Abstract
Background
Transurethral resection of bladder tumor (TUR-BT) followed by chemoradiation (CRT) is a valid treatment option for patients with muscle-invasive bladder cancer (MIBC). This study aimed to investigate the efficacy of a tetramodal approach with additional regional hyperthermia (RHT).
Methods
Patients with stages T2–4 MIBC were recruited at two institutions. Treatment consisted of TUR-BT followed by radiotherapy at doses of 57–58.2 Gy with concurrent weekly platinum-based chemotherapy and weekly deep RHT (41–43 °C, 60 min) within two hours of radiotherapy. The primary endpoint was a complete response six weeks after the end of treatment. Further endpoints were cystectomy-free rate, progression-free survival (PFS), local recurrence-free survival (LRFS), overall survival (OS) and toxicity. Quality of life (QoL) was assessed at follow-up using the EORTC-QLQ-C30 and QLQ-BM30 questionnaires. Due to slow accrual, an interim analysis was performed after the first stage of the two-stage design.
Results
Altogether 27 patients were included in the first stage, of these 21 patients with a median age of 73 years were assessable. The complete response rate of evaluable patients six weeks after therapy was 93%. The 2-year cystectomy-free rate, PFS, LRFS and OS rates were 95%, 76%, 81% and 86%, respectively. Tetramodal treatment was well tolerated with acute and late G3–4 toxicities of 10% and 13%, respectively, and a tendency to improve symptom-related quality of life (QoL) one year after therapy.
Conclusion
Tetramodal therapy of T2–T4 MIBC is promising with excellent local response, moderate toxicity and good QoL. This study deserves continuation into the second stage.
Introduction
Radiotherapy (RT) with concurrent chemotherapy is a good treatment option for patients with inoperable muscle-invasive bladder cancer (MIBC) to preserve organ function. Overall survival (OS) rates after concurrent chemoradiation (CRT) range from 50 to 63% after five years and 75–82% of the patients can retain their bladder [Citation1–3]. The reported complete response (CR) rates after CRT range from 66% to 82% [Citation4–6]. Importantly, quality of life (QoL) after CRT is good as in a series of 60 patients, 85% reported no significant side effects and in excess of 75% of patients not only retained their bladder but also normal bladder function [Citation7].
Deep regional hyperthermia (RHT) is a well-known radio- and chemosensitizer [Citation8–11]. This concept has been confirmed in a randomized study where patients with MIBC were treated with either radiotherapy alone or radiotherapy in combination with RHT [Citation12]. The CR rates differed considerably between patient groups treated with and without RHT (73% and 51%), albeit without a significant difference in OS [Citation12].
Tetramodal therapy consisting of transurethral resection of bladder tumor (TUR-BT) followed by CRT and RHT is a compelling concept to further improve bladder-preserving therapy. In a cohort of 369 patients with high-risk bladder cancer of stages Ta, Tis, T1, and T2 treated with TUR-BT followed by CRT, 79 patients also received RHT [Citation3]. In the latter group, the clinical CR rate was 87%. Interestingly, additional RHT increased 5-year OS from 64% with CRT alone to 87% (p = 0.0001) and long-term bladder preservation was significantly improved in patients treated with CRT plus RHT (p = 0.006). We report the results of a prospective phase IIB study, performed in two European HT centers, which aimed to investigate the efficacy of tetramodal therapy in patients with MIBC.
Materials and methods
Trial design
The study was designed as a single-arm multicenter phase IIB study to evaluate the efficacy of the tetramodal treatment approach.
Patient selection criteria
The main inclusion criteria were histologically confirmed urothelial (transitional cell) cancers of the bladder, no previous pelvic radiotherapy and no other prior or concurrent malignancy, TNM stages T2–4, Nx, M0, age ≥18 years, KPS ≥ 70%, medical inoperability or patients who declined radical cystectomy. Laboratory requirements (within 14 days prior to enrollment) included absolute granulocytes > 1.5 × 109/L, platelets > 75 × 109/L, creatinine clearance > 60 ml/min (Cockroft Formula).
The main exclusion criteria were any metal implants in the anatomical area to be heated, electronic cardiac devices and uncontrolled cardiac disease. Disease staging included either an MRI or CT of the abdomen and a CT thorax. Written informed consent was obtained prior to the registration of the patients in the study.
