Hosni M, Petruzziello A, et al. Complete pathologic response after two-stage cytoreductive surgery with HIPEC for bulky pseudomyxoma peritonei: proof of concept. Int J Hyperthermia. 2020;37(1):585–591.
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13(2):267–272.
- Elias D, Laurent S, Antoun S, et al. Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2003;27(4):407–412.
- Marcotte E, Dubé P, Drolet P, et al. Hyperthermic intraperitoneal chemotherapy with oxaliplatin as treatment for peritoneal carcinomatosis arising from the appendix and pseudomyxoma peritonei: a survival analysis. World J Surg Onc. 2014;12(1):332.
- Marcotte E, Sideris L, Drolet P, et al. Hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from appendix: preliminary results of a survival analysis. Ann Surg Oncol. 2008;15(10):2701–2708.
- Piché N, Leblond FA, Sidéris L, et al. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis: a study of the effect of heat on intraperitoneal oxaliplatin using a murine model. Ann Surg. 2011;254(1):138–144.
- Kocsis A, Markóczy Z, Agócs L, et al. [Pseudomyxoma of the pleura and of the peritoneum – case report of a rare disease]. Magy Seb. 2012;65(1):24–26.
