Abstract
Rationale Current hepatic locoregional therapies are limited in terms of effectiveness and toxicities. Given promising pre-clinical results, a first in-human trial was designed to assess the technical effectiveness and safety profile of histotripsy, a noninvasive, non-thermal, non-ionizing focused ultrasound therapy that creates precise, predictable tissue destruction, in patients with primary and secondary liver tumors.
Methods A multicenter phase I trial (Theresa Study) was performed in a single country with 8 weeks of planned follow-up. Eight of fourteen recruited patients were deemed eligible and enrolled in the study. Hepatic histotripsy, was performed with a prototype system (HistoSonics, Inc., Ann Arbor, MI). Eleven tumors were targeted in the 8 patients who all had unresectable end-stage multifocal liver tumors: colorectal liver metastases (CRLM) in 5 patients (7 tumors), breast cancer metastases in 1 (1 tumor), cholangiocarcinoma metastases in 1 (2 tumors), and hepatocellular carcinoma (HCC) in 1 (1 tumor). The primary endpoint was acute technical success, defined as creating a zone of tissue destruction per planned volume assessed by MRI 1-day post-procedure. Safety (device-related adverse events) through 2 months was a secondary endpoint.
Results The 8 patients had a median age of 60.4 years with an average targeted tumor diameter of 1.4 cm. The primary endpoint was achieved in all procedures. The secondary safety profile endpoint identified no device-related adverse events. Two patients experienced a continuous decline in tumor markers during the eight weeks following the procedure.
Conclusions This first-in-human trial demonstrates that hepatic histotripsy effectively destroys liver tissue in a predictable manner, correlating very well with the planned histotripsy volume, and has a high safety profile without any device-related adverse events. Based on these results, the need for more definitive clinical trials is warranted. Trial Registration: Study to Evaluate VORTX Rx (Theresa). NCT03741088. https://clinicaltrials.gov/ct2/show/NCT03741088
Histotripsy, a new noninvasive, non-thermal, non-ionizing focused ultrasound therapy, safely created a zone of tissue destruction in the liver that correlated very well with the pre-defined planned tissue destruction volume.
In this first human trial histotripsy was well tolerated with no histotripsy device-related adverse events and its primary endpoint of acute technical success was achieved in all 8 enrolled patients with primary or secondary liver tumors.
This new locoregional therapy for patients with liver tumors is safe and effective, warranting further trials.
KEY POINTS
Introduction
Over approximately the last two decades, locoregional therapies, such as percutaneous thermal-based ablation, radiation therapy, and intraarterial chemo- or radioembolization, have been incorporated into most treatment guidelines for primary and/or metastatic hepatic malignancies [Citation1–4]. Despite a large body of evidence supporting the use of such therapies, each approach has considerable drawbacks, including the degree of invasiveness [Citation5–7], the high dose of radiation [Citation8], the lack of visualization of the ablation effect in real-time, and the variability in local tumor control [Citation9–11]. Consequently, new effective therapies are sought. Histotripsy may be one such therapy since it is a noninvasive, non-thermal, and a non-ionizing energy therapeutic modality that allows real-time visualization of the tissue effect.
Histotripsy uses short (<50 microseconds), alternating, high-amplitude pulses arising from focused ultrasound to generate inertial acoustic cavitation in tissues. When a repetitive negative focal pressure with enough force occurs in tissue, a bubble cloud (cluster of microbubbles) forms, expands and then collapses thousands of times per second, imparting severe stresses on surrounding cells and tissues to produce cellular destruction of the target tissue. With sufficiently high pressure and adequate number of pulses, the target tissue can be completely destroyed leaving a fluid homogenate that has no recognizable cellular structures [Citation12]. The tissue damage is precise at the histologic level and can be visualized by diagnostic ultrasound in real-time. The system has a software controlled micro-positioning system that is co-axially aligned with the diagnostic ultrasound transducers so that a target of any size and shape can be treated [Citation13]. Earlier studies conducted in animals demonstrated the ability of histotripsy to effectively treat tumors in small animals and to destroy soft tissue in large animals with precisely controlled energy delivery [Citation12–15]. Given such promising pre-clinical results, a first in-human trial was designed to assess the technical effectiveness and safety profile of histotripsy in patients with primary and secondary liver tumors.
