Abstract
Purpose
To compare the efficacy and safety of intravenous anesthesia (IV) with local anesthesia (LA) in patients undergoing ultrasound (US)-guided radiofrequency ablation (RFA) of thyroid nodules.
Methods
50 patients with American Society of Anesthesiologists classification grades I–II undergoing US-guided thyroid RFA were enrolled and randomly (1:1) divided into IV (conscious sedation with Ramsay Sedation Scale [RSS] scores of 2–3 with an anesthesiologist) and LA (subcutaneous anesthesia with lidocaine without an anesthesiologist) groups. Pre-, intra- and post-procedural blood pressure (BP) (SBP0/DBP0, SBP1/DBP1, and SBP2/DBP2), intra- and post-procedural pain (NRS1 and NRS2), ablated area volume, treatment time and adverse events were analyzed and compared.
Results
Age, sex, weight, number, nature, volume of nodules, and SBP0/DBP0 showed no difference between both groups. 11 and 0 patients’ SBP1/DBP1 were elevated in the LA and IV groups. NRS1 differed between both groups. 6 patients in the LA group had moderate or severe pain, but none in the IV group. No between-group difference in SBP2/DBP2, NRS2, ablation completion rate and ablated volume was noted. The median procedure duration differed from 1109 (176) s in IV group and 723 (227) s in LA groups. There was no increased incidence of adverse events in IV group.
Conclusions
IV with RSS scores of 2–3 maintained intra-procedural BP and relieved intra-procedural pain better, without affecting the ablation efficacy and increasing complications. Despite increased treatment time, IV is a potential option for patients undergoing US-guided RFA of thyroid nodules.
Introduction
Ultrasound (US)-guided radiofrequency ablation (RFA) for benign thyroid nodules and papillary thyroid microcarcinomas (PTMCs) is a minimally invasive treatment modality that has been rapidly developed recently. RFA has shown good efficacy and safety and can resolve cosmetic issues of the neck caused by thyroid nodules [Citation1–21]. Local anesthesia (LA) is the recommended method of anesthesia in thyroid RFA, according to the 2017 Korean Society of Thyroid Radiology (KSThR) guidelines, 2018 China guidelines and other guidelines and expert consensus [Citation3,Citation4,Citation7,Citation8,Citation19]. However, we observed that some patients still experienced symptoms, such as pain or elevated blood pressure (BP), even with adequate LA. Several clinical studies have confirmed that pain is the most common adverse event of thyroid ablation, and it occurs in 2.6–100% of thyroid RFA [Citation1–6,Citation8–16,Citation22–24]. Severe pain forces the surgeon to reduce the ablation power or stop the procedure, affecting the effectiveness of the treatment [Citation9,Citation24]. The adverse effects of elevated BP seem to be more easily overlooked than pain. Studies have reported that 5–10% of patients have moderate or severe hypertension during ablation [Citation24]. It may be underestimated as it is transient and does not require treatment. Pain and elevated BP may increase the risk of bleeding or hematoma and also increase the intolerance to treatment, which may affect the safety and effectiveness of ablation. Therefore, in addition to LA, other anesthesia techniques are urgently required to improve patient comfort. This is necessary for patients with poor coordination, poor pain tolerance, excessive anxiety about treatment and with potential risk of elevated BP.
Intravenous anesthesia (IV) is widely used for invasive or noninvasive examination and treatment of outpatients, such as endoscopy and gynecological examination, due to its advantages of convenience, rapid induction, quick recovery and fewer side effects [Citation25–35]. This technique is also widely used in hyperthermia of deep organs, such as the liver, kidney, lungs and superficial organs, such as the prostate and veins [Citation27–35]. However, systematic studies on the effectiveness and safety of IV in US-guided thyroid ablation are inadequate.
This study primarily aimed to evaluate the anesthetic effect of IV in US-guided RFA of thyroid nodules compared with LA. Furthermore, we analyzed the efficacy and adverse events of IV to explore the suitable population.
Subjects and methods
The First Affiliated Hospital of Dalian Medical University’s institutional review board provided approval for this study. The protocol number is PJ-KS-KY-2020-61. All the subjects were given informed consent prior to entering the study.
