?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
To evaluate the long-term survival benefits of high intensity focused ultrasound (HIFU) ablation in patients with hepatocellular carcinoma (HCC) combined with portal vein tumor thrombus (PVTT).
Methods
The data of patients with HCC-PVTT treated with HIFU from January 2014 to December 2019 were retrospectively analyzed. All patients received HIFU ablation for both PVTT and liver tumor in one session. Perioperative adverse events (AEs) were recorded, and follow-up was performed postoperatively. The Kaplan–Meier method was used for survival analysis.
Results
Median follow-up was 13.75 ± 1.31 months. A total of 144 patients (male/female: 122/22, age: 54.15 ± 11.84 years old) were included in the study. A total of 267 liver tumors (tumor number: 1.87 ± 1.65, range 1–10) were treated with HIFU. The mean ± SD diameter of viable liver tumors was 100.98 ± 61.65 mm. The reported postoperative AEs of HIFU were skin edema (93.75%), local pain (69.44%) and fever (7.64%). There was no liver failure, gastrointestinal bleeding or perioperative death. The median overall survival (OS) time was 14 months, while the cumulative survival rates of 0.5, 1, 2 and 3 years were 79.0%, 58.6%, 33.3% and 5.9%, respectively. The median OS of PVTT types I, II and III was 22, 13 and 14 months, respectively, and the difference was not statistically significant (p > 0.05).
Conclusion
HIFU is a minimally invasive method for HCC-PVTT with fewer complications, which could prolong the OS. Patients with PVTT type III could benefit more from HIFU, compared to types I and II.
Background
Primary liver cancer primarily hepatocellular carcinoma (HCC), has high morbidity and mortality rates all over the world, especially in East Asia. In China, it is the fourth most common type of cancer in newly diagnosed cases and the third leading cause of cancer-related mortality [Citation1].
Portal vein tumor thrombus (PVTT) is a frequent and mortal complication of HCC. The mean overall survival (OS) time of patients with HCC-PVTT is only 2.7 months without therapy [Citation2–4]. These patients are prone to rapid disease progression, intrahepatic metastasis within a short time interval, portal hypertension, jaundice, peritoneal effusion and other symptoms, causing serious impact on the OS and quality of life [Citation2].
HCC-PVTT is classified as stage C, according to the Barcelona Liver Cancer Staging System (BCLC), and stage III per the China Liver Cancer Staging System (CNLC) [Citation5,Citation6]. The Expert Consensus of Liver Cancer with Portal Vein Thrombus in China (2018) recommends the adaptation of a multidisciplinary therapy plan for patients with HCC-PVTT who have no opportunity for radical surgical resection [Citation2]. This plan includes palliative surgery, interventional embolization, targeted therapy, radiotherapy, chemotherapy, local ablation, immunotherapy, etc.
High intensity focused ultrasound (HIFU) ablation is reported to be a safe and effective treatment for liver cancer [Citation7–10] and is recommended by the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (China, 2019 version) [Citation2]. It has also been reported that HIFU is safe and effective for HCC-PVTT [Citation11–14]. However, the follow-up period of these studies so far is less than 3 years. Therefore, we do this study to investigate the long-term survival benefits of HIFU therapy in patients with HCC complicated with PVTT.
Materials and methods
Data
Patients, ethics approval and consent to participate
HCC patients with PVTT received HIFU treatment from 1 January 2014 to 31 December 2019 were included in this study, which was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China) (538-2020) and performed in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients prior to the study.
Classification standard of PVTT (Cheng’s standard) [Citation15]
Type I0: tumor thrombus formation is found under microscope.
Type I: tumor thrombus involving segmental branches of portal vein or above.
Type II: tumor thrombus involving the left and right branches of portal vein.
Type III: tumor thrombus involving the main portal vein.
Type IV: tumor thrombus involving the superior mesenteric vein.
Inclusion criteria and exclusion criteria
Inclusion criteria: (1) HCC diagnosed pathologically or clinically, clinical diagnosis is one of the important diagnostic methods for primary liver cancer, which is recommended by the Standardization for Diagnosis and Treatment of Primary Hepatic Carcinoma (2019 Edition). This guideline recommends a clinical diagnostic roadmap for HCC, including HCC risk factors, imaging features and serological molecular markers (AFP, PIVKA II, etc.) [Citation6]. (2) PVTT diagnosed by enhanced MR or CT or contrast-enhanced ultrasound. (3) Child-Pugh A/B, PS score 0–2 points. (4) At least one measurable tumor. (5) Technical indications for HIFU treatment: lesions with 1 cm or over in diameter can be ablated, while visualized by localization, image fusion and real-time evaluation of HIFU treatment device system [Citation16].