Patient treatment
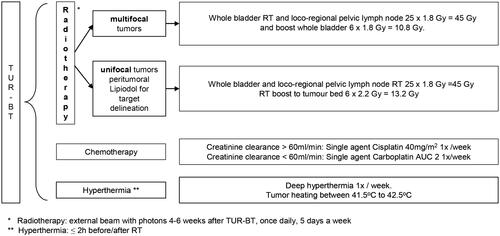
The study treatments are represented schematically in . Patients were treated with TUR-BT followed by daily RT combined with weekly chemotherapy and RHT four to six weeks after TUR-BT. RT was applied using either 3 D-conformal or intensity modulated radiotherapy (IMRT) techniques with 6 megavoltages (MV) photon beams. A CT simulation in the supine position with an empty rectum and bladder was required for treatment planning. For unifocal tumors, a boost was planned on an additional CT scan performed with a full bladder. The gross target volume (GTV) comprised the macroscopic bladder tumor visible on CT/MRI/cystoscopy. The 'CTV pelvis' included the GTV, whole bladder, regional lymph nodes (obturator, external + internal iliac), proximal urethra, prostate + prostatic urethra in men. The PTV was created by expanding the CTV by a 1.5–2 cm margin. Boost volumes (entire bladder or partial bladder) were defined as a 0.5 cm GTV to CTV expansion and a 1.5 cm CTV to PTV expansion. RT was administered once a day, five days a week, to a total dose of 45 Gy (25 × 1.8 Gy) to the whole bladder. Patients with multifocal tumors received a boost to the whole bladder of 12 Gy (6 × 2 Gy), whereas patients with unifocal tumors received a boost of 13.2 Gy (6 × 2.2 Gy) to the tumor bed, marked with ethiodized oil (Lipiodol, Guerbet, France).
Patients received weekly chemotherapy with cisplatin or carboplatin for a minimum of six and a maximum of seven cycles. Cisplatin with 40 mg/m2 was given intravenously according to the institutional protocol. If the creatinine clearance was <60 ml/min, weekly carboplatin (AUC2) was administered instead of cisplatin. Deep RHT was administered in accordance with the quality assurance guidelines published by the European Society for Hyperthermic Oncology (ESHO) [Citation13,Citation14]. The BSD 2000/3 D system with the Sigma-60 or Sigma-Eye phased array applicator (BSD Medical Cooperation/Pyrexar, Salt Lake City, UT, USA) was used. RHT was performed once a week for a minimum of six and a maximum of seven sessions. After an induction period of approximately 30 min, RHT was delivered for 60 min. Thermal mapping with multichannel thermometry probes was mandatory to measure the temperature achieved at the reference points. The temperature probes were placed in the rectum, bladder, vagina (for female patients) and additionally on the anal margin (rima ani). The temperature was measured at 10-second intervals, starting before treatment and stopping five minutes after switching off the radiofrequency power. Based on these measurements, the Cumulative Equivalent Minutes (CEM43 °C) were calculated to describe the thermal dose applied to the bladder [Citation15]. According to protocol, HT was initiated within two hours prior to or after RT.
Evaluation of toxicity and quality of life (QoL)
The acute and late genitourinary (GU) and gastrointestinal (GI) toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v.4.03). In addition, RHT-induced symptoms were also monitored. The acute toxicities were documented weekly during therapy and six weeks after completion of therapy. The late toxicity was assessed during the follow-up examinations.
QoL was measured with the general module QLQ-C30 (version 3.0) and MIBC-specific module QLQ BLM30 of the EORTC QoL questionnaires. Scoring was performed according to the manual provided by the EORTC. Patients completed these questionnaires before and after therapy. In addition, QoL scores were collected at six weeks and during each follow-up visit after completion of therapy. The QLQ-C30 scores were compared with the reference values for genito-urinary cancer published by EORTC Quality of Life Group members [Citation16].
Follow-up
Follow-up visits were scheduled after six weeks and 6, 12 and 24 months after completion of tetramodal therapy and included physical examination, toxicity assessment according to CTCAE v.4.03, tumor status assessments and QoL (QLQ-C30 and QLQ-BLM30). The radiologic assessment was performed according to CT/MRI RECIST (version 1.1) [Citation17].