Patients
Fifty cases were included in this prospective study. The inclusion criteria were as follows: patients (1) aged 18 − 80 years, (2) who met the indications for thyroid RFA and (3) had American Society of Anesthesiologists classification grades I–II. The exclusion criteria were as follows: patients with (1) chronic pain, (2) long-term alcoholism or taking tranquilizers or antipsychotic drugs, (3) malignant nodules infiltrating the capsule or the edge of the nodule <2 mm from the capsule, (4) nodules compressing the trachea or retrosternal tubercle and (5) hypertension in which BP was not controlled below 140/90 mmHg. Office Excel (Software, Washington, US) was used to generate random numbers for each case and sort them. Twenty-five cases with smaller random number were assigned to LA group, and 25 cases with larger random number were assigned to IV group. The results were monitored by an external supervisor.
Anesthesia methods
The IV group was anesthetized by an anesthesiologist. The patients were instructed to fast before the treatment, and venous access was established through the forearm cephalic, brachial or noble vein. The nasal oxygen flow was 3 L/min. Intravenous midazolam (initial dose, 0.03 mg/kg) was inducted. Propofol (4–12 mg/kg/h) and remifentanil (1.2–7.2 μg/kg/h) intravenous pump was inducted until the end of the procedure. According to the Ramsay Sedation Scale (RSS), the sedation effect was controlled using the RSS scores of 2–3, keeping the patient cooperative, oriented and tranquil during the procedure and maintaining a quick response to gentle taps or commands. The cough reflex of the patient was maintained throughout the course of anesthesia. Ramosetron was routinely administered to prevent nausea and vomiting [Citation25–26]. Intravenous medication was stopped at the end of the treatment so that the patients quickly awakened after the procedure.
The patients in the LA group were anesthetized by the physician performing ablation. After routine disinfection and draping, 10 ml of 1% lidocaine was injected into the skin puncture site and thyroid capsule trans-mid-neck under US guidance [Citation4,Citation8]. When the patient had severe pain, 2–5 ml of 1% lidocaine was supplemented locally.
Ablation was discontinued when patients experienced unbearable pain.
Radiofrequency equipment and technique
A color Doppler US system (LOGIQ E9, GE) with a 9-L high-frequency linear probe was used to monitor and guide the ablation procedure. RF generator (LDRF-120S, Lide, Mianyang) and 17 G radiofrequency electrode with 0.7 − 1.0-cm active tip (LDDJS3-0080050B, Lide, Mianyang) were used. A patient monitoring system (iMEC12, Mindray, Shenzhen) was used.
The ablation procedure was performed by a US physician with over 20 years of experience in interventional US and 10 years of experience in thyroid RFA. Hydrodissection techniques were used after anesthesia and before ablation, if necessary [Citation19,Citation36]. The vital structures around the thyroid were kept more than 5 mm away from the capsule by injecting 5% glucose through a 22-G needle. For ablation, the power for the ablation was maintained at 40–50 W. Moving-shot or fixed-applicator technique was used based on the tumor characteristics [Citation37]. The target for ablation for a benign nodule was the entire nodule. Nodules that were completely solid or mixed with ≥ 90% solid components were directly ablated. For the predominantly solid nodules containing 50–89% of solid components, the cystic portion was aspirated using a 20–23-G puncture needle before the ablation. For nodules with > 50% fluid content, the fluid was withdrawn first, followed by saline flushing and ablation [Citation38]. The target for ablation for a malignant nodule was the entire tumor and the area at least 2 mm from the original tumor margin. The ablation procedure was terminated when the target lesion was completely ablated ().
Figure 1. Ablation methods for different nodules. (A–C) Nodules that were completely solid or mixed with 90% solid components were directly ablated. The ablation target is the entire nodule. (D–F) For nodules with more than 50% fluid content, the fluid was withdrawn first, followed by saline flushing and ablation. The ablation target is the entire nodule. (G–I) Malignant nodules are ablated directly, and the target for ablation was the entire tumor and the area at least 2 mm from the original tumor margin.