Exclusion criteria: (1) PVTT type IV. (2) Child-Pugh C, PS score 3. (3) Contraindications for anesthesia and HIFU treatment, such as active bleeding, severe cardiovascular and cerebrovascular diseases, acute infection, severe pulmonary dysfunction, etc. (4) Patients living abroad for a long time. (5) HIFU device limitations: The lesion located in a position cannot be reached by the focus of the ultrasound transducer; No safe or effective ultrasound pathway for HIFU treatment due to the occlusion of bony structures or the most important organs; the lesion cannot be effectively covered by the focal field of the HIFU device [Citation16].
HIFU treatment
HIFU device
Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) is equipped with a diagnostic ultrasound probe and a therapeutic transducer. The diagnostic ultrasound probe (3.5–5.0 MHz) enables real-time ultrasound imaging to determine lesion location and monitor the entire treatment process during HIFU treatment. The transducer parameters (100–300 mm in diameter, 100–250 mm in focal length, 0.5–2 MHz in frequency, 1000–20,000 W/cm2 in focusing peak intensity) are key for the conversion of electric energy into ultrasonic energy and achieving ultrasonic focusing. The focal area is an ellipsoid (3 × 8 mm). The aforementioned parameters have been described previously [Citation17,Citation18].
HIFU operation procedure
Preoperative plans of HIFU treatment should include intrahepatic tumor lesions and PVTT. Some patients need to follow a divided treatment plan or a partial ablation plan for intrahepatic lesions, e.g., when the diameter of intrahepatic tumor lesions is large (>15 cm), the edge of the lesions is too close to the cavity organs (such as stomach, intestine and gallbladder) (<0.5 mm), or when the lesion is complicated with severe cirrhosis or improved Child-Pugh grade B liver function after medical treatment. HIFU treatment (treatment power is less than 400 W, lasting for 2–3 s, interval of 3 s) is performed mainly by spot scanning supplemented with line scanning. The ultrasonic probe, equipped with the HIFU apparatus, is used to monitor the effect and safety of HIFU treatment in a real-time. The end of treatment is marked by mass gray-scale changes in the target area that cover the whole lesion. In absence of mass gray-scale changes during HIFU, the end of treatment is indicated by the lack of microbubble filling with sulfur hexafluoride microbubble (SonoVue, Bracco International B.V., J20180005, Gorizia, Italy) contrast-enhanced ultrasound (CEUS) in the lesions.
Other therapies
Local therapeutics
TACE/TAE and 125I seed implantation are often used as adjuvant treatments to HIFU ablation. Their efficacy against HCC-PVTT has been reported by many researchers [Citation2,Citation19–21]. TACE/TAE should be performed by interventional specialists 1–4 weeks before HIFU to treat intrahepatic neoplasms. 125I seed implantation can be performed by HIFU doctors in collaboration with nuclear medicine doctors 0–2 days before HIFU ablation, which is mainly for treating portal vein cancer thrombus.
Systemic therapy
If there are no contraindications, HBsAg positive patients are treated with antiviral drugs (entecavir or tenofovir) [Citation6,Citation22]. Targeted therapy is possible with oral Sorafenib (400 mg, bid), or lenvatinib (8 m, weight <50 kg or 12 mg, weight >50 kg; qd). Pembrolizumab or nivolumab or camrelizumab can be used as an immunodrug (PD-1) of choice for immunotherapy (200 mg, iv, Q3W). Systemic therapy should be performed regularly and continuously until the onset of resistance, intolerable side effects, or until patient death [Citation6].
Follow-up and efficacy evaluation
Follow-up and efficacy evaluations were conducted by querying case records and telephone interviews. The number of viable liver tumor lesions and the maximum diameter of each lesion were measured and recorded. The sum of viable liver tumors lesions was calculated prior to the initiation of the entire treatment plan. The first follow-up was performed 2–4 weeks after HIFU ablation, and the perioperative survival status was recorded. If the patient had died, the cause of death was recorded. Patients were followed up with every 3 months to record their survival. Follow-up ended at the patient’s death or at the end of the follow-up period, i.e., 31 December 2019. Patients’ survival time (from the completion of HIFU ablation to the end of follow-up) and survival status at the end of follow-up were recorded.
Adverse events
Adverse events (AEs) within seven days after HIFU ablation were recorded. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0.
