Abstract
Objective
Investigate the relationships between endopelvic fascial edema and its influencing factors after ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation of uterine fibroids.
Methods
This retrospective study included 688 women with uterine fibroids treated by USgHIFU; based on post-treatment MRI, the patients were divided into two groups: endopelvic fascial edema group and nonedema group. The specific location of fascial edema of each patient was also recorded. Fascial edema and fibroid features and treatment parameters were set as the dependent and independent variables, respectively, and the correlations were studied using univariate and multivariate analyses. The relationship between the pain-related adverse events and location of fascial edema was analyzed by χ2 and fisher's exact tests.
Results
Edema and nonedema groups had 556 and 112 patients, respectively. Among the edema patients, posterior fascial edema incidence was the highest. Multifactorial analysis showed that the energy efficiency factor (EEF), fibroid location, and enhancement type were positively associated with endopelvic fascial edema (p < 0.05), while the distance from dorsal surface of the fibroid to sacrum was negatively correlated (p < 0.001). Patients with anterior, posterior and perirectal, and right lateral fascial edemas were associated with lower abdominal pain, sacrococcygeal pain, and leg numbness/pain, respectively.
Conclusion
Post-USgHIFU ablation, patients were prone to developing endopelvic fascial edema, and some of them experienced pain-related adverse events. The fibroid location, its types of contrast enhancement, the distance from the dorsal surface of the fibroid to the sacrum, and EEF were the influencing factors resulting in the endopelvic fascial edema after USgHIFU ablation.
Introduction
Uterine fibroids, also known as leiomyomas, are the most common benign gynecologic tumors, causing serious impact on health and quality of life [Citation1,Citation2]. With a significant advance in the minimally-invasive and noninvasive technology, high-intensity focused ultrasound (HIFU) is gradually being used for the treatment of uterine fibroids; it is a totally noninvasive procedure and associated with safety, effectiveness, and lower risk of complications [Citation3,Citation4]. This manipulation can be performed under ultrasound guidance, i.e. ultrasound-guided HIFU (USgHIFU) [Citation5].
Magnetic resonance imaging (MRI) is widely used for the postoperative follow-up of USgHIFU ablation because of its excellent soft tissue resolution that can assess the target and surrounding tissue. Some studies showed that the sacrococcygeal fascial edema, which is significantly associated with postoperative sacrococcygeal pain, can be observed in MRI in certain patients after USgHIFU [Citation6]. However, the endopelvic fascia is the continuous connective tissue network that covers the structures of the pelvic cavity and contains many fascia and space other than the sacrococcygeal region, such as the parietal component (covered musculoskeletal structures such as the pelvic floor and wall), visceral component (attached to the pelvic organs, including the bladder, uterus, and rectum), and connective tissue linking these two components [Citation7]. Injury to fascia may irritate or compress the underlying nerves causing pain or paresthesia in the corresponding area [Citation8]. Moreover, the surrounding fasciae may also be involved in the pathophysiology of pelvic floor disorders and play an essential role in the support and suspension of the female pelvic floor structures [Citation7,Citation9].
Previously, few studies have investigated postoperative edema of the overall endopelvic fascia. In this study, we evaluated the endopelvic fascia post-USgHIFU, and investigated the factors that results in endopelvic fascial edema. A better understanding of these factors will be helpful to improve the evaluation procedure for pre-USgHIFU, predict therapeutic risk factors, and provide effective assistance for optimal postoperative care.
Materials and methods
Patients
This study was approved by the Ethics Committee of Chongqing Medical University in Chongqing, China (approval number 2021-548). Informed consent requirement was waived due to the retrospective nature of the study. From November 2017 to May 2021, data of a total of 668 women who underwent USgHIFU ablation for uterine fibroids at our institution were collected. The inclusion criteria for HIFU therapy were as follows: (1) symptomatic or asymptomatic uterine fibroids requiring urgent treatment, and (2) patients who had the ability to communicate during the ablation. On the other hand, the exclusion criteria were: (1) incomplete preoperative or postoperative pelvic MRI data, (2) endometriosis or other serious gynecological diseases, (3) history of high-dose radiotherapy for lower abdominal malignancies, and (4) pregnancy.