Materials and methods
Trial design
This prospective, non-randomized, multi-center, feasibility trial was designed to evaluate the safety profile and technical efficacy of histotripsy for the destruction of primary and metastatic liver tumors. The trial protocol and statistical analysis plan can be found in Supplement 1 and Supplement 2. The Agencia Española de Medicamentos y Productos Sanitarios (approval 651/17/EC) and the Ethics Committees for Investigation of Medicinal Products at the participating hospitals approved the trial. Written informed consent was obtained from all participants prior to the trial procedure.
Participants
Two clinical sites recruited patients between July 2018 and May 2019. Follow-up was completed in July 2019. Patients were screened for eligibility criteria under two protocol revisions (eTable 1, Supplement 3). The investigators requested, and the sponsor approved a protocol revision after the first two patient treatments to allow multiple tumor histotripsies, since the safety profile was within the normal limits, and patients could potentially benefit from additional tumor treatment. Patients who were not suitable candidates for any other therapies including surgery or locoregional therapies or who refused other therapies were deemed eligible for enrollment into the study. All patients had to have end-stage liver tumors and exhausted other therapeutic options.
Histotripsy procedure
All histotripsy procedures were performed using an investigational device (VORTX Rx, Histosonics, Inc., Ann Arbor, MI). The system is a portable (AC-powered) device designed to create an acoustic cavitation bubble cloud utilizing focused ultrasound. Ultrasonic pulses (700 kHz) of microsecond (<20 μs) duration are delivered at low-duty-cycle (<1%) and high peak negative pressures (>10 MPa) to induce controlled inertial acoustic cavitation at a known focal zone (‘bubble cloud’) [Citation12]. The bubble cloud, ∼4 mm × 4 mm × 8 mm in this trial, produces complete mechanical cellular destruction at the focal point [Citation12]. The therapy transducer contains a co-axially aligned diagnostic ultrasound probe allowing real-time tumor targeting, bubble cloud visualization, histotripsy monitoring, and immediate post-histotripsy verification. The therapy transducer is attached to a software-controlled micro-positioning system enabling fully automated treatment of a pre-planned ablation volume. Histotripsy requires coupling the therapy transducer to the patient's skin, with cooled, degassed water that is held in a drape (Ioban, 3 M, St. Paul, MN) attached to the skin surface.
Each histotripsy treatment, performed under general anesthesia, consisted of lesion localization using a free-hand diagnostic ultrasound, marking the skin overlying the treatment window, application of a water bath on the skin for acoustic coupling, then placement of the therapy transducer into the water bath. The micro-positioner was used to center the target within the planned histotripsy treatment volume prior to histotripsy and to evaluate the edges of the planned volume to ensure complete treatment coverage of the tumor with a margin. Margins were determined by the investigator per site standard of care; 5 mm margins were used for hepatocellular carcinoma (HCC) and 1 cm for all others.
Following planning, ramped test pulses were placed at fixed locations in the planned histotripsy volume (center, superior, inferior, anterior, posterior, left, and right) to determine the cavitation threshold, i.e., the minimum energy delivery needed for bubble cloud formation. Subsequently, automated complete tissue destruction was performed by the micro-positioner moving the therapy transducer continuously to position the bubble cloud within the pre-planned treatment volume. Throughout each histotripsy procedure, the operators were able to visualize the bubble cloud to ensure proper energy delivery and correct location. The procedures were performed under general anesthesia with fixed tidal volume to minimize liver motion. During targeting, prior to treatment, the variation in movement of the liver was included in the treatment area to ensure full coverage including the indicated. The therapy transducer and water bath were removed upon completion of the histotripsy, the patient was extubated, recovered in a post-anesthesia care unit for approximately 1 h and then admitted for observation in the hospital.
Outcomes
The primary endpoint of the study was the determination of the acute technical success of a single treatment using the VORTX Rx® histotripsy device, as assessed on magnetic resonance (MR) imaging using a 1.5 T MR scanner with maximum slice thickness of 5 mm one day post procedure. Determination of acute technical success was based on the ability to create an ablation zone as visualized on MR imaging 1-day post-procedure that correlated well with the pre-defined planned ablation volume (PAV), a three-dimensional volume, determined by the investigator in the planning phase of the software workflow [Citation16].