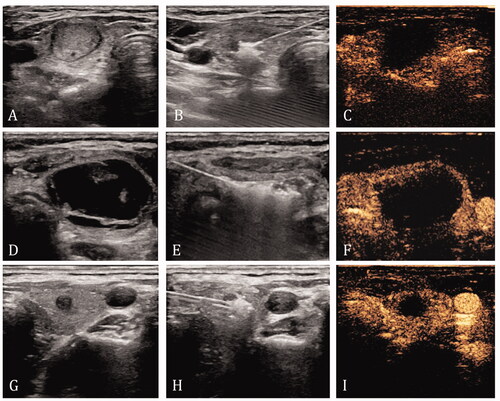
Two minutes after ablation, contrast-enhanced US (CEUS) (SonoVue, Bracco, Milan, Italy) was performed to evaluate whether the ablation range is reached and the ablated volume.
Data collection
Data regarding sex, age, weight, number of nodules, cytology and composition of the nodules were collected from the patient.
BP measured 30 min before the procedure was recorded as SBP0/DBP0. BP measured 10 min after the beginning of the procedure was recorded as SBP1/DBP1. BP measured 30 min after the completion of the procedure was recorded as SBP2/DBP2. The intra- and post-procedural pain was evaluated by internationally accepted Numerical Rating Scale (NRS), and the pain level was scored from 0 to 10, with 0 being the absence of pain and 10 being the maximum intensity. When patients woke up after the procedure, they were asked to rate the pain during the procedure and record it as the intra-procedural pain (NRS1). At 30 min after the procedure, we asked the patient to re-score the pain at the time and recorded it as the post-procedural pain (NRS2).
The ablated volume was calculated by measuring the three meridians corresponding to each non-enhanced area by CEUS after ablation [Citation4,Citation37] ().
Treatment duration refers to the time from the beginning of anesthesia to the end of the procedure. The type and severity of adverse events during and within 2 h after the procedure were assessed. Standard definitions and reporting criteria were used [Citation37].
Objectives of the study
The primary objective of this study was to determine whether elevated BP and pain occurred, which reflects the effect of anesthesia.
According to the classification of BP in the Chinese guidelines for the prevention and treatment of hypertension and Common Terminology Criteria for Adverse Events version 5.0, when the levels of SBP1/DBP1 and SBP2/DBP2 are higher than that of SBP0/DBP0, it is considered that the patient’s BP is elevated. If the levels of SBP1/DBP1 and SBP2/DBP2 do not change or are lower than that of SBP0/DBP0, the patient’s BP is well-controlled ().
Table 1. Classification and definition of blood pressure levels.
The patient’s NRS1 and NRS2 were calculated by patients with scores ranging from 0 to 10, with 0 being the absence of pain and 10 being the maximum intensity. Moreover, scores 1–3, 4–6, and 7–9 were considered mild, moderate and severe pain. When the patient had moderate or severe pain, an event of pain was considered to have occurred.
The secondary objective was to assess the efficacy, including the ablation completion rate, ablated volume and treatment time. Treatment completion was defined as when the target lesion was completely ablated.
In addition, the occurrence and severity of complications and side effects were observed.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 17.0 (IBM Corporation, Armonk, NY). Normally distributed data are expressed as mean (standard deviation), and non-normally distributed data are expressed as median (interquartile range). Differences in characteristics between the two groups were analyzed using the Kruskal–Wallis and Mann–Whitney U tests. Categorical data are presented as frequencies and analyzed using Fisher’s exact test. Two-sided p-values <0.05 were considered statistically significant.
Results
From June 2020 to September 2020, 50 patients undergoing US-guided thyroid RFA at the Ultrasound Intervention Center of the First Affiliated Hospital of Dalian Medical University were included in this study. Thus, 25 patients were included in each group. Clinical and demographic findings of the study population and characteristics of the enrolled nodules are summarized in .
Table 2. Baseline characteristics of patients and nodules undergoing thyroid RFA.
There was no significant difference between the two groups in sex, age, weight, number of nodules, nodule nature, composition and nodules volume (p > 0.05).