Adverse events: (1) skin toxicity immediately after HIFU ablation, including skin edema and burns. (2) Pain (NRS scores), 1–3 for mild pain, 4–6 for moderate pain, 7–10 for severe pain. (3) Hepatic failure. (4) Fever, including: low fever 37.3–38 °C, moderate fever 38.1–39 °C, high fever 39.1–41 °C, ultra-high fever >41 °C. (5) Bleeding, including perioperative gastrointestinal bleeding, tumor rupture bleeding and portal vein vascular rupture bleeding. (6) Perioperative death, the cause being recorded. (7) Others.
Statistical analysis
Statistical analysis was performed using IBM SPSS 25.0 (IBM, Armonk, NY). The measured data are expressed as and the rate (percentage) of counting data is expressed. Kaplan–Meier’s method was used for survival analysis, reverse Kaplan–Meier’s method was used for calculating median follow-up, and the log-rank test was used for differences between groups. p< 0.05 was considered statistically significant.
Results
Patient characteristics
Median follow-up was 13.75 ± 1.31 months. A total of 182 (182/623) HCC patients with PVTT received HIFU ablation during the study period. One hundred and forty-four out of all the patients met the inclusion criteria (male/female: 122/22). The flowchart of enrolled patients with the exclusion criteria is shown in . The mean age of the patients was 54.15 ± 11.84 years (27–85 years old). A total of 267 liver tumors were treated with HIFU ablation. The mean tumor number of the patients was 1.87 ± 1.65 (1–10), and the mean sum of the maximum diameter of viable liver tumors was 100.98 ± 61.65 mm (16.11–352.29 mm). More characteristics of the patient are shown in .
Table 1. Patient characteristics (n = 144).
HIFU ablation parameters
All patients received HIFU treatment for liver tumor lesions combined with coexisting PVTT. Except for eight patients (5.56%) who received HIFU treatments twice, all other patients were treated with HIFU once. The HIFU treatment parameters included: average power of 371.42 ± 55.00 W (range 100–450 W), mean sonication time of 1581.99 ± 1289.39 s (108–6149 s), and mean time of HIFU surgery is 122.71 ± 67.47 min, and total therapeutic energy of 594,505.35 ± 503,620.28 J (15,300–2,533,388 J). Intravenous anesthesia and general anesthesia were used in 11 (7.64%) and 133 (92.4%) patients, respectively.
Adverse events of HIFU
The main AEs after HIFU ablation were skin edema (93.75%) and pain (69.44%). Other rare AEs included fever (7.64%), abdominal distention (3.47%), skin burn (2.78%), peritonitis (1.39%), loss of appetite (1.39%), etc.; CTCAE ≤3 (5.0 version) (). No serious AEs, such as liver failure, bleeding, including perioperative gastrointestinal bleeding, tumor rupture bleeding, portal vein rupture bleeding, or death, were observed during the perioperative period. Among the four patients with skin burn, two patients with grade III skin burn were cured by local surgical debridement, and the other two did not require any intervention. Complications like fever, peritonitis, intractable hiccups, abdominal distention, loss of appetite, and acute kidney injury went away within 2 weeks after HIFU treatment. Pain and skin edema occurred in the HIFU ablation area. Except for severe pain, these patients did not require any special treatment and, in most cases, resolved spontaneously within 2 weeks after HIFU treatment.
Table 2. Perioperative adverse events of HIFU ablation.
Survival
Overall survival
The median OS (MOS) time of the 144 patients with HCC combined with PVTT was 14 months. The cumulative survival rates after 0.5, 1, 2 and 3 years were 79.0%, 58.6%, 33.3% and 5.9%, respectively. The mean OS time was 20.29 ± 2.75 (95%CI: 14.91–25.67) months ().
Table 3. Overall survival of PVTT and its subgroups.
Subgroup analysis
Subgroup of PVTT type
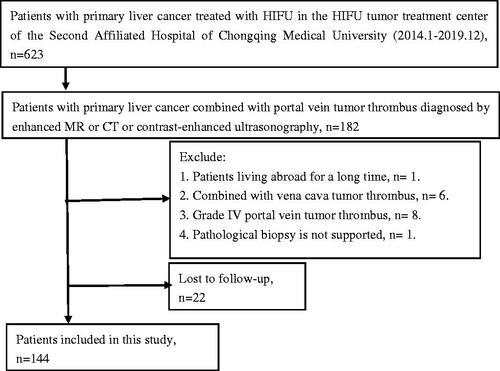
In the subgroup of PVTT type, the median subgroup OS was 22 months for type I, 13 months for type II and 14 months for type III. The mean OS and cumulative survival rates of 0.5, 1, 2 and 3 years are shown in . There was no significant difference in OS among subgroups of types I, II and III PVTT (p > 0.05) (). In another subgroup analysis, there were 103 cases of branch tumor thrombus (type I + II) and 41 cases of main tumor thrombus (type III). The MOS was 14.0 months in patients with branch tumor thrombus (type I + II). The cumulative survival rate is shown in . There was no significant difference in OS between the two subgroups (Log-rank test, χ2=2.43, p= 0.622) ().