The size and shape of the PAV are dependent on the dimensions of user-defined contours (target and margin). The diameter within the rendered volume ‘d’ represented the diameter of an inscribed sphere, the largest diameter that is completely contained within the PAV. Using 1-day post-ablation MRI images of the ablation zone, three diameters were measured by the central reader: x = orthogonal anterior-posterior (AP) in the axial plane; y = transverse measurement in the axial plan (TRV); and z = cranio-caudal measurement made in the coronal plane (CC). All measurements were performed in the portal venous phase of the MR imaging protocol [Citation17]. The Primary Endpoint was met if the minimum diameter of the actual ablation zone was greater than or equal to ‘d minus 5 mm’ (to take into consideration the MRI slice thickness and measurement error).
Prespecified secondary endpoints included:
Assessment of the safety profile using the National Cancer Institute Common Terminology Criteria for Adverse Events NCI CTCAE v4.0 [Citation18]. All adverse events for the duration of the trial were recorded with assessments occurring post-procedure and at 1-day, 1-week, 1-, and 2-months. Preexisting conditions were not reported unless exacerbated and laboratory abnormalities and abnormal vital signs were only considered an adverse event (AE) if they were: clinically significant in the investigator’s judgment, induced clinical signs or symptoms, or required medical intervention or a change in concomitant therapy. All serious (per ISO 14155) AEs were reviewed by an independent Data Safety Monitoring Board (DSMB) to determine device-relatedness (i.e., related to the histotripsy system). The safety profile of the histotripsy device was assessed based on all AEs that were probably or definitely related to the device. As per the clinical study protocol, a favorable safety profile was defined as the absence of any major bleeding requiring transfusion within 48 hours of the treatment, visceral perforation due to the device, a major bile duct injury or death directly resulting from the device.
Assessment of local tumor progression (LTP) and the involution of the ablation zone by MRI at 1-week, 1- and 2-months, post-procedure [Citation19].
Assessment of liver function (liver function tests) at 1-week, 1- and 2-months post-procedure.
Assessment of a possible immune response to the treatment (including: CD3+, CD4+, CD8+, CD45+, CD16+, CD56+ and CD19+, C-reactive protein [CRP], complement C3, C4 and CH50, immunoglobulins [IgG, IgM, IgA], interleukin-6 [IL-6]) at 1-week, 1- and 2-months post-procedure.
Evaluation of quality of life by using Quality of Life scores assessed at baseline, 1-, and 2-months post-procedure using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 scores [Citation20].
Assessment of pain and analgesic requirements post- procedure using a 100 mm visual analog scale (VAS) (where 0 means ‘no pain’ and 100 ‘the maximum pain possible’) at 24 hours and 1-week post-procedure.
All imaging assessments including the primary endpoint, LTP, and involution of the histotripsy zone were conducted by an independent central reader radiologist (Department of Radiology at the University of Wisconsin School of Medicine and Public Health, Wisconsin, USA). The endpoints were revised to account for the protocol change to permit multiple histotripsies within a patient (see eTable2, Supplement 3).
Additionally, a post hoc analysis was performed using quantitative European Association for the Study of Liver (qEASL) to measure non-histotripsy treated tumor response at 1-day, 1-week, 1-, and 2-months and was performed using semi-automated 3D tumor segmentation application (Multi-Modality Tumor Tracking application, IntelliSpace Portal version 12; Philips Healthcare, Haifa, Israel) as described in detail previously [Citation21–25]. The accuracy and reader-independent reproducibility of the semiautomatic tumor segmentation software has been confirmed previously [Citation23]. The whole tumor volume was calculated from the segmentation, and a cubic reference region of interest (ROI) comprising 1 cm3 of the healthy/normal appearing liver parenchyma was manually selected as a reference for normalization to calculate the relative enhancement within the tumor after which qEASL proprietary algorithm was applied on the 3D tumor volume. The qEASL analysis was performed by two independent readers neither of whom participated in the histotripsy and the mean values from two readers were used in analysis.