Anesthesia effect
Blood pressure
The SBP0/DBP0 in the IV and LA groups were 129.76 ± 17.074/79.92 ± 10.758 and 127.28 ± 11.092/82.52 ± 9.143 mmHg, respectively. There was no significant difference in the pre-procedural BP between both groups (p > 0.05). The SBP1/DBP1 in the IV and LA groups were 125.52 ± 14.477/74.48 ± 8.823 and 138.16 ± 17.892/87.60 ± 11.229 mmHg, respectively. The SBP2/DBP2 in the IV and LA groups were 126.32 ± 13.993/75.76 ± 9.692 and 132.88 ± 13.258/80.52 ± 7.338 mmHg, respectively ( and ).
Figure 2. Changes in blood pressure between two groups IV group: intravenous anesthesia group; LA group: local anesthesia group; T0: 30 min before the procedure; T1: 10 min after the procedural beginning; T2: 30 min after the procedure.
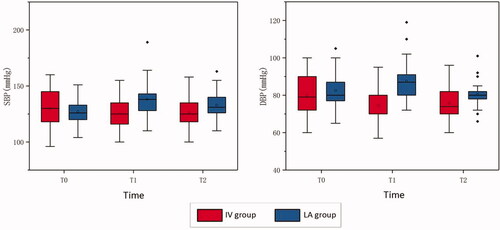
Table 3. Perioperative blood pressure classification of patients in the IV and LA groups.
Intra-procedural BP upgrades were observed in 11 (44%) and 0 (0%) patients in the LA and IV groups, respectively. There was a significant difference in SBP1/DBP1 between both groups (p = 0.000). In the LA group, 9/20 cases with normal baseline BP had intra-procedural BP upgraded, and the proportion of escalation was 45%. Moreover, 2/5 cases with abnormal baseline BP had intra-procedural BP upgraded, with a 40% upgrade rate ().
Table 4. Intra-procedural blood pressure changes in the IV and LA groups.
Post-procedural BP upgrades were observed in 5 (20%) and 0 (0%) patients in LA and IV groups, respectively. No significant difference was observed in the post-procedural BP between both groups (p = 0.05).
Pain
The NRS1 and NRS2 in both groups are depicted in and . In the IV group, there was no moderate or severe pain during the treatment, and the highest NRS score was 3, indicating mild pain. In the LA group, six patients (24%) experienced moderate or severe pain during the procedure. The highest NRS score was 7. The pain during the procedure was mainly tingling or varied pain in the ipsilateral lower neck, face, teeth, head, shoulders, chest and back. There was a significant difference between both groups (p = 0.022). Regarding NRS2, no patients reported moderate or severe pain in either group.
Table 5. Perioperative pain of patients in the IV and LA groups.
Efficacy
Except for one patient in the LA group who stopped the procedure due to intolerable pain and failed to complete the treatment, the remaining 49 patients completed the treatment according to the preoperative plan. Treatment completion rates in the LA and IV groups were 96% and 100%, respectively, with no statistical difference (p = 1.000).
The ablated volume was 1.38 (2.54) and 1.08 (3.73) mL in the IV and LA groups, respectively, with no statistical difference (p = 0.689).
The treatment durations of both groups were significantly different (p = 0.000). The median times were 1109 (176) s and 723 (227) s in the IV and LA groups, respectively. The treatment duration was longer with IV than with LA.
Complications
Complications were classified according to the Society of Interventional Radiology classification. Five major complications were observed, including three cases of hoarseness, one case of hematoma and one case of vasovagal reaction. Two cases of hoarseness occurred in the IV group, and one case occurred in the LA group; they received medication with a short duration of hospitalization (48 h) and recovered within one month. Vasovagal reaction occurred in the LA group, and the patient experienced bradycardia, hypotension and defecation, which were relieved after therapy. The patients were observed in the hospital for 48 h after the procedure. One case of hemorrhage was observed in the LA group, with US revealing the presence of characteristic linear hypoechoic surrounding the thyroid lobe. The hypoechoic range did not increase after mild pressure was applied to the neck. Hemorrhage completely resolved within seven days. Seven minor complications were observed. Two patients experienced dizziness, one case experienced nausea and one experienced snoring in the IV group, whereas three patients experienced dizziness in LA group. None of the above patients required therapy, and these minor complications were relieved after observation.