Figure 2. (A) Kaplan–Meier’s curves show OS of PVTT type I, II and III. (B) Kaplan–Meier’s curves show OS of main (type III) and branch (type (I + II)) PVTT.
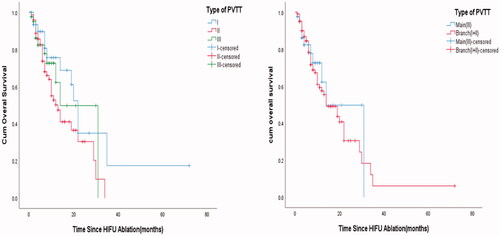
Table 4. OS and AEs of various treatment options for HCC complicated with PVTT.
Subgroup of other therapy
In the subgroup of targeted treatment and/or immunotherapy and non-targeted treatment nor immunotherapy, the mean and MOS were 21.92 ± 2.48 (95%CI: 17.06–26.78) and 22.0 months, respectively, in 31 patients (21.53%) receiving targeted treatment and/or immunization. In the non-targeted treatment nor immunotherapy group (113 patients, 78.47%), the mean and MOS were 19.23 ± 2.97 (95%CI: 13.41–25.06) and 14.0 months, respectively. There was no significant difference in OS between the two subgroups (log-rank test, χ2 = 3.799, p = 0.051) (). In the subgroup of TACE/TAE (n = 109, 75.69%) and non-TACE/TAE (n = 35, 24.31%), there was no significant difference in OS between the two subgroups (log-rank test, χ2=0.112, p = 0.738) (). However, the mean and median tumor sizes in the TACE/TAE group were 105.84 ± 5.20 (95%CI: 95.54–116.15) and 98.56 mm, respectively. In the non-TACE/TAE group, the mean and median tumor sizes were 85.82 ± 13.43 (95%CI: 58.52–113.11) and 54.23 mm, respectively. There were significant differences in tumor size between the two subgroups (nonparametric test, Z = 3.393, p= 0.001) (). In another subgroup of I25I seed-HIFU (n = 76, 52.78%) and non-125I seed-HIFU (n = 68, 47.22%), there was no significant difference in OS between the two subgroups (log-rank test, χ2=1.75, p= 0.186) ().
Figure 3. (A) Kaplan–Meier’s curves show OS of targeted and/or immunotherapy and non-targeted nor immunotherapy. (B) Kaplan–Meier’s curve shows the OS of TACE/TAE and non-TACE/TAE. (C) Boxplots show the tumor size of TACE/TAE and non-TACE/TAE. (D) Kaplan–Meier’s curve shows the OS of 125I seed-HIFU and non-125I seed-HIFU.
Discussion
There are 466,100 new diagnosed liver cancer cases and 422,100 cancer-related deaths each year in China [Citation1]. Compared to other hepatic blood vessels, the portal vein is more susceptible to tumor thrombus formed by HCC (44.0–62.2%) [Citation34]. Many researchers have found PVTT to be an independent risk factor for survival of HCC patients [Citation30,Citation31,Citation35,Citation36]. The treatment of HCC-PVTT is difficult and has a MOS of HCC-PVTT of only 2.7 months [Citation2–4] without therapy. Currently, there is no unified treatment scheme for patients. HCC-PVTT is classified as stage C BCLC in European and American countries, for which targeted therapy with sorafenib is the only recommended treatment [Citation5].