Statistical analysis
Given this was the first in-human trial of the VORTX Rx investigational device for the treatment of liver tumors, a feasibility design was chosen. Since no comparator group exists and the sample size calculation could not be based on previously published data as this was the first in human study, a single arm design with a sample size of up to 10 patients was chosen. A DSMB was responsible for halting the trial or making recommendations regarding protocol revisions based on its ongoing assessment of the AEs and device malfunctions during the trial.
Patients who underwent histotripsy with the investigational device were included in the analysis. Unless otherwise specified, quantitative variables were described with median and interquartile range (IQR) and qualitative variables were described using absolute frequencies (N, %) using only the population without missing data. Analysis of parameter evolution were presented descriptively as mean and standard deviation (SD); additionally, analysis was conducted via a general linear model. Inferential analysis of ablation zone volume was also conducted by paired t-test or Wilcoxon test based on the variable distribution. A two-sided p-value of <.05 was considered significant.
Results
Study population
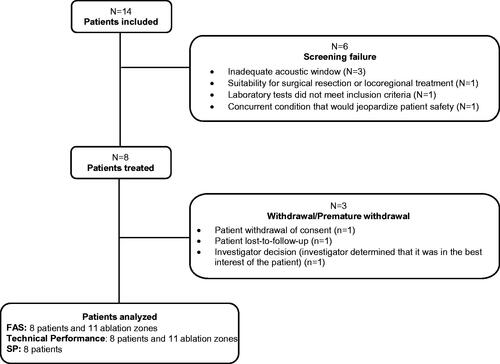
Eight patients were ultimately enrolled and treated out of 14 patients initially screened (five females, three males) (median age 60.4; IQR 52.6–74.7, 25% ECOG 0, 75% ECOG 1) (). In total, eleven tumors (median 1.4 cm, range 0.9 to 1.6 cm) were targeted in these 8 patients: 7 colorectal metastases (CRM) in 5 patients, 2 metastatic tumors in one patient with cholangiocarcinoma, and one metastatic tumor in one patient with breast cancer and one patient with one primary HCC tumor (see detailed patient and tumor characteristics in , eTables 3 and 4, Supplement 3). Three patients withdrew from the study including one who was lost to follow-up ().
Table 1. Patient and tumor characteristics.
Primary endpoint
The study met its primary endpoint of acute technical success in all 11 tumors treated with histotripsy. The median minimum measured diameter (d) of each treatment with histotripsy was 2.4 cm (IQR 2.2–2.6) as measured on MRI 24 h post treatment. The median difference between the planned ablation zone volume (PAV) and final ablation zone volume was 3.2 mm3 (IQR 1.6–8.2); this value supports the finding that the treatment was not excessive. Additional technical parameters are described in .
Table 2. Histotripsy ablation procedure.
Secondary endpoints
There were no adverse events determined to be probably or definitely device-related, giving the histotripsy procedure an excellent safety profile. Although there were 2 serious adverse events classified as CTCAE grade 3, neither of these events were deemed related to the device or procedure (one had a dental abscess 32 days post treatment and the other hypocalcemia from Crohn’s disease that required hospitalization 22 days post treatment). All other non-hepatic AEs were grade 1–2 and resolved fully within 1 week (see eTable 10, Supplement 3).
Liver function elevations were observed in all patients and expected given histotripsy’s mechanism of action that completely destroys hepatocytes. Indeed, 4 patients developed grade 3 procedure-related transaminase elevation (greater than 5 times the upper limits of normal; 3 patients AST, 1 patient ALT) but the elevations were transient and returned to baseline by 1-week post-procedure. Other patients experienced grade 1–2 transient elevations in AST and ALT 24 h post histotripsy, but they returned to baseline by the 1-week timepoint assessment and remained within normal limits for the remainder of the trial. The remaining components of the liver function tests (GGT, Alkaline phosphatase, total bilirubin, Albumin, Prothrombin, and INR) did not significantly vary from baseline at any of the follow-up timepoints (eTable 7, Supplement 3). The independent central reader radiologist identified one perfusion defect and one small portal vein thrombosis adjacent to the treatment area; both were deemed clinically insignificant and therefore not considered an adverse event by the site according to the study protocol.