Tracheal or vascular injury, Horner syndrome, irreversible hoarseness, nodule rupture, infection, skin burns or other ablation complications were not observed in both groups ().
Table 6. Perioperative pain of patients in the IV and LA groups.
Discussion
US-guided thermal ablation of solid tumor has rapidly developed recently. RFA is a type of thermal ablation treatment with shorter treatment time, fewer complications and better efficacy and has been recognized in the treatment of benign thyroid nodules [Citation1–5,Citation9,Citation38]. Although the indications of RFA for PTMCs remain controversial, accumulating evidence indicates that RFA can effectively eliminate lesions in non-surgical patients [Citation10–21].
LA with perithyroidal lidocaine injection is the most commonly used anesthesia method for thyroid RFA, which has been recommended in the 2018 China expert consensus, 2017 KSThR guidelines, 2020 Asian Conference on Tumor Ablation recommendations and 2020 European Thyroid Association Clinical Practice Guideline [Citation3,Citation4,Citation8,Citation19]. This technique involves injecting anesthetics under the skin and around the thyroid capsule to block the sensory nerves within the range [Citation22]. The advantage of LA is that it is effective and performed without an anesthesiologist. It reduces the duration and economic costs of surgeons and patients. However, some studies have reported that 5–10% of patients have moderate or severe hypertension and 2.6–100% have pain during ablation even with adequate LA [Citation1–6,Citation8–16,Citation22–24]. Moreover, pain and elevated BP may increase the risk of bleeding or hematoma, which may affect the safety and effectiveness of ablation. The possible reasons for this are as follows: First, the patient may have a stress response due to treatment, activating the pituitary-adrenal system and secreting a large amount of catecholamine hormones, causing vasoconstriction and elevated BP. Second, thyroid cells destroyed by ablation release a large amount of thyroxine into the blood, which may increase sympathetic nerve excitation and further increase BP [Citation24]. These systemic responses may affect the accuracy and safety of ablation, and some patients stop the treatment [Citation9,Citation38].
Total IV refers to the use of multiple intravenous anesthetics to complete the induction and maintenance of anesthesia. It has been widely used for invasive or noninvasive examination and treatment of outpatients, such as endoscopy and gynecological examination, due to its advantages of short-acting, rapid metabolism, no accumulation and controllability. At present, it has also emerged in interventional US, especially in hyperthermia in deep organs, such as liver, kidneys, and lungs and superficial organs, such as the prostate and vein [Citation27–35]. However, whether IV is suitable for thyroid ablation remains controversial. The 2017 KSThR and Korean Society of Radiology guidelines do not recommend its use for thyroid ablation because previous studies have mentioned that general anesthesia or sedation may delay the detection of complications [Citation4,Citation39]. The 2016 AACE/ACE/AME guidelines recommend conscious sedation with diazepam combined with LA [Citation6]. The 2020 European Thyroid Association (ETA) and 2021 European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe guidelines recommend that mild conscious sedation may be helpful for keeping the patient relaxed throughout the procedure to avoid frequent swallowing and head movements [Citation18–19]. Some studies have explored the application of IV in children who cannot cooperate in thyroid ablation or combined with LA, which was successful in terms of safety [Citation27,Citation40]. Undoubtedly, the rational use of appropriate depth of IV is also a potential option.
This study innovatively applied IV to thyroid ablation, observed its anesthetic and therapeutic effects and systematically compared it with LA. Considering the balance between sedative effect and over sedation, the study set the goal of anesthesia as RSS scores of 2–3, which keeps the patient cooperative, oriented and tranquil during the procedure and maintains a quick response to gentle taps or commands [Citation22]. The advantage of maintaining this depth of anesthesia is that patients can wake up quickly after treatment, which does not increase the postoperative observation time of outpatients. More importantly, it maintains the patient’s cough reflex so that adverse effects, such as tracheal or nerve damage, can be detected early [Citation41].