USgHIFU ablation
All USgHIFU procedures were performed using a clinical extracorporeal JC200 USgHIFU therapy system (Chongqing Haifu Medical Technology Co., Ltd. Chongqing, China). The therapeutic ultrasound beam was generated by an ultrasound transducer with a frequency of 0.8 MHz, a focal length of 15 cm, and a diameter of 20 cm. This system was also equipped with a Mylab 70 ultrasound imaging device (Esaote, Genova, Italy) to provide real-time imaging for localizing fibroids and monitoring treatment.
During the treatment, the patient was placed in a prone position on the treatment table with the abdominal wall in contact with circulating degassed water in a tank. HIFU was performed under intravenous sedation with fentanyl and midazolam hydrochloride; the patients remained awake throughout the procedure and could communicate with the physician for reporting any intraoperative discomfort or pain. Any such feedback was recorded. The procedure was started from the posterior part of the leiomyomas using a spot scan with a power setting between 300 − 400 W, with a focus to the boundary of the leiomyoma at a distance of at least 1 cm. The ultrasound power was adjusted according to patients’ feedback and changes in the grayscale of the ultrasound image. Treatment was terminated when all signs of blood flow disappeared or grayscale changes in the target tissue were observed on color Doppler ultrasound. The sonication power, sonication time, treatment intensity, the therapeutic dose (TD), and energy-efficiency factor (EEF, J/mm3, the energy required to ablate per unit volume of fibroid) were recorded.
Following the Society of Interventional Radiology (SIR) classification system, the severity of postoperative adverse events, including the lower abdominal pain, sacrococcygeal pain, lower limb pain, and vaginal discharge, was recorded. The classifications were evaluated as follows: (1) class A: no therapy, no consequence; (2) class B: nominal therapy or no consequence, including overnight admission for observation only; (3) class C: therapy and minor hospitalization (<48 h) required; (4) class D: major therapy required, including an unplanned increase in the level of care or prolonged hospitalization (>48 h); (5) class E: permanent adverse sequelae; and (6) class F: death [Citation10].
MRI evaluation
MRI was performed on a 1.5 T MR system (uMR570, United Imaging Medical Ltd., Shanghai, China) before and 1 − 2d after the therapy. Standard T1-weighted imaging (T1WI) (repetition time/echo time (TR/TE), 500/13 ms, voxel size 1.7 × 1.3 × 5.0 mm, thickness 5 mm, axial plane), T2WI (TR/TE, 5300/100 ms, voxel size 1.0 × 1.0 × 5.0 mm, thickness 5 mm, axial plane), fat-suppressed T2WI (TR/TE, 4729/75 ms, voxel size 1.0 × 1.0 × 5.0 mm, thickness 5 mm, sagittal plane), diffusion weighted imaging (DWI) (TR/TE, 4800/98.8 ms, voxel size 3.0 × 3.0 × 5.0, thickness 5 mm, b values 0 and 800 s/mm2, axial plane)and contrast-enhanced sequences (TR/TE, 6.23/2.91 ms, voxel size 1.7 × 1.2 × 5.0 mm, thickness 5 mm, axial plane) were performed on all patients.
The MR images of each patient were evaluated by two experienced radiologists, and in case of controversy, the final decision was made by the chief doctor of the department. The three-dimensional diameters of the non-perfused area and the leiomyoma were measured on the contrast-enhanced MR images: longitudinal (D1), anterior-posterior (D2), and transverse (D3). Non-perfused volume (NPV) and fibroid volume were calculated based on the following equation: V = 0.5233 × D1 × D2 × D3, and some variables were calculated based on treatment parameters, including treatment intensity (s/h, sonication time required to ablate fibroids per hour) and NPV ratio (NPV to fibroid volume).