In the past 5 years, surgical treatment of liver cancer complicated with type I and type II PVTT has been recommended by consensus among Chinese experts, who believe that a radical cure is possible [Citation2]. Su et al. found that the MOS time of surgical resection for HCC-PVTT patients is 18 months (types I + II + III) [Citation15]. TACE and HAIC are commonly used in the non-operative treatment of HCC with PVTT, often in combination with other treatment regimens, such as sorafenib, apatinib, DEB, radiotherapy, 125I seed, RFA, MWA, etc., which have been studied by many researchers. In these studies, the MOS is reported to be 7.9–19 months [Citation19,Citation20,Citation23,Citation24,Citation26,Citation28,Citation30]. Radiotherapy is one of the therapeutic strategies for HCC-PVTT patients and can also be combined with other therapeutic options. Radiotherapy includes external radiotherapy (3DCRT, IMRT, SBRT) and internal radiotherapy (e.g., 125I seed implantation therapy and YI-90 microsphere therapy (TARE)). Some studies report a MOS of 9–13.3 months [Citation15,Citation25–27,Citation32,Citation33]. Systemic therapies, such as targeted therapy (sorafenib, lenvatinib, apatinib, etc.), PD-1 therapy (pembrolizumab, nivolumab, camrelizumab, etc.), chemotherapy and supportive therapy, are recommended for the treatment of HCC at stage BCLC-C and CNLC-III. The MOS has been reported as 7.2–14.8 months in some researches [Citation19,Citation29,Citation31].
Clinical studies have demonstrated good clinical effects of high-intensity focused ultrasound ablation in the treatment of both small and large liver cancer [Citation9–12,Citation18,Citation37]. Meta-analyses have shown that HIFU combined with TACE can safely improve the long-term survival rate [Citation7,Citation38,Citation39]. However, there are few studies on HIFU therapy for HCC-PVTT patients. For instance, Zhu et al. reported that PVTT were treated with HIFU [Citation8]. Subsequently, other researchers, with follow-up of 12–36 months, found that HIFU treatment of HCC-PVTT was safe and effective and could prolong the survival of patients [Citation12–14]. In this study, we conducted a 6-year retrospective study of HIFU therapy on 144 patients with HCC-PVTT whose mean OS was 20.29 ± 2.75 years (95%CI: 14.91–25.67), MOS was 14 months, and cumulative survival rates at 0.5, 1, 2 and 3 years were 79.0%, 58.6%, 33.3% and 5.9%, respectively. We found that these results are similar to those of other scholars, indicating that HCC patients with PVTT could benefit from HIFU treatment.
HBV infection is the main cause of primary liver cancer in China [Citation40], and antiviral therapy is the main treatment for HCC complicated with HBVsAg positive, as recommended by liver cancer treatment guidelines [Citation6,Citation22,Citation41]. In our study, 127 of the 144 patients (88.19%) were HBVsAg positive and received anti-HBV therapy.
In recent years, targeted therapy and PD-1 therapy have become the recommended systemic therapies for advanced liver cancer with reported clinical efficacy () [Citation20,Citation23,Citation29,Citation30]. In our study, only a small proportion of patients (31 patients, 21.53%) received targeted and/or immunotherapy. Only recently (2018), new antitumor drug therapy specifications were released in China [Citation42]. Since only some targeted or immunotherapy drugs were available for medical insurance reimbursement, some HCC-PVTT patients failed to receive any targeted therapy or treatment plan. In this study, subgroup analysis revealed a MOS time of 22 and 14 months in patients who received and did not receive targeted nor immunotherapy, respectively. The observed difference in OS between the two subgroups did not reach statistical significance (p= 0.051). Although there may be some bias due to the large difference in the number of cases between the two groups (31 vs. 113), we believe that patients can benefit more from combination therapy.
TACE is recommended for the treatment of HCC with PVTT. However, because the efficacy of TACE largely varies, experts suggest combining it with other therapies [Citation2,Citation43]. Some researchers reported that TACE-HIFU in the treatment of HCC with PVTT achieved good clinical effects without serious AEs [Citation12,Citation13,Citation44]. TACE is the first-choice therapy for patients with unresectable liver cancer, liver tumor lesions with abundant blood supply, or large lesions. TACE/TAE treatment before HIFU ablation can increase the therapeutic effect [Citation37]. HIFU alone can also achieve good ablation results in small lesions or tumors with poor blood supply [Citation45]. In our study, 109 patients (75.69%) were treated with TACE/TAE before HIFU. In the HIFU + TACE/TAE group, the average and median viable tumor diameters were 105.84 ± 5.20 and 98.56 mm, respectively. In the HIFU + non-TACE/TAE group, the mean and median tumor diameters were 85.82 ± 13.43 and 54.23 mm, respectively. The difference in the tumor size between the two subgroups was statistically significant (nonparametric test, Z = 3.393, p= 0.001), unlike the difference in OS (log-rank test, χ2=0.112, p= 0.738). The combination therapy achieved an equal survival benefit for patients with different tumor sizes.