Endpoints and study design
The primary endpoint of the study was CR six weeks after treatment assessed by cystoscopy, urine cytology and CT or MRI of the abdomen. If the cytology and cystoscopy were negative, this was considered CR. The secondary endpoints of the study were 'sustained CR at six months after completion of therapy', functional bladder preservation, toxicity evaluation and long-term QoL according to the EORTC QLQ-C30 and QLQ-BLM30 questionnaires. Additional secondary endpoints were 2-year progression-free survival (PFS) and OS from time of study inclusion to evidence of progression or death. Furthermore, local recurrence-free survival (LRFS) was analyzed. Patients with no disease progression or who were alive at the time of follow-up were censored for PFS, LRFS and OS.
Study design and sample size
The study design was Simon's two-stage minimax design [Citation18]. In the first phase, 27 eligible patients were to be included with the condition that if more than 21 positive CRs were achieved, the trial could be terminated. Otherwise, the trial would continue with the inclusion of 13 additional patients.
The null hypothesis was based on historical data, which show a CR rate of 75% six weeks after completion of therapy for patients with MIBC treated with TUR-BT and cisplatin-based CRT [Citation4,Citation5]. The alternative hypothesis assumed that tetramodal treatment could attain complete remission in 90% of patients. The study was planned with a 10% significance level (one-sided) and 90% power.
Due to slow accrual, the trial committee decided to perform an unplanned analysis after stage 1 of the trial with a total of 27 patients (21 eligible) to decide whether recruitment should continue. The results of this analysis are presented here.
Statistical analysis
Descriptive and additional statistical analyses of the trial data were performed with IBM SPSS Statistics 25 software (IBM, New York, NY, USA) and R statistical software v.4.0.3 (R Core Team, 2020). The Wilson method for calculating confidence intervals for proportions was applied. A two-tailed paired t-test with a significance level of 5% was used to analye the QoL data. Survival endpoints were analyzed using the Kaplan-Meier method and the survival R package.
Results
Patient characteristics
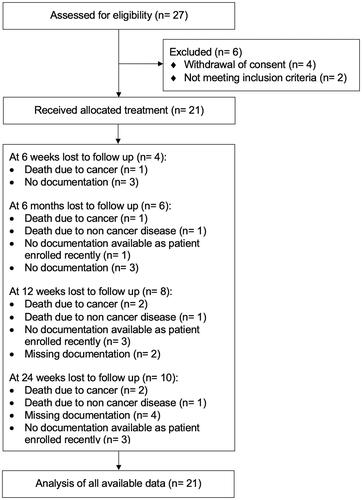
Between June 2013 and April 2021, 27 patients were recruited at Cantonal Hospital Aarau (seven patients), Switzerland and Charité Universitätsmedizin Berlin (20 patients), Germany. Of these 27 patients, four patients withdrew their consent during follow-up and two patients had to be excluded because they did not meet the study's inclusion criteria (). The median age of all patients was 73 (range: 54–82) years, with a preponderance of male (14/21, 67%) patients. Patient and treatment characteristics are summarized in .
Table 1. Patient- and treatment-related characteristics.
Protocol treatment adherence
The planned radiotherapy dose was delivered to 20/21 (95%) patients. Chemotherapy was delivered with a median of five (range: 1–7) cycles using either cisplatin (six patients), carboplatin (10 patients) or cisplatin followed by carboplatin (five patients). In a patient with known myelodysplastic syndrome, chemotherapy was discontinued after the first cycle due to pancytopenia. The median number of RHT sessions was six (range: 3–7). At least five sessions of RHT could be delivered in 17/21 (81%) of patients. RHT was discontinued in two patients due to patient refusal. Treatment was stopped prematurely in one patient after 54 Gy, three chemotherapy cycles and three RHT sessions because of the detection of multiple lung metastases. In two patients, treatment was interrupted but not discontinued. The first patient needed surgical treatment of spinal empyema after which CRT was continued without RHT. The second patient developed grade 3 obstructive pyelonephritis, which required prolonged hospitalization.