The following data were recorded based on MR images: the distance from ventral/dorsal surface of the fibroid to skin/sacrum, thickness of abdominal wall (measured as shown in ), position of uterus (anteverted and anteflexed, anteverted and retroflexed, retroverted and anteflexed, retroverted and retroflexed), location of fibroids (anterior, posterior, lateral, fundus), lateral location of fibroids (evaluated on the slice with maximal fibroid area on axial T2WI, right, left, mid-position), type of fibroids (submucosal, intramural, subserosal), the signal intensity of T2WI (hypointense [signal intensity is lower than that of the skeletal muscle or similar], isointense [signal intensity higher than that of the skeletal muscle but lower than the myometrium], hyperintense [signal intensity similar to or higher than that of the myometrium]), degree of fibroid enhancement (slight, moderate, significant), and number of ablations [Citation11]. Fascial edema was exhibited as fascial thickening, the hyperintense signal on T2WI, and may show linear hyperintensity on enhancement sequences. According to the postoperative MR findings, 668 patients were divided into the following two groups: (1) endopelvic fascial edema and (2) nonedema. Furthermore, based on the spatial location of the fasciae, the endopelvic fascia were divided into anterior wall, posterior wall, left lateral wall, right lateral wall, peri-vesical, peri-uterine, and perirectal fasciae (shown in ), and recorded the edema at each site.
Figure 1. MRI sagittal view of a patient with uterine fibroid. (a) the distance from ventral surface of the fibroid to skin, (b) the distance from dorsal surface of the fibroid to sacrum, (c) thickness of abdominal wall.
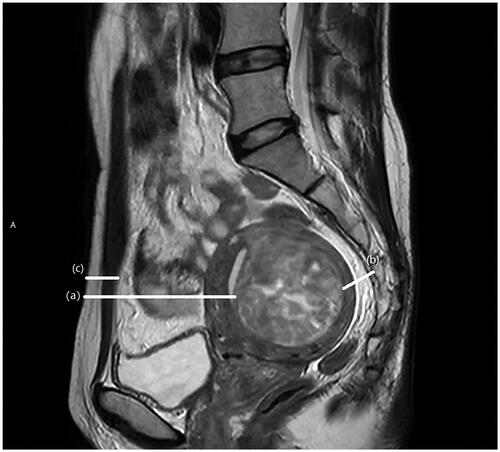
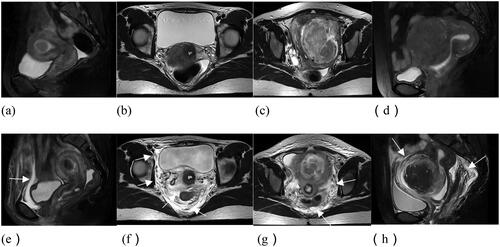
Figure 2. The location of the endopelvic fascial edema. (a-d) MR images before USgHIFU ablation. (e) Anterior wall fascial edema; (f) posterior wall fascial, right lateral wall fascial, peri-vesical fascial, and perirectal fascial edema; (g) posterior wall fascial, left lateral wall fascial edema; (h) peri-uterine fascial, posterior wall fascial, and perirectal fascial edema.
Statistical analysis
Data were analyzed using SPSS version 26.0 (IBM, Chicago, IL, USA). The normal and skewed distribution measures were expressed as means ± standard deviations and median and inter quartile range, respectively, while the count data were expressed as frequencies. Endopelvic fascial edema was set as the dependent variable, while fibroid features and treatment parameters were set as the independent variables. Independent sample t-test, Mann − Whitney test, and χ2 test were used to determine the statistical significance of differences between the two groups. All the variables with p < 0.05 in univariate analysis were selected for binary logistic regression in the multivariate analysis after being tested for collinearity. The relationship between the postoperative pain-related adverse events and location of fascial edema was analyzed by χ2 and Fisher's exact tests. p values were judged significant if <0.05.