Radioactive particle implantation is an alternative method for the local treatment of HCC and PVTT [Citation2,Citation6]. Some studies have reported that TACE/TAE+125I seed implantation has better clinical efficacy than TACE in the treatment of HCC complicated with PVTT [Citation21,Citation46]. Yang et al. reported 1st- and 2nd-year cumulative survival rates of 47.4% and 7.9% for HCC-PVTT patients treated by HIFU combined with 125I seed implantation, respectively, with a mean OS of 11.6 ± 3.0 months. The MOS of patients with types I, II and III PVTT was 13.5, 7.0 and 4.0 months, respectively. No serious complications, such as massive bleeding, tumor embolus shedding and acute liver failure, were observed during surgery or 72 h post-operation [Citation14]. In our study, a total of 76 patients (52.78%) received 125I seed therapy before HIFU, including 15 patients (48.39%) with type I PVTT, 38 patients (52.78%) with type II PVTT and 23 patients (56.10%) with type III PVTT. There was no significant difference between the HIFU+125I seed group and the HIFU group in OS (log-rank test, χ2=1.75, p= 0.186). Both subgroups had an equal OS benefit.
Most researchers believe that there is a significant difference between the degree of invasion and prognosis of PVTT, especially type III tumor thrombus invading the main portal vein [Citation15,Citation19]. Some researchers reported a MOS of 4.5–7.9 months for HCC with PVTT treated with 3D-CRT, hepatectomy, TACE and other treatments [Citation15,Citation19,Citation28,Citation29,Citation32]. Interestingly, in our retrospective study, it was found that the MOS of PVTT was 22, 13 and 14 months for types I, II and III, respectively. displays the mean survival time and cumulative survival rate at 0.5, 1, 2 and 3 years, indicating that the three types of PVTT had the same clinical benefit. In particular, HIFU therapy for HCC patients with type III PVTT achieved a good clinical benefit and prolonged survival time. Cheng et al. reported that the growth characteristics of PVTT are reverse flow centrifugal development, with an average growth rate of 0.5 ± 0.1 cm3/m and a monthly development length of 1.2 ± 0.4 cm [Citation47]. Zhang found that 3 months after treating PVTT with HIFU and 125I, the mean and median invasion distances of tumor thrombus were 5.94 and 0 mm, respectively [Citation48]. Importantly, the growth rate of PVTT decreased after treatment, which is beneficial to maintain liver function and blood circulation. This result supports the clinical benefits of HIFU and 125I treatment for type III HCC-PVTT patients.
Obviously, the adverse vascular events associated with HIFU ablation in PVTT patients have been the focus of attention. No HIFU-related blood vessel events have been revealed by morphology examinations (enhanced CT/MRI) or functional examination after HIFU ablation for liver and pancreatic cancer [Citation11,Citation49,Citation50]. Therefore, we believe that HIFU treatment of PVTT does not increase portal vein adverse vascular events. In our study, we found no HIFU-related portal vein AEs, such as portal vein rupture and hemorrhage or upper gastrointestinal bleeding associated with portal hypertension, which indicates that HIFU is safe for the treatment of HCC-PVTT patients.
In our retrospective study, postoperative AEs after HIFU treatment were mainly local toxic skin reactions in the treatment area, including skin edema (93.75%) and local pain (69.44%). The incidence of other AEs was low, and no AEs above CTCAE grade 4 occurred. This finding is similar to that reported by Illing et al. [Citation51]. According to the incidence of AEs of other treatment regimens, as shown in , the patients included in our study were older (>60 years old, 27.78%), with large liver tumor lesions (intrahepatic lesions >100 cm, 41.67%), accompanied by extrahepatic metastatic lesions (20.83%) (). However, these adverse factors did not lead to an increase in AEs after HIFU ablation has small AEs and can be used in combination with other therapies to achieve a good survival benefit for patients with HCC complicated with PVTT.
Despite promising results, this study has some shortcomings. Since it is a single-center retrospective study, the possibility of potential selection bias cannot be ruled out. Second, the long-term survival benefit of patients with HCC combined with PVTT treated by HIFU ablation was investigated without comparison to patients who did not receive HIFU ablation.
Conclusion
The comprehensive modality that combines high-intensity focused ultrasound ablation with systemic therapy is an effective treatment strategy with high safety and few complications, which could prolong the OS of patients with HCC and PVTT.
Author contributions
Conception and design of the project (BJ, ZH and KZ), performance of experiments, obtainment and analysis of data (XC, YhM, JZ, WY, CbJ, LfR), writing of the manuscript (XC), and critical revision of the manuscript (KZ). All authors read and approved the final manuscript.
Disclosure statement
The authors have no conflict of interest related to this publication.