In the primary results of this study, IV with the anesthesia target of RSS scores of 2–3 has the advantages of reducing intra-procedural pain and maintaining intra-procedural BP compared with LA. It may possibly minimize the potential risks of hemorrhage and cerebrovascular diseases during the procedure and improve patients’ comfort effectively. It is related to the pharmacological effects of intravenous anesthetics for anti-anxiety, reducing the excitability of the central system and reducing stress. The absence of difference in BP and pain between the two groups post-procedure proves that the anesthetic effect can be eliminated in a short period without increasing recovery time.
In this study, 44% of patients with LA had intra-procedural BP upgrade. This may reveal two things. First, LA has shortcomings in controlling intra-procedural BP. Second, the proportion is slightly higher than the previously reported 5–10%, proving that intra-procedural BP with LA is indeed underestimated by researchers [Citation24]. This event should be given more attention in daily work and in future research.
In the secondary results of this study, one patient in the LA group terminated the treatment in advance due to severe pain and did not achieve the treatment goal. We found that this may be caused by the variation of the vagal nerve on the affected side, which is accidental. Therefore, combined with the results of no difference in ablation completion rate and ablated area volume between the two groups, we believe that different anesthesia methods do not affect the ablation efficacy.
The treatment time in the IV group was longer. The main reason for the long duration of IV is that it takes time for the anesthetic agent to take effect and reach the anesthesia goal. In the LA group, the following conditions may prolong the treatment duration: (1) some patients have multiple nodules located in different glandular lobes and require multiple anesthesia, and (2) some patients need additional injections of anesthetics due to intra-procedural pain. Therefore, we believe that the increased time caused by IV could be accepted when multiple nodules or large nodules are planned to be treated.
Major and minor complications occurred with both methods of anesthesia. Although there is no evidence to prove that the incidence of adverse events is different due to the small number of cases, we observed slightly different types of complications in both groups. Patients with IV have specific symptoms of post-procedural nausea and intra-procedural snoring. These conditions are considered to be caused by the application of intravenous anesthetics. The most common side effects of propofol and remifentanil are gastrointestinal reactions, such as nausea and vomiting. Snoring may be caused by excessive sedation or obesity (body mass index, 28.1 kg/m2). This suggests that individualized medication dosage and close anesthesia care are significantly important during IV induction.
This prospective cohort study has some limitations. First, the difference between the complications of the two anesthesia methods is to be further studied, as the sample size was limited. Second, limited by ethical considerations and individualized anesthetic medication, we did not design a comparison of different depths of IV. Third, considering the rapidity of anesthetic drug metabolism and the consistency of ablation methods, we did not compare the long-term efficacy of ablation therapy. Nevertheless, more evidence-based studies are required to fully exploit the advantages of IV in the ablation of thyroid nodules.
Conclusion
Compared with LA, IV with RSS scores of 2–3 shows greater advantages in maintaining intra-procedural BP and relieving intra-procedural pain in patients undergoing thyroid RFA. IV does not affect the ablation efficacy, and there is no evidence that the incidence of adverse events will increase, which is highly dependent on the anesthesiologists. Despite the increased treatment time, IV may be a potential option for patients who have anxiety, sensitivity to pain and potential risk of elevated BP.
Acknowledgments
The authors thank anesthesiologist Dr. Yong Luan and Mr. Xinye Song for providing anesthesia services to patients, as well as teachers and editors who provided opinions on article revision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321–1325.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Thyroid tumor ablation experts group of Chinese medical doctor association, Chinese association of thyroid oncology, interventional ultrasound committee of Chinese college of Interventionalists, et al. Expert consensus on thermal ablation for thyroid benign nodes, microcarcinoma and metastatic cervical lymph nodes (2018 edition). China Oncol. 2018;27(10):768–773.
- Kim JH, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Lim JY, Kuo JH. Thyroid nodule radiofrequency ablation: complications and clinical follow Up. Tech Vasc Interv Radiol. 2022;25(2):100824.
- Gharib H, Papini E, Garber JR, AACE/ACE/AME Task Force on Thyroid Nodules, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):622–639.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Ha EJ, Baek JH, Che Y, et al. Radiofrequency ablation of benign thyroid nodules: Recommendations from the asian conference on tumor ablation task force. Ultrasonography. 2021;40(1):75–82.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Kim JH, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroid carcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33(2):212–219.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34(5):611–616.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation technique for the treatment of papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Bernardi S, Palermo A, Grasso RF, et al. Current status and challenges of US-Guided radiofrequency ablation of thyroid nodules in the long term: a systematic review. Cancers (Basel). 2021;13(11):2746.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–−185.