Results
Baseline characteristics and fibroid features of patients
A total of 668 patients with uterine fibroids treated with USgHIFU were included in this study, and 1519 fibroids were ablated. The endopelvic fascial edema () and non-edema groups had 556 (83.2%) and 112 (16.8%) patients, respectively. Of all patients who presented with endopelvic fascial edema, 217 (32.5%), 496 (74.3%), 264 (39.5%), 200 (29.9%), 113 (16.9%), 50 (7.5%), and 78 (11.7%) had anterior wall, posterior wall, right lateral wall, left lateral wall, peri-vesical, peri-uterine, and perirectal fascial edemas, respectively.
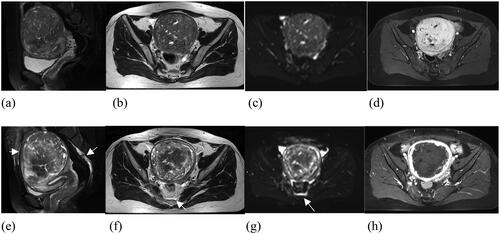
Figure 3. A 44 years old patient with fibroid. (a-d) MRI images before USgHIFU ablation. (e-h) MRI images after USgHIFU ablation. (a) Pre-USgHIFU a sagittal T2WI image showed a posterior wall fibroid that is almost hyperintense on T2WI. (e-f) Fascial edema was shown as a high-intensity signal on T2WI and diffusion-weighted imaging (arrow).
The mean age of the 668 study patients was 40 ± 6.67 years (range 18 − 57), while the mean values of fibroid volume and maximum diameter were 133.62 ± 142.51 cm3 and 6.49 ± 2.01 cm, respectively. The median distance from the dorsal surface of the fibroid to the sacrum was 21.74 mm (interquartile range: 12.69–36.17 mm) in the edema group, which was significantly smaller than that in the non-edema group (median: 35.34 mm, interquartile range: 19.87–53.00 mm) (p < 0.01). The location of fibroids in the edema group was 190 (28.4%), 180 (26.9%), 152 (22.8%), and 34 (5.1%) in the anterior wall, posterior wall, lateral wall, and fundus, and 56 (8.4%), 13 (1.9%), 29 (4.3%), and 14 (2.1%) in the nonedema group, respectively. And the distance from the ventral surface of the fibroid to the skin, maximal diameter of the fibroid, fibroid volume, location of the fibroid, type of the fibroid, the fibroid’s signal of T2WI, and degree of enhancement were significantly different between the two groups (p < 0.05, ), while the differences in age, thickness of abdominal wall, lateral location of fibroids, number of ablated fibroids, and location of the uterus between the two groups were not statistically significant ().
Figure 4. Violin plot analysis of each quantitative parameter between the edema and non-edema groups (a–i). White dash lines represent median values. TD: therapeutic dose; EEF: energy efficiency factor
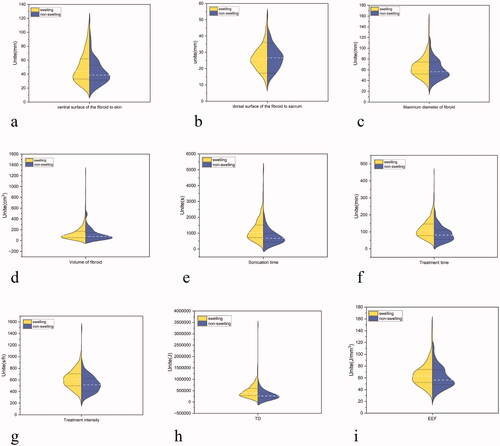
Table 1. Univariate analysis to evaluate the relationship between the endopelvic fascial edema and fibroid features.
Comparison of USgHIFU treatment parameters between the two groups
The median values of EEF in the edema and non-edema groups were 4.58 J/mm3 (range 3.29 − 6.18) and 3.00 J/mm3 (2.27 − 4.71), respectively. As shown in , significant differences were observed in the treatment time, treatment intensity, sonication time, TD, and EEF between the two groups (p < 0.05). On the other hand, no significant difference was observed in the sonication power, NPV, and NPV ratio level between the two groups (p > 0.05).
Table 2. Univariate analysis to evaluate the relationship between the endopelvic fascial edema and ultrasound ablation parameters.