Patients who had untreated residual disease did not experience any unanticipated increase in tumor growth, nor were new tumors identified during the follow-up period. At 1-week and 1-month post-procedure, LTP occurred at two histotripsy sites (2/10 lesions, 20%). One of the progressing tumors was mistargeted due to poor ultrasound visualization requiring targeting using vascular landmarks. This resulted in part of the tumor left inadvertently outside the ablation zone. Growth of an adjacent untreated tumor in another patient made it impossible to distinguish local progression from adjacent tumor ingrowth and was classified as LTP. No further LTP was noted out to 2-months ().
Figure 2. Representative qEASL image. Contrast-enhanced MRIs obtained at baseline (A) and 24 hours (B), 1 week (C), 1 month (D), and 2 months (E) posttreatment in a patient with diffuse colorectal liver metastases who had undergone multiple types of therapy including surgical resection and several lines of systemic chemotherapy. A small lesion in segment 2 measuring 1 cm in diameter was treated with histotripsy. The three-dimensional measurement known as qEASL (quantifiable European Association for the Study of Liver) was performed using a semiautomatic tumor segmentation software (Philips Healthcare). The qEASL algorithm quantified the tumor diameter, absolute tumor volume, and enhancing tumor volume as absolute numbers as well as the percentage of the enhancing tumor volume. In a previous radiology-histopathology study, excellent correlation between the appearance on MR imaging and the findings at pathology both in terms of tumor viability (enhancing on MRI) and necrosis (lack of enhancement on MRI) confirmed the usefulness of qEASL mapping to assess tumor response [Citation22,Citation23].
![Figure 2. Representative qEASL image. Contrast-enhanced MRIs obtained at baseline (A) and 24 hours (B), 1 week (C), 1 month (D), and 2 months (E) posttreatment in a patient with diffuse colorectal liver metastases who had undergone multiple types of therapy including surgical resection and several lines of systemic chemotherapy. A small lesion in segment 2 measuring 1 cm in diameter was treated with histotripsy. The three-dimensional measurement known as qEASL (quantifiable European Association for the Study of Liver) was performed using a semiautomatic tumor segmentation software (Philips Healthcare). The qEASL algorithm quantified the tumor diameter, absolute tumor volume, and enhancing tumor volume as absolute numbers as well as the percentage of the enhancing tumor volume. In a previous radiology-histopathology study, excellent correlation between the appearance on MR imaging and the findings at pathology both in terms of tumor viability (enhancing on MRI) and necrosis (lack of enhancement on MRI) confirmed the usefulness of qEASL mapping to assess tumor response [Citation22,Citation23].](/cms/asset/bd32d144-7a05-48a8-a6a7-4e8d7a824aec/ihyt_a_2112309_f0002_c.jpg)
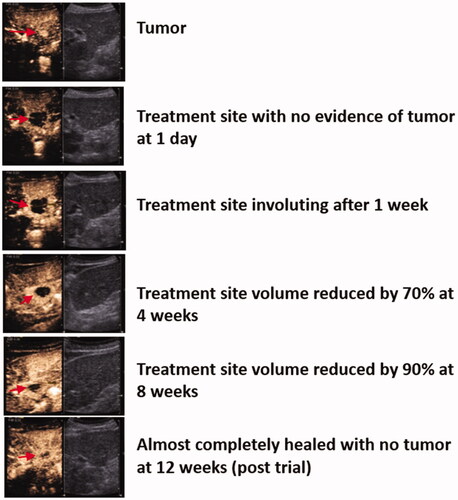
Post-ablation involution () did not show a statistically significant reduction in lesions using the general linear model (N = 6) at 24-h (10.6 ± 6.3 cm3) versus 1-week (8.5 ± 9.6 cm3), 1-month (5.9 ± 10.0 cm3) and 2-months (4.8 ± 9.4 cm3) (p = .06) since only six lesions were available for analysis. Using the appropriate statistical analysis for the small sample size (n = 10), the ablation zone had a statistically significantly reduction after 1 week (15.4 vs. 12.1 cm3; p = .040) and 1 month (15.4 vs. 7.7 cm3; p = .019), respectively (eTable 5, Supplement 3).
Figure 3. Involution of treatment zone. Tumor and treatment zone (red arrow) at each time point on contrast-enhanced ultrasound.
Immunological parameters are reported in eTable 6, Supplement 3; There were no statistical differences noted in immunologic parameters measured (eTable 7, Supplement 3).