Complete response
In 15/21 patients, response data were available for the early six-week time point. Of these, 14/15 patients (93%) achieved a CR. The proportion analysis of CR at six weeks and 6, 12 and 24 months after treatment is summarized in . A detailed graphical illustration of the disease status of all 21 patients at different time points is shown in . The patient who did not achieve a CR after six weeks received a lower CEM43 °C than the median. Due to the high variability of thermal dose per patient and the small number of patients enrolled in the study at this point, no statistically significant effect of thermal dose and number of total sessions on the treatment response could be found.
Figure 3. Disease status of study patients after 1.5, 6, 12 and 24 months. Each line represents one patient, n = 21. The time point 0 refers to the end of treatment. A red column between 0 and 1.5 months indicates complete response (CR) at 1.5 months with the assumption that CR was achieved sometimes within this 6-week time period. Similarly, a blue column starting after 1.5 months indicates confirmed local or locoregional recurrence at the 6-month time point. Locoregional recurrences occurred in 4 patients, 3 local and 1 regional. In two patients information about disease status during follow-up is missing, one of the two patients died early after treatment. For each patient, HT treatment data are listed (left); in the first column: mean of values of total CEM43 °C during the treatment course and in the second column: number of total HT sessions. n.r.: not reported.
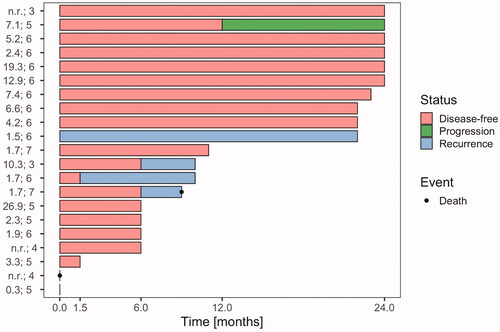
Table 2. Complete response (CR) rates six weeks, 6, 12 and 24 months after treatment.
Acute and late toxicity
Acute and late GI and GU toxicities are presented in and Citation4, respectively. The overall rate of acute G3–4 toxicity was 10% (2/21). As described above, one grade 4 spinal empyema and one grade 3 pyelonephritis were observed during treatment. In addition, we observed 67% (14/21) grade 2 urogenital (GU) and 48% (10/21) grade 2 gastrointestinal (GI) acute toxicities. The most frequent chemotherapy-induced acute toxicity was an intermittent decrease in glomerular filtration rate in 14% (3/21) of patients.
Table 3. Acute and late genitourinary (GU) toxicity.
GI and GU toxicities six weeks after treatment were examined in 17 patients. 54% and 6% of patients developed grade 2 GU and GI toxicities, respectively. No patient had grade 3 toxicity six weeks after treatment. Other toxicity symptoms at this time point were fatigue grade 1 (2 patients) and abdominal pain grade 1 (2 patients).
Late GU and GI toxicities were evaluated in 15 patients six months after treatment as summarized in and . The overall rate of late G3 toxicity was 13% (2/15) including one patient with noninfectious cystitis and one patient with erectile dysfunction. No late grade 4 toxicity has been observed. The rates of G2 GU and GI late toxicities were 14% (2/15) and 2% (3/15), respectively.
Table 4. Acute and late gastrointestinal (GI) toxicity.
The toxicity assessments at one and two years were limited by low patient numbers. The follow-up data at 1 year show that only 18% (2/13) of patients had grade 2 GI toxicity. Similarly, 20% (2 patients) of patients had grade 2 GI toxicity at the 2-year follow-up time point.
Assessment of QoL
QoL (EORTC-QLQ-C30 and QLQ-BLM30) questionnaires were returned by 18 patients prior to treatment, 15 patients after treatment, 14 patients after six weeks follow-up, 15 patients after six months, 11 patients after 12 months and 11 patients after 24 months. The assessments of the qualitative outcome at baseline, before treatment and after one year are shown in , based on the answers of nine patients with QoL data available for both time points.
Table 5. Quality of life using QLQ-C30 and QLQ-BLM30.
A higher mean score for functional scales and global QoL in QLQ-C30 reflects a better level of functioning, in contrast, a higher mean score for symptoms reflects more problems. In comparison to the reference values for functional scales in patients with genitourinary cancer published by the EORTC for Global Health Status, our patient cohort shows the similar or better quality of life except for emotional and cognitive functioning where the scores were slightly below the reference values [Citation16]. With regard to the severity of symptoms, our cohort reported lower scores for fatigue, nausea & vomiting, pain, appetite loss, constipation and financial difficulties but worse scores for dyspnea, insomnia and diarrhea in comparison to the EORTC reference values.