Multivariate analysis of influencing factors of pelvic fascia edema
The collinearity diagnostics indicated that the sonication time, treatment time, and TD were collinear; the sonication time and treatment time were eliminated stepwise according to the variance inflation factors (VIF). Therefore, the distance from the ventral/dorsal surface of the fibroid to skin/sacrum, maximal diameter of the fibroid, volume, fibroid location, type of fibroid, T2WI, enhancement degree, treatment intensity, TD, and EEF were included as independent variables in the binary logistic regression for analysis. Eventually, of the 11 independent variables included in the model, EEF, the distance from dorsal surface to sacrum, location of fibroid, and degree of enhancement were significantly associated with endopelvic fascial edema. The larger value of EEF indicated higher risk of endopelvic fascial edema (p < 0.001, odds ratio [OR] 1.292, 95% confidence interval [CI] 1.147 − 1.457). The distance from the dorsal surface to the sacrum was negatively correlated with the risk of fascial edema, while the posterior wall fibroid and degree of enhancement were positively correlated with fascial edema (p < 0.05, ).
Table 3. Multivariable binary logistic regression analysis to evaluate the correlation of fascial edema with the significant factors of univariate analysis.
Adverse events
According to the SIR criteria, the classification of observed postoperative adverse events is shown in . There were 296 (44.3%), 109 (16.3%), and 3 (0.4%) cases of Class A, B, and C events, respectively, in the two groups; no class D, E, or F events occurred in this study. Among the Class A adverse events, the main adverse event was vaginal discharge (180 [26.9%] cases; 27.0% (150/556) and 26.8% (30/112) of patients in the edema and non-edema groups, respectively). The incidences of lower abdominal pain, sacrococcygeal pain, and lower limb numbness/pain were 7.9%, 4.7%, and 1.3% in the edema group and 7.1%, 2.7%, and 0.9% in the non-edema group, respectively. Further, nine (1.6%) patients in the edema group and 3 (2.7%) in the non-edema group showed odynuria. One patient experienced proctalgia. Among the class B events, 9.1% of patients had postoperative lower abdominal pain (8.8% [49/556] and 10.7% [12/112] of patients in the edema and non-edema groups, respectively). The incidence of sacrococcygeal pain and lower limb numbness/pain was 2.9% and 1.8% and 2.7% and 0% in the edema and non-edema groups, respectively. In class C, 3 patients with fascial edema had urinary retention. No significant difference was observed in adverse events between the two groups (p > 0.05).
Table 4. Summary of postprocedural adverse events.
Relationship between pain-related adverse events and the location of endopelvic fascial edema
Among 668 patients who underwent USgHIFU for uterine fibroids, 113 (16.9%), 47 (7.0%), 23 (3.4%), 12 (1.8%), and 1 (0.15%) patients showed lower abdominal pain, sacrococcygeal pain, lower limb numbness/pain, odynuria, and proctalgia, respectively. Some of the pain-related adverse events were associated with the location of endopelvic fascial edema (). The results of the χ2 tests showed that the proportion of patients with anterior wall fascial edema who developed lower abdominal pain was 24.9% (54/217). Further, the risk of lower abdominal pain was higher in patients with anterior wall fascial edema (χ2 = 14.5, p < 0.001). On the other hand, the incidence of sacrococcygeal pain was lower in patients with anterior wall fascial edema (χ2 = 8.96, p = 0.003). The proportion of sacrococcygeal pain was 8.9% (44/496) in patients with posterior wall fascial edema, and the risk of sacrococcygeal pain was higher in patients with posterior wall fascial edema (χ2 = 9.91, p = 0.001). The sacrococcygeal pain was significantly associated with perirectal fascial edema (χ2 = 27.65, p = 0.000), while the incidence of lower limb numbness/pain was higher in patients with right lateral wall fascial edema (χ2 = 11.67, p = 0.001).
Table 5. Relationship between the pain-related adverse events and location of endopelvic fascial edema.