The mean global score (SD) of the QLQ-C30 questionnaire was 67.7 ± 12.9 (N = 8) at baseline, 61.1 ± 22.2 (N = 6) at one month, and 65.0 ± 25.3 (N = 5) at two months (eTable 8, Supplement 3); there were no significant differences in any dimension (eTable 9, Supplement 3).
Median pain scores on a 100-point scale were 0 (N = 8, IQR 1–10) 24 h post-histotripsy and 30.0 (N = 7, IQR 0–40) 1-week post-histotripsy. Five of eight patients required non-narcotic pain medication in the first week (paracetamol and/or dexketoprofen), but no patient required narcotic pain medications.
Post hoc analyses
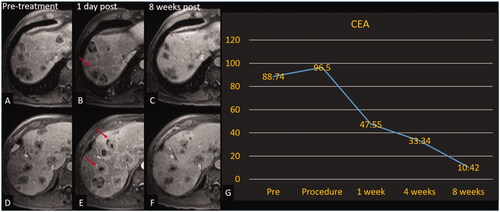
A post hoc descriptive assessment was performed on patients who had untreated residual disease. Two of seven patients (one patient lost-to follow-up excluded in calculation) (28.6%) had positive effects recorded on non-treated tumors. A patient with multifocal HCC experienced stable non-targeted disease at each follow-up time point and a decline in alpha fetoprotein (AFP) beginning one week following the procedure through the 8-week follow-up time point. The second patient had one of many colorectal metastases treated. After an initial increase in size and enhancement of non-target tumors at one day, there was a continuous decrease in non-target tumor diameters and a reduction of carcinoembryonic antigen (CEA) beginning one-week post-treatment through the eight-week follow-up period (). During this period the patient did not receive chemotherapy, immunotherapy or any locoregional intervention. This reaction around off-target tumors could indicate a systemic anti-tumor response that warrants further exploration.
Figure 4. Histotripsy of CRLM with decreasing CEA. Patient with colorectal cancer with involution of the non-treated tumors. Treated tumor is not shown. Axial contrast-enhanced MR images obtained at 2 different slices; pretreatment (A, D), 1-day post treatment (B, E), and 8-week post-treatment (C, F). Red arrows (B, E) denote tumors with increased peripheral enhancement (reaction around off-target tumors that could indicate a systemic anti-tumor response) compared with pretreatment images. (G) Graph of CEA over the course of the trial for this patient.
The qEASL analysis demonstrated consistent results to the post-treatment MRI analysis. A representative qEASL color map () is overlaid on the subtracted MRI, showing the enhancing portions of the tumor in red/yellow before treatment and complete lack of enhancement after treatment consistent with a complete response to histotripsy despite the seemingly increase in size of the treated lesion which is typical of locoregional therapy creating a margin of tissue destruction.
Discussion
This first in-human feasibility trial demonstrates the technical effectiveness and excellent safety profile of histotripsy in patients with advanced primary and secondary liver cancer, confirming the pre-clinical data. The histotripsy device performed consistently and reliably in destroying all targeted liver tumors precisely and without liver toxicities or other significant adverse events. Although this clinical trial was limited to eight patients, the results generated by a totally noninvasive focused ultrasound technology are impressive and warrant further evaluation in larger patient cohorts.
Unlike all other locoregional therapies, histotripsy is unique because it is simultaneously non-thermal, non-ionizing, and noninvasive, properties that are helpful when treating tumors. The lack of thermal energy deposition, for example, reduces the risk of injuries to the body wall and adjacent viscera or structures. Conversely, cooling from vascular structures (heat sink) that has plagued thermal ablative technologies does not apply in the case of histotripsy since the mechanism underlying its destructive power in tissue is purely mechanical.
Another advantage of histotripsy is that the treatment effect can be monitored real-time under ultrasound. This ensures that the target is indeed treated appropriately while minimizing if not eliminating the risk of injury to adjacent tissue. This is precisely what we were able to demonstrate in this limited human study where histotripsy destroyed tissue thoroughly in a predictable manner since the zone of tissue destruction correlated well with the prescribed or planned destruction zone. In that regard, the results of this first human study matched the results published in multiple pre-clinical studies where 100% technical success was achieved [Citation12–15,Citation26].