Analysis of QLQ-C30 showed no increase in mean global health status score in patients immediately before therapy and at 12 months afterwards. In addition, a trend of a decrease in symptom scores indicated improvement of different symptoms (). In general, patients’ symptoms were not statistically different and thus remained stable one year after therapy.
The QLQ-BLM30 is a supplementary module specific for patients with MIBC and comprises 30 questions classified into five domains. Higher scores on the scales and items of the QLQ-BLM30 should be interpreted as a greater symptomatic burden, with the exception of the sexual function scale and sexual enjoyment where higher scores represent the better function. For urinary problems, all patients completed items 31–37 at baseline, which measure urinary problems in patients without urostomy.
The analysis of EORTC-QLQ-C30 showed no increase in mean global health status in patients 12 months after end of treatment. In contrast, a trend of a decrease in symptom scores indicated improvement of various symptoms (). In general, patients’ symptoms were not statistically different and thus remained stable one year after therapy.
Survival outcomes
The median follow-up for all patients was 11 months (range: 0–28). The cystectomy-free survival after two years was 95%. The 2-year PFS was 76% (see ). Three patients had a local recurrence at six weeks, six months and one year, respectively. The patient with local recurrence at six months was treated with cystectomy; the other two patients had local resections. One patient developed a lymph node recurrence one year after treatment but was free from any tumor one year thereafter, at 24 months of follow-up.
Figure 4. 2-year (a) progression-free survival, (b) overall survival and (c) local recurrence-free survival rates.
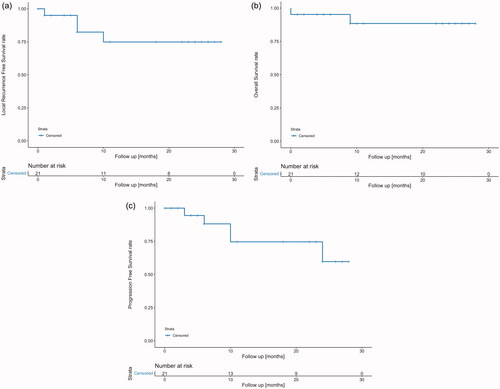
The 2-year OS was 86% (see ). At the time of data analysis, two patients had died from progressive cancer. One of these patients died after completion of therapy but before the first follow-up due to rapid general disease progression with extensive lung metastases. The 2-year LRFS was 81% (see ). In total, four patients had recurrent disease.
Discussion
We performed a prospective phase II study investigating tetramodal therapy in stage T2–T4 MIBC. The early results of our study showed a CR rate at six weeks of 93%. In addition, the bladder preservation rate at 2 years was 95%, 2-year PFS, LRFS and OS rates were 76%, 81% and 86%, respectively, and the treatment-related toxicity was mild. In contrast to previous studies, our study is the first to include QoL measures, the analysis of which showed a trend to improved symptom-related QoL at one year. Although the sample size of this analysis is too small to reach any solid statistical conclusions, we consider the results to be very promising for this cohort of inoperable and comorbid patients and have decided to proceed to the second stage of our phase IIB study despite low patient accrual during the first stage.
Several clinical studies have previously investigated the effect of heating tumor tissues at 43 °C in combination with chemotherapy or RT in bladder cancer including two randomized studies. In the first randomized study performed by Colombo et al. [Citation11], a higher local control rate of 82.9% was shown when patients with early (Ta-T1) bladder cancers were treated with mitomycin C in combination with local microwave-based hyperthermia in comparison to 42.5% achieved by treatment with mitomycin C alone. The other randomized study by a Dutch group was performed in the pre-concurrent chemotherapy era and compared RT with RT in combination with RHT in patients with unresectable pelvic tumors [Citation12]. In the subcohort of patients with bladder cancer, CR rates as assessed by cytology three months after treatment were 51% after RT and 73% after combined treatment (p < 0.0001). Two large retrospective studies investigated the effect of trimodal therapy (CRT + TUR-BT) in bladder cancer. In the first study by Chauvet et al. [Citation4], 109 patients with localized MIBC (36% stages T3 and T4) who were not candidates for cystectomy were treated with TUR-BT followed by CRT. RT was given at doses of 55–60 Gy similar to our study, and cisplatin was given daily in weeks 1 and 5. The CR was 70% and the 4-year OS 42%. In the second study by Rödel et al. [Citation5], 415 patients, predominantly with T2–T4 tumors, were treated using the trimodal approach. In this series, CR was achieved in 72% of patients using the same time point six weeks after treatment to assess local response.