As shown in , the lateral location of fibroids was significantly associated with lateral fascial edema (p < 0.05). The incidence of lower limb numbness/pain differed by the lateral location of fibroids, with a greater incidence of lower limb numbness/pain in right-sided fibroids (5.5%) than that of the left-sided fibroids (1.3%) (p <0.05).
Table 6. Relationship between the lateral location of fibroids and parietal fascial edema/lower limb discomfort.
Discussion
Many studies have demonstrated the safety and effectiveness of USgHIFU therapy [Citation12,Citation13]. However, owing to the complexity of the lesion and human body, the reflection and scattering of ultrasound may lead to deposition of energy in the acoustic channel and in the adjacent tissues, producing a risk of tissue damage from thermal effects. In the present study, among 556 patients who underwent MRI-based observation of endopelvic fascial edema after USgHIFU, 496 (496/668; 74.3%) showed posterior wall fascial edema, consistent with the results of Zhang et al. [Citation6]. Although a few previous studies have investigated the postoperative MR findings of the sacrum and soft tissues of the sacrococcygeal region [Citation6,Citation14,Citation15], the endopelvic fascia is a continuous structure that contains other more fasciae. Moreover, the pelvic floor fasciae may play a critical role in chronic pelvic pain [Citation7]. From this perspective, the inclusion of the intact endopelvic fascia in the observation is necessary for the study of postoperative adverse events. In this study, we fully evaluated the overall presentation of the endopelvic fascia by MRI and analyzed the various factors influencing fascial edema. Further, we analyzed the relationship between the site of fascial edema and pain-related adverse events.
We found that other sites of edema besides the posterior wall included the anterior wall, right parietal wall, left parietal wall, peri-vesical, peri-uterine, and perirectal fasciae. The incidence of posterior wall fascial edema was the highest possibly due to the fact that the posterior fascia, adjacent to the sacrum, is affected by both the direct heat conduction from sacral energy deposition and ultrasonic energy absorption in the posterior acoustic field, resulting in significant energy accumulation and an increased risk of thermal injury. Because the endopelvic fascia is a continuous structure composed of fibrous connective tissue of various densities, the remaining sites of the fascia in the acoustic field will also be affected by the focal energy, absorbing, and reflecting the ultrasound; thus, producing edema.
In a recent study, investigators reported that the distance from the fibroid to the sacrum was strongly correlated with the localized edema of endopelvic fascia due to HIFU treatment [Citation6]. In this study, we found that the distance from the ventral surface of the fibroid to the skin, the distance from the dorsal surface of the fibroid to the sacrum, maximal diameter, fibroid volume, location of the fibroid, type of the fibroid, the signal of T2WI, degree of enhancement, and treatment parameters were significantly different between the fascial edema and non-edema groups. However, multivariate analysis showed that only the EEF, fibroid location, distance from the dorsal surface of the fibroid to the sacrum and degree of enhancement were associated with endopelvic fascial edema. EEF refers to the energy required to ablate per unit volume of fibroid and is a quantitative indicator of energy delivery during the HIFU, reflecting the ease of ablation of uterine fibroids. Fibroids with significant enhancement, high signal intensity on T2WI, and more posterior location require more sonication time as well as increased TD and EEF during HIFU ablation, and hence, associate with a higher risk of sacral thermal injury [Citation16]. The results of our study showed that in comparison to the nonedema group, the fibroids in the edema group were more posteriorly located, had a higher signal in T2WI, strongly enhanced, and required more energy for treatment. These results suggest that the ablation of fibroids in the edema group was more difficult, required more energy to achieve a satisfactory therapeutic effect, and increased the chance of edema from thermal effect to the fascia, which validates the theoretical basis of previous studies. Yu et al. [Citation17] demonstrated that pretreatment with oxytocin could decrease the fibroid hemoperfusion, increase energy deposition efficiency, and reduce the TD required for ablation. Therefore, for fibroids requiring high energy for ablation, oxytocin may be administered preoperatively that may decrease the total energy requirement for ablation and may reduce the likelihood of endopelvic fascial edema.