The accuracy of the device in destroying a volume consistent with what the physician intended (i.e., PAV) is documented with 100% accuracy in this study. However, being able to identify the target with 100% accuracy is limited by the use of ultrasound since many tumors are not visible with this modality. In the present study, even though the targeted area of one lesion was treated appropriately, the complete elimination of the tumor was missed because it was not clearly visible by ultrasound and required use of landmarks for the targeting. This clearly represents an area for future improvement by using fusion technologies and/or cone-beam CT to enhance lesion identification.
The constant monitoring of the destructive process of histotripsy from ultrasound also added to the safety profile of this technology as demonstrated in this study. No serious adverse events that could be attributed to the procedure took place a remarkable finding given that the patient population treated had advanced disease, been subjected to multiple lines of therapy and had no further therapeutic options.
Finally, a surprising finding in our trial was the positive off-target effects observed in two patients. Although, the possibility of an enhanced immunological response from histotripsy has already been reported [Citation27–32], it is noteworthy that both patients, who had been heavily treated with multiple lines of chemotherapy (study exclusion required discontinuation of chemotherapy for at least two weeks and immunotherapy at least four weeks prior to histotripsy treatment), experienced a decrease in the size of untreated liver tumors and a commensurate decline in tumor markers after histotripsy was performed on a single, small liver tumor. A recent rodent implanted liver tumor model presented evidence that complete tumor regression was observed in 9/11 (81%) by only partially histotripsying the implanted tumors with no evidence of local recurrence of metastasis up to 12-week post-treatment as compared to 0/11 (0%) in the non histotripsied control animals (all of which experienced local tumor progression and intra-hepatic metastases) [Citation33]. These findings of a possible abscopal effect remain unproven at the present time, but warrants further exploration in the clinical setting.
Our trial has several limitations. The patient population enrolled was heterogeneous and included various types of malignancies. Different types of solid malignancies may respond differently to histotripsy. Enrolled patients were in the end stages of their disease and had undergone multiple therapies prior to histotripsy. This patient population is standard for a first-in-human trial but limits any conclusions, particularly regarding long-term effectiveness and the interaction with other therapies. Nevertheless, the absence of serious safety issues is encouraging given the advanced stage of disease in the patient population. The trial did not evaluate the potential impact of underlying cirrhosis, steatosis, post-chemotherapy fibrosis, and acute chemotherapy-induced inflammation on the efficacy of histotripsy; however, the pre-determined targeted tissue volume was successfully treated in all cases, as reflected by 100% technical success. The device used for the trial was an investigational prototype device that is not intended for broad commercial clinical use, but improved histotripsy devices are currently in development. Finally, the trial had a short follow-up period because of the necessity to evaluate the technology in patients who were end stage. Consequently, there were limitations in the ability to assess the durability of histotripsy, local recurrence rate, or disease-free survival.
Conclusions
In summary, this first-in-human trial demonstrates that hepatic histotripsy can effectively destroy a targeted tissue volume with no device-related adverse events in a small number of patients. Given histotripsy’s novel, noninvasive approach, more extensive multicenter clinical trials validating these results are warranted.
Supplemental Material
Download PDF (2.6 MB)Disclosure statements
Dr. J. Vidal-Jove is consultant for Advanced Microbubbles Inc., for Chongqing Haifu Medical Technology Co. Ltd., and for Histosonics Inc.
Dr. Serres holds no competing interests.
Eli Vlaisavljevich is a former employee, shareholder, and consultant for HistoSonics, Inc.
Jon Cannata is an employee and shareholder of HistoSonics, Inc.
Alex Duryea is an employee and shareholder of HistoSonics, Inc.
Ryan Miller is an employee and shareholder of HistoSonics, Inc.
Dr. Merino holds no competing interests.
Dr. Velat holds no competing interests.
Yossi Kam is an employee of Philips Healthcare, ICAP-EDI.
Ryan Bolduan is an employee and shareholder of HistoSonics, Inc.
Dr Amaral is an employee and shareholder of HistoSonics, Inc and of Venture Investors, a Venture Capital firm supporting Histosonics.
Dr. Hall is a consultant for and shareholder of HistoSonics, Inc.
Dr. Xu is a consultant for, is a shareholder of, and has research support from HistoSonics, Inc.