- Mauri G, Hegedüs L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–−197.
- Tufano RP, Pace-Asciak P, Russell JO, et al. Update of radiofrequency ablation for treating benign and malignant thyroid nodules. The future is now. Front Endocrinol (Lausanne). 2021;12:698689.
- Ding Z, Chen J, Chen Z, et al. Efficacy and safety of thermal ablation for treating lymph node metastasis from papillary thyroid carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:738299.
- Morelli F, Ierardi AM, Biondetti P, et al. The importance of subcapsular anesthesia in the anesthesiological management for thyroid radiofrequency ablation. Med Oncol. 2020;37(4):22.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Wang JF, Wu T, Hu KP, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J (Engl). 2017;130(11):1361–1370.
- Nimmo AF, Absalom AR, Bagshaw O, et al. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): joint guidelines from the association of anaesthetists and the society for intravenous anaesthesia. Anaesthesia. 2019;74(2):211–224.
- Chinese Medical Association Anesthesiology Branch Expert Consensus Working Group on Total Intravenous Anesthesia Expert consensus on total intravenous anesthesia. Chin J Anesthesiol. 2016;36(6):641–649.
- Piccioni F, Poli A, Templeton LC, et al. Anesthesia for percutaneous radiofrequency tumor ablation (PRFA): a review of current practice and techniques. Local Reg Anesth. 2019;12:127–137.
- Kim HJ, Park BK, Chung IS. Comparison of general anesthesia and conscious sedation during computed tomography-guided radiofrequency ablation of T1a renal cell carcinoma. Can Assoc Radiol J. 2018;69(1):24–29.
- Zhou W, Arellano RS. Thermal ablation of T1c renal cell carcinoma: a comparative assessment of technical performance, procedural outcome, and safety of microwave ablation, radiofrequency ablation, and cryoablation. J Vasc Interv Radiol. 2018;29(7):943–951.
- Puijk RS, Ziedses des Plantes V, Nieuwenhuizen S, et al. Propofol compared to midazolam sedation and to general anesthesia for percutaneous microwave ablation in patients with hepatic malignancies: a single-center comparative analysis of three historical cohorts. Cardiovasc Intervent Radiol. 2019;42(11):1597–1608.
- Prud’homme C, Deschamps F, Moulin B, et al. Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia. 2019;36(2):37–45.
- Wu J, Lu Y, Cao X. Different effects of oxycodone and remifentanil in patients undergoing ultrasound-guided percutaneous radiofrequency ablation of hepatic cancer: a randomized trial. Drug Des Devel Ther. 2019;13:365–372.
- Ramsay CR, Adewuyi TE, Gray J, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(49):1–490.
- Aydin AM, Gage K, Dhillon J, et al. Focal bipolar radiofrequency ablation for localized prostate cancer: safety and feasibility. Int. J. Urol. 2020;27(10):882–889.
- Brittenden J, Cotton SC, Elders A, et al. Clinical effectiveness and cost-effectiveness of foam sclerotherapy, endovenous laser ablation and surgery for varicose veins: results from the comparison of laser, surgery and foam sclerotherapy (CLASS) randomised controlled trial. Health Technol Assess. 2015;19(27):1–342.
- Souza KP, Rahal A, Jr, Volpi EM, et al. Hydrodissection and programmed stop sedation in 100% of benign thyroid nodules treated with radiofrequency ablation. Eur J Radiol. 2020;133:109354.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicenter prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Bernardi S, Lanzilotti V, Papa G, et al. Full-thickness skin burn caused by radiofrequency ablation of a benign thyroid nodule. Thyroid. 2016;26(1):183–184.
- Hong MJ, Sung JY, Baek JH, et al. Safety and efficacy of radiofrequency ablation for nonfunctioning benign thyroid nodules in children and adolescents in 14 patients over a 10-year period. J Vasc Interv Radiol. 2019;30(6):900–906.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol (Lausanne). 2021;11:575152.