We found that in comparison to anterior fibroids, fascial edema is more likely to occur when the fibroid is located in the posterior wall. Further, larger distance from the dorsal surface of the fibroid to the sacrum was associated with a lower risk of fascial edema that was an independent protective factor for fascial edema. According to previous studies, as the distance between the transducer and the target increased, more refraction, reflection, absorption, and scattering occurred when the ultrasound beam crossed the acoustic pathway, resulting in energy attenuation due to a rising amount of tissue in front of the focal point [Citation18,Citation19]. Therefore, fibroids located in the anterior wall or closer to the abdominal wall were more likely to achieve the required ablation volume at the same energy [Citation20]. With the increase in dose and propagation distance of the ultrasound beam, the ultrasound-tissue interaction in the acoustic field becomes more complex, and the risk of adverse events also increases. Meanwhile, the sacrum is often perpendicular or nearly perpendicular to the HIFU beam axis during the procedure, and the physical properties of the ultrasound beam and the high impedance of the bone make it susceptible to deposition and reflection of energy. During ablation, the sacrum continues to heat up, and heat can spread to adjacent tissues causing fascial edema. Therefore, the focal point of the acoustic beam should be as far away from the sacrum as possible during the procedure, and timely adjustments should be made based on intraoperative patient feedback.
Contrast-enhanced scans can reflect the blood perfusion of the tissue. Gong et al. [Citation21] demonstrated that the ease of HIFU ablation was related to the blood supply of uterine fibroids, and fibroids with mild enhancement were better ablated than those with the moderate/significant enhancement. It is because the flowing blood may redistribute or take away the heat on the target area, resulting in inefficient energy deposition, a phenomenon known as the ‘cooling effect’ [Citation18]. Our results showed that the greater degree of fibroid enhancement was related to the higher risk of fascial edema. Therefore, according to the principle of HIFU ablation, tumors with less fibrous tissue and an abundant blood supply are less sensitive to HIFU, and the ablation needs increased TD. During this process, more energy propagates along the acoustic channel, and the acoustic beam can form ectopic small focal points and deposit energy in nontarget tissues (such as the endopelvic fascia) within the acoustic channel, producing a heating effect and causing edema.
Safety has always been a major concern for the USgHIFU treatment. We reported adverse events comparable to that observed in previous studies [Citation22,Citation23]. Our results showed that 113 (16.9%) patients developed lower abdominal pain after ablation and anterior wall fascial edema was significantly associated with it. This may be related to the soft tissue injury of the abdominal wall due to intraoperative energy deposition in the acoustic channel. A total of 47 patients experienced sacrococcygeal pain, and patients with posterior wall fascial edema had a higher risk of developing sacrococcygeal pain, which is consistent with the findings of Li et al [Citation15]. Furthermore, 17 patients with sacrococcygeal pain had perirectal fascial edema, suggesting that local soft tissue injury in the sacrococcygeal region may cause this pain. Further, our results showed that 73.9% (17/23) of the 23 patients who presented with lower limb numbness/pain had right lateral fascial edema and the fibroids leaning to the right side were more likely to present with lower limb numbness/pain after the procedure. This result is consistent with clinical experience, and we consider that this may be caused by sacral nerve irritation. However, these pain-related adverse events resolved within one week of treatment, and fascial edema gradually decreased during the follow-up period. We consider that endopelvic fascial edema represents an immediate thermal effect in the majority of patients after ablation, with only a small percentage of patients experiencing severe injury accompanied by pain. Complications will be reduced further as USgHIFU technology and equipment are optimized, and it is expected to become a truly noninvasive treatment.
This study has the limitation of data selection bias due to the retrospective design. Furthermore, the present study did not quantitatively grade the degree of endopelvic fascial edema in each patient, and the sample size could be expanded in future to accurately assess the severity of postoperative fascial edema with quantitative data.