Dr. Lee is a consultant and has research support from Ethicon, Inc., has patents and royalties with Medtronic, Inc., and research support, stock options, and a board of directors role for Histosonics, Inc.
Dr. Ziemlewicz is a consultant and receives research support from Neuwave Medical and is a shareholder and receives research support from Histosonics.
Additional information
Funding
References
- Bruix J, Sherman M. American association for the study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Benson AB, D’Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;17(4):302–310.
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369.
- Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw. 2018;16(6):693–702.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Livraghi T, Meloni F, Solbiati L. et al. Collaborative Italian group using as. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35(4):868–874.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2007;47(1):82–89.
- Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61(7):1100–1101.
- Xu Z, Hall TL, Vlaisavljevich E, et al. Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int J Hyperthermia. 2021;38(1):561–575.
- Smolock AR, Cristescu MM, Vlaisavljevich E, et al. Robotically assisted sonic therapy as a non-invasive nonthermal ablation modality: proof of concept in a porcine liver model. Radiology. 2018;287(2):485–493.
- Worlikar T, Vlaisavljevich E, Gerhardson T, et al. Histotripsy for non-invasive ablation of hepatocellular carcinoma (HCC) tumor in a subcutaneous xenograft murine model. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:6064–6067.
- Vlaisavljevich E, Owens G, Lundt J, et al. Noninvasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model. Ultrasound Med Biol. 2017;43(6):1237–1251.
- Longo KC, Knott EA, Watson RF, et al. Robotically Assisted Sonic Therapy (RAST) for noninvasive hepatic ablation in a porcine model: Mitigation of body wall damage with a modified pulse sequence. Cardiovasc Intervent Radiol. 2019;42(7):1016–1023.
- Coenegrachts K. Magnetic resonance imaging of the liver: new imaging strategies for evaluating focal liver lesions. World J Radiol. 2009;1(1):72–85.
- Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. May 28, 2009 (v4.03: June 14, 2010.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Arraras JI, Arias F, Tejedor M, et al. The EORTC QLQ-C30 (version 3.0) quality of life questionnaire: validation study for Spain with head and neck cancer patients. Psychooncology. 2002;11(3):249–256.
- Tacher V, Lin M, Duran R, et al. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. 2016;278(1):275–284.
- Chapiro J, Wood LD, Lin M, et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273(3):746–758.
- Duran R, Chapiro J, Frangakis C, et al. Uveal melanoma metastatic to the liver: the role of quantitative volumetric Contrast-Enhanced MR imaging in the assessment of early tumor response after transarterial chemoembolization. Transl Oncol. 2014;7(4):447–45523.
- Sahu S, Schernthaner R, Ardon R, et al. Imaging biomarkers of tumor response in neuroendocrine liver metastases treated with transarterial chemoembolization: can enhancing tumor burden of the whole liver help predict patient survival? Radiology. 2017;283(3):883–894.
- Zhao Y, Duran R, Bai W, et al. Which criteria applied in multi-phasic CT can predict early tumor response in patients with hepatocellular carcinoma treated using conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc Intervent Radiol. 2018;41(3):433–442.
- Schuster TG, Wei JT, Hendlin K, et al. Histotripsy treatment of benign prostatic enlargement using the Vortx Rx system: initial human safety and efficacy outcomes. Urology. 2018;114:184–187.
- Qu S, Worlikar T, Felsted AE, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer. 2020;8(1):e000200.
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26(305):234–241.
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322.
- Sugimoto K, Kakimi K, Takeuchi H, et al. Irreversible electroporation versus radiofrequency ablation: comparison of systemic immune responses in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2019;30(6):845–853 e6.
- Pandit H, Hong YK, Li Y, et al. Evaluating the regulatory immunomodulation effect of irreversible electroporation (IRE) in pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26(3):800–806.
- Hendricks-Wenger A, Sereno J, Gannon J, et al. Histotripsy ablation alters the tumor microenvironment and promotes immune system activation in a subcutaneous model of pancreatic cancer. IEEE Trans Ultrason Ferroelect Freq Contr. 2021;68(9):2987–3000.
- Worlikar T, Zhang M, Ganguly A, et al. Impact of histotripsy on development of intrahepatic metastases in a rodent liver tumor model. Cancers. 2022;14(7):1612.