Data availability statement
All data are available upon request.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Cheng SQ, Chen MS, Cai JQ, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 edition). Chin J Dig Surg. 2019;35(4):737–743.
- Li S, Wei W, Guo R, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol. 2013;30(4):696.
- Shu-Hong L, Zhi-Xing G, Cheng-Zuo X, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev. 2013;14(8):4759–4763.
- Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona Clinic Liver Cancer Staging System. World J Gastroenterol. 2015;21(36):10327–10335.
- Wu MC, Tang ZY, Liu YY, et al. Standardization for diagnosis and treatment of primary hepatic carcinoma (2019 edition). Chin J Pract Surg. 2020;40(2):121–138.
- Li Z, Mi DH, Yang KH, et al. TACE combined with HIFU for primary hepatic carcinomas: a meta-analysis. J Evid Based Med. 2013;13(5):292–299.
- Zhu H, Chen WZ, Li KQ, et al. Preliminary clinical observation on interventional oily chemoembolization guided by US combined with HIFU for treatment of embolization in portal vein. Chin J Ultrasound Med. 2004;20(1):77–79.
- Fukuda H, Numata K, Nozaki A, et al. Hyperecho in ultrasound images during high-intensity focused ultrasound ablation for hepatocellular carcinomas. Eur J Radiol. 2011;80(3):e571–e575.
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253(5):981–987.
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19(2):437–445.
- Ma R, Zhu H, Gong JP. Survival analysis of transcatheter arterial chemoembolization combined with high intensity-focused ultrasound in the treatment of liver cancer based on propensity score matching method. China Oncol. 2018;28(4):282–289.
- Cui L, Liu XX, Jiang Y, et al. Comparative study on transcatheter arterial chemoembolization, portal vein embolization and high intensity focused ultrasound sequential therapy for patients. Asian Pac J Cancer Prev. 2012;13(12):6257–6261.
- Yang X, Yuan GB, Wang YC, et al. Clinical value of 125I seeds implantation combined with high intensity focused ultrasound for the treatment of portal vein tumor thrombus from hepatocellular carcinoma. Chin J Nucl Med. 2015;35(3):182–185.
- Su F, Chen KH, Liang ZG, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2018;7(9):4387–4395.
- Expert committee for the formulation of technical specifications for clinical application of focused ultrasound ablation, Chinese Medical Doctor Association, Lang JH, Expert consensus on technical specifications for clinical application of focused ultrasound ablation (2020 edition). Natl Med J China. 2020;100(13):974–977.
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235(2):659–667.
- Huang L, Zhou K, Zhang J, et al. Efficacy and safety of high-intensity focused ultrasound ablation for hepatocellular carcinoma by changing the acoustic environment: microbubble contrast agent (SonoVue) and transcatheter arterial chemoembolization. Int J Hyperthermia. 2019;36(1):244–252.
- Tang Q, Huang W, Liang J, et al. Efficacy and safety of transarterial chemoembolization in elderly patients of advanced hepatocellular carcinoma with portal vein tumor thrombus: a retrospective study. Front Oncol. 2021;11:646410.
- Li Y, Li H, Hu H, et al. Efficacy and safety of transcatheter arterial chemoembolization combined with either (125)I seed implantation or apatinib in hepatocellular carcinoma with portal vein tumor thrombosis: a retrospective comparative study. J Cancer Res Ther. 2020;16(7):1691–1697.
- Zhang ZH, Zhang W, Gu JY, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: a propensity-score analysis. J Vasc Interv Radiol. 2018;29(8):1085–1093.
- Wang GQ, Duan ZP, Zhuang H, et al. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Chin J Hepatol. 2019;27(12):938–961.
- Cao Y, Sun T, Guo X, et al. Sorafenib versus apatinib both combined transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a comparative retrospective study. Front Oncol. 2021;11:673378.
- Zhang XX, Wang C, Zhao W, et al. Efficacy and safety of DEB-TACE combined with apatinib in the treatment of hepatocellular carcinoma with portal vein tumor thrombus. J Interv Radiol. 2021;30(3):282–287.
- Munire A, Tan Y, Wang HF, et al. Analysis of therapeutic effect and prognostic factors of IMRT combined with sorafenib in the treatment of hepatocellular carcinoma with portal vein thrombosis. Tianjin Med J. 2021;49(5):514–519.
- Ohkoshi-Yamada M, Kamimura K, Shibata O, et al. Efficacy and safety of the radiotherapy for liver cancer: assessment of local controllability and its role in multidisciplinary therapy. Cancers. 2020;12(10):2955.