In conclusion, in this study, a significant number of patients showed endopelvic fascial edema after USgHIFU treatment of uterine fibroids; the incidences were more common after the treatment of fibroids with larger EEF, higher enhancement, smaller distance of dorsal surface to sacrum, or located in the posterior wall. Moreover, the location of endopelvic fascial edema was significantly associated with the postoperative pain-related adverse events. Therefore, in order to further reduce the incidence of endopelvic fascial edema, its influencing factors must be fully considered to optimize the USgHIFU treatment regimen for patients with uterine fibroids.
Supplemental Material
Download PDF (271.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Furong Lv and Zhibo Xiao, upon reasonable request.
Additional information
Funding
References
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
- Machado-Lopez A, Simón C, Mas A. Molecular and cellular insights into the development of uterine fibroids. IJMS. 2021;22(16):8483.
- Liu L, Wang T, Lei B. High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a Meta-analysis. Eur Radiol. 2022;32(2):1195–1204.
- Management of symptomatic uterine leiomyomas: ACOG practice bulletin, number 228. Obstetrics and Gynecology. 2021;137(6):e100–e115.
- Anneveldt KJ, van 't Oever HJ, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Zhang Y-J, Xiao Z-B, Lv F-R, et al. MRI evaluation of endopelvic fascial swelling and analysis of influencing factors in patients with uterine fibroids after high-intensity focused ultrasound ablation. Int J Hyperthermia. 2020;37(1):175–181.
- Roch M, Gaudreault N, Cyr MP, et al. The female pelvic floor fascia anatomy: a systematic search and review. Life (Basel). 2021;11(9):900.
- Ercoli A, Delmas V, Fanfani F, et al. Terminologia anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol. 2005;193(4):1565–1573.
- Maher C, Baessler K, Glazener CMA, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews. 2004;(4):CD004014.
- Cardella JF, Kundu S, Miller DL, Society of Interventional Radiology, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl):S189–S91.
- Yang MJ, Yu RQ, Chen WZ, et al. A prediction of NPVR >/= 80% of Ultrasound-Guided High-Intensity focused ultrasound ablation for uterine fibroids. Front Surg. 2021;8:663128
- Yu L, Zhu S, Zhang H, et al. The efficacy and safety of MR-HIFU and US-HIFU in treating uterine fibroids with the volume <300 cm(3): a Meta-analysis. Int J Hyperthermia. 2021;38(1):1126–1132.
- Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of Ultrasound-Guided High-Intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221.
- Cun J-p, Fan H-j, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2019;36(1):21–28.
- Li D, Gong C, Bai J, et al. Analysis of magnetic resonance signal intensity changes in the sacrococcygeal region of patients with uterine fibroids treated with high intensity focused ultrasound ablation. Int J Hyperthermia. 2020;37(1):404–413.
- Yang Z, Zhang Y, Zhang R, et al. A case-control study of high-intensity focused ultrasound combined with sonographically guided intratumoral ethanol injection in the treatment of uterine fibroids. Journal of Ultrasound in Medicine: official Journal of the American Institute of Ultrasound in Medicine. 2014;33(4):657–665.
- Yu SC, Cheung EC, Leung VY, et al. Oxytocin-Augmented and Non-Sedating High-Intensity-Focused ultrasound (HIFU) for uterine fibroids showed promising outcome as compared to HIFU alone or uterine artery embolization. Ultrasound Med Biol. 2019;45(12):3207–3213.
- Marinova M, Ghaei S, Recker F, et al. Efficacy of ultrasound-guided high-intensity focused ultrasound (USgHIFU) for uterine fibroids: an observational single-center study. Int J Hyperthermia. 2021;38(2):30–38.
- Fan HJ, Zhang C, Lei HT, et al. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine (Baltimore). 2019;98(10):e14566.
- Liu Z, Gong C, Liu Y, et al. Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2018;34(1):77–86.
- Gong C, Lin Z, Lv F, et al. Magnetic resonance imaging parameters in predicting the ablative efficiency of high-intensity focused ultrasound for uterine fibroids. Int J Hyperthermia. 2021;38(1):523–531.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Fan HJ, Cun JP, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35(1):534–540.