The tetramodal approach has recently been investigated in a large retrospective analysis by a group from Erlangen in Germany [Citation3]. Of 369 patients with pTa, pTis, pT1 and pT2 bladder cancer, 79 patients received tetramodal therapy. Treatment response was evaluated four to six weeks after treatment with TUR-BT. The overall CR rate in the patients treated with the tetramodal approach was 87% and the 10-year bladder preservation rate was 96%. Interestingly, the use of RHT in addition to CRT and TUR-BT did not improve the CR rate (87% vs 86%) but increased the 5-year OS from 64% to 87% (p = 0.0001) suggesting a systemic immune-modulating effect of RHT. In addition to the less advanced tumor stages, the study of Merten et al. [Citation3] also differed from our study in terms of younger patient age (67 vs 75 years). Therefore, our results are complementary to those published by the Erlangen group as we included elderly patients with more advanced MIBC.
Although T2–4 stage tumors could be included, the majority of patients in our study had T2 tumors and only one patient had a T4 tumor. This might be considered a major limitation as trimodal therapy might be considered sufficient to control T2 tumors. Rödel et al. [Citation5], reported CR rates of 80% in a cohort of 389 patients with T2 tumors, of whom 50% developed local relapse. In a French retrospective analysis of patients with exclusively T2 N0 bladder cancers, Peyromaure et al. [Citation19] reported a CR rate after CRT of 74.4% and a salvage cystectomy rate of 25.6%. According to these results, we believe that there is also room for improvement in the control of T2 MIBC through the addition of hyperthermia.
The median number of RHT sessions in our study is higher as compared with Merten et al. (6 versus 5) [Citation3]. In the earlier analysis of the same patient cohort by Wittlinger et al., a significant correlation between the number of RHT sessions and the probability of OS was observed [Citation20]. Due to the low patient number and the lack of statistical power, we cannot confirm this with our data.
We did not find a statistically significant correlation between thermal dose and clinical outcome. Possible reasons for this are the low patient number and that the value for the thermal dose was not defined in the study protocol due to the lack of current data as to the requisite value for RHT of bladder cancer. Furthermore, the thermal dose might vary with the location of the probe in the bladder. Recently, strong effects of RHT on clinical outcomes have been demonstrated for the combination of RT and RHT in cervical cancer [Citation21] and recurrent breast cancer [Citation22], in both cases based on retrospective analyses of large patient cohorts. The standardization of temperature metrics, their measurement, their recording as well as the definition of temperature target values are important research goals in the field of hyperthermia. Of note, preclinical data show that even at the low temperature of 39–40 °C, pleiotropic biological effects occur which sensitize the tumor to RT and chemotherapy, as summarized by Oei et al. [Citation23].
The tolerability of the tetramodal approach in our study was good because only one patient had acute grade 4 toxicity (empyema) and another patient had grade 3 pyelonephritis, however, the latter was most likely secondary to obstructive tumor growth. These toxicity outcomes are comparable to Merten et al. who reported acute toxicity of grade 3–4 in only five patients treated with RHT [Citation3]. Importantly, for the first time, our study investigated QoL measures for the tetramodal approach. Analysis of EORTC-QLQ-C30 questionnaires showed no increase in the mean global health status at 12 months when compared to pretreatment scores. Regarding symptom-related QoL, a general decrease in symptom scores suggested improvement of various symptoms (), although the differences did not reach statistical significance due to a low number of patients. In comparison to the published reference values from the EORTC, the patients included in our study had a better quality of life except for cognitive and emotional functioning. This could be explained by the high patient age. With regard to symptoms, our cohort had less fatigue and gastrointestinal symptoms such as nausea & vomiting, pain, appetite loss and constipation, which further underlines the good tolerability of our pelvic treatment. One small series retrospectively compared QoL after radical cystectomy with that after bladder preservation [Citation24]. QoL appeared to be better after bladder preserving management, mainly because of better sexual activity. A comparative cross-sectional study also suggested that urinary symptoms and sexual function are better after RT in comparison with cystectomy [Citation25].