- Li Z, Si G, Jiao DC, et al. Portal vein stenting combined with (125)I particle chain implantation followed by as(2)O(3) in the treatment of hepatocellular carcinoma with portal vein tumour thrombus. Biomed Res Int. 2020;2020:1–7.
- Liang J, Ge NJ, Guo XL, et al. Clinical analysis of percutaneous radiofrequency ablation combined with TACE in treatment of hepatocellular carcinoma with type III portal vein tumor thrombus. J Hepatobil Surg. 2020;28(5):345–348.
- Yuan G, Cheng X, Li Q, et al. Safety and efficacy of camrelizumab combined with apatinib for advanced hepatocellular carcinoma with portal vein tumor thrombus: a multicenter retrospective study. Onco Targets Ther. 2020;13:12683–12693.
- Ni JY, Sun HL, Luo JH, et al. Transarterial chemoembolization and sorafenib combined with microwave ablation for advanced primary hepatocellular carcinoma: a preliminary investigation of safety and efficacy. Cancer Manag Res. 2019;11:9939–9950.
- Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478.
- Jia Z, Jiang G, Tian F, et al. A systematic review on the safety and effectiveness of yttrium-90 radioembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Saudi J Gastroenterol. 2016;22(5):353–359.
- Lee SU, Park JW, Kim TH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190(9):806–814.
- Zhang ZM, Lai EC, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8–16.
- Li CX, Zhang H, Wu XF, et al. Clinical efficacy and prognostic factors analysis following curative hepatectomy for hepatocellular carcinoma patients with different China liver cancer staging. Chin J Surg. 2021;59(2):134–143.
- Wei J, Cui W, Fan W, et al. Unresectable hepatocellular carcinoma: transcatheter arterial chemoembolization combined with microwave ablation vs. combined with cryoablation. Front Oncol. 2020;10:1285.
- Zhu BR, Li J, Gai LH, et al. Clinical analysis of focused ultrasound ablation combined with TACE in the treatment of larger than 10 cm hepatocellular carcinoma. Chin J Interv Radiol. 2016;4(2):86–90.
- Cao H, Xu Z, Long H, et al. Transcatheter arterial chemoembolization in combination with high-intensity focused ultrasound for unresectable hepatocellular carcinoma: a systematic review and meta-analysis of the Chinese literature. Ultrasound Med Biol. 2011;37(7):1009–1016.
- Zhao J, Zhang H, Wei L, et al. Comparing the long-term efficacy of standard and combined minimally invasive procedures for unresectable HCC: a mixed treatment comparison. Oncotarget. 2017;8(9):15101–15113.
- Wang M, Wang Y, Feng X, et al. Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China North areas: experience of the Chinese National Cancer Center. Int J Infect Dis. 2017;65:15–21.
- Prevention of Infection Related Cancer (PIRCA) Group, Specialized Committee of Cancer Prevention and Control, Chinese Preventive Medicine Association, et al. Strategies of primary prevention of liver cancer in China: Expert Consensus (2018). Zhonghua Zhong Liu Za Zhi. 2018;40(7):550–557.
- Guidelines for the clinical application of new anti-tumor drugs (2018 edition). J Multidisciplinary Cancer Manage. 2019;5(1):35–54.
- Ajit Y, Sudarsan H, Saumya G, et al. Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival. Oman Med J. 2014;29(6):430–436.
- Kim J, Chung DJ, Jung SE, et al. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma <5 cm: comparison with transarterial chemoembolisation monotherapy—preliminary observations. Br J Radiol. 2012;85(1018):e940–e946.
- Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257(4):686–692.
- Yang M, Fang Z, Yan Z, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140(2):211–219.
- Cheng SQ, Wu MC, Cheng HY, et al. Growing features of the tumor thrombus of portal vein in hepatocellular carcinoma: report of 130 cases. Chin J Curr Adv Gen Surg. 2003;2:103–105.
- Zhang B. Observation of the local efficacy of high intensity focused ultrasound combined 125I implantation therapy in the treatment of portal vein tumor thrombus[D]. Chongqing: Chongqing Medical University; 2016.
- Strunk HM, Lützow C, Henseler J, et al. Mesenteric vessel patency following HIFU therapy in patients with locally invasive pancreatic cancer. Ultraschall Med. 2018;39(6):650–658.
- Guo X, Zhu H, Zhou K, et al. Effects of high-intensity focused ultrasound treatment on peripancreatic arterial and venous blood vessels in pancreatic cancer. Oncol Lett. 2020;19(6):3839–3850.
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890–895.