There are several limitations to our study such as the small sample size, the fact that the study was analyzed prematurely and the incomplete data regarding clinical response at six weeks in six patients. However, the latter might be a too early time point for assessing treatment response due to persisting post-treatment inflammation and for logistical reasons in this inoperable and comorbid patient cohort. In addition, three of our patients first achieved CR after six months. A significant shortcoming of our study is the absence of systematic post-treatment bladder biopsies during follow-up because some recurrences might not be detected by cystoscopy alone. In our study, a biopsy was only performed in case of suspicious lesions observed during cystoscopy. This procedure was determined in collaboration with the urologists and seemed to be adequate for this inoperable cohort of patients with limited additional treatment options.
Conclusion
Tetramodal therapy is promising with excellent local treatment response, very moderate toxicity and a tendency for improved symptom-related QoL in inoperable patients with locally advanced MIBC. Based on these results, we have entered the second stage of our phase IIb study despite low patient accrual during the first stage. We propose that this strategy deserves further clinical investigation in a multicenter setting.
Ethic statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee (protocol code 2011/076).
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Weiss C, Wolze C, Engehausen DG, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24(15):2318–2324.
- Rödel C, Weiss C, Sauer R. Trimodality treatment and selective organ preservation for bladder cancer. J Clin Oncol. 2006;24(35):5536–5544.
- Merten R, Ott O, Haderlein M, et al. Long-Term experience of chemoradiotherapy combined with deep regional hyperthermia for organ preservation in high-risk bladder cancer (Ta, Tis, T1, T2). Oncologist. 2019;24(12):e1341–e1350.
- Chauvet B, Brewer Y, Felix-Faure C, et al. Concurrent cisplatin and radiotherapy for patients with muscle invasive bladder cancer who are not candidates for radical cystectomy. J Urol. 1996;156(4):1258–1262.
- Rödel C, Grabenbauer GG, Kühn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20(14):3061–3071.
- James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–1488.
- George L, Bladou F, Bardou VJ, et al. Clinical outcome in patients with locally advanced bladder carcinoma treated with conservative multimodality therapy. Urology. 2004;64(3):488–493.
- Hill SA, Denekamp J. The response of six mouse tumours to combined heat and X rays: implications for therapy. Br J Radiol. 1979;52(615):209–218.
- Overgaard J. Fractionated radiation and hyperthermia: experimental and clinical studies. Cancer. 1981;48(5):1116–1123.
- Ohtsubo T, Saito H, Tanaka N, et al. Enhancement of cisplatin sensitivity and platinum uptake by 40 degrees C hyperthermia in resistant cells. Cancer Lett. 1997;119(1):47–52.
- Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21(23):4270–4276.
- van der Zee J, González D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch deep hyperthermia group. Lancet. 2000;355(9210):1119–1125.
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14(2):125–133.
- Bruggmoser G, Bauchowitz S, Canters R, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol. 2012;188(Suppl 2):198–211.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values manual. 2nd ed. Brussels, Belgium: EORTC Quality of Life Group; 2008. p. 427.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10.
- Peyromaure M, Slama J, Beuzeboc P, et al. Concurrent chemoradiotherapy for clinical stage T2 bladder cancer: report of a single institution. Urology. 2004;63(1):73–77.
- Wittlinger M, Rödel CM, Weiss C, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol. 2009;93(2):358–363.
- Kroesen M, Mulder HT, van Holthe JML, et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother Oncol. 2019;140:150–158.
- Bakker A, Tello Valverde CP, van Tienhoven G, et al. Post-operative re-irradiation with hyperthermia in locoregional breast cancer recurrence: temperature matters. Radiother Oncol. 2022;167:149–157.
- Oei AL, Kok HP, Oei SB, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020;163–164:84–97.
- Caffo O, Fellin G, Graffer U, et al. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer. 1996;78(5):1089–1097.
- Henningsohn L, Wijkström H, Dickman PW, et al. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol. 2002;62(2):215–225.