Abstract
Objective
To explore the learning curve of high intensity focus ultrasound (HIFU) treatment for breast fibroadenoma.
Methods
A database of 110 patients with 255 breast fibroadenomas who underwent HIFU treatment at two different clinical centers (Center 1 and 2) were retrospectively analyzed. The learning curves of HIFU treatment for breast fibroadenoma were drawn by CUSUM analysis in two centers, respectively. According to the inflection point of the learning curves, the treatment was divided into two groups: initial phase and consolidation phase. HIFU treatment parameters were compared between two groups. The effectiveness and safety results were also evaluated.
Results
The inflection points of the learning curves were the 60th treatment in Center 1 and the 65th treatment in Center 2. The screening time, treatment time, sonication time and hyperechoic scale change time were significantly shorter in consolidation phase than those in initial phase of the two centers (p < 0.05). There were no differences in non-perfused volume (NPV) ratio and energy effect factor (EEF) between the two groups in Center 1, while in Center 2, these above-mentioned results in consolidation phase led to a greater improvement than those in initial phase. There was no difference of Visual Analogue Scale (VAS) scores and no adverse event observed in both centers.
Conclusion
HIFU treatment for breast fibroadenoma was effective and safe. The learning curve of HIFU treatment for breast fibroadenoma can be completed after treating 60–65 tumors without increasing the safety risk.
Introduction
Breast fibroadenoma is one of the most common benign breast lesions that mainly affects 20–30 years old young females with overall incidence rate of 2.2% in this age group [Citation1–4]. Nearly 25% of asymptomatic women were reported to have fibroadenomas [Citation5]. Although the growth of fibroadenoma is usually slow and the potential of malignant change rate have been reported as rare as 0.02–0.125%, active clinical intervention is recommended for symptomatic fibroadenoma to alleviate anxiety regarding for physical discomfort and cosmetic concern [Citation6].
Currently, the common treatments for breast fibroadenoma are open surgical excision and vacuum-assisted breast biopsy (VABB) technique [Citation7]. Although open surgical excision could entirely remove the mass, it may result in scar formation with the potential for keloids in some patients, as well as breast volume loss and potential for nipple areolar distortion or displacement, causing poor breast cosmesis; VABB is a surgical alternative with small skin incision for the management of fibroadenoma up to 3 cm in size, but it also has the risks of hemorrhage, infection, even extensive resection with unnecessary removal of normal tissue [Citation3,Citation8,Citation9].
In the past two decades, various minimally- and noninvasive ablation techniques have been developed rapidly, such as radio frequency ablation (RA), microwave ablation (MWA), laser ablation (LA), cryoablation, as well as high intensity focused ultrasound (HIFU) [Citation7]. HIFU is a truly novel noninvasive ablative technique which has been applied to treat breast cancer and fibroadenoma [Citation10]. With the advantages of satisfactory cosmesis with no skin scar and no breast tissue loss, a short recovery time, and no bleeding risk, HIFU is thought to be particularly suitable for treating breast fibroadenoma [Citation11].
Recently, HIFU has been used in many studies for breast tumors. Some scholars found breast tumor cells with presence of coagulative necrosis after HIFU treatment [Citation12]. The first multi-center study was published in 2015 by Kovatcheva et al., in which HIFU treatment was performed on 42 patients with 51 fibroadenomas [Citation13]. Their trial showed that the fibroadenoma volume reduction achieved 72.5% after 12 months and no adverse event was reported. Since the effectiveness and safety of HIFU treating fibroadenoma have been demonstrated by previous studies, the skill learning of HIFU, as a novel technique, is another important aspect for the training of clinical practitioners. The cumulative summation (CUSUM) technique is a common tool to analyze the learning curve of a surgical skill, which was initially used to monitor the trend of continuous variation in the industrial sector and has been adopted in the medical field since 1970s [Citation14,Citation15]. A deep understanding of the learning curve on HIFU can not only help this noninvasive technique to be conducted with increased effectiveness and safety, but also can help doctors under the standard training without unnecessary time-consuming repetition. Since there was no study reported the learning curve on HIFU treatment of breast fibroadenoma by CUSUM analysis, this study aims to evaluate the learning curve of HIFU treatment for breast fibroadenoma by using of CUSUM analysis in a multi-center clinical trial.
Materials and methods
Patients
This study was approved by the ethics committee of the First Affiliated Hospital with Nanjing Medical University (2020-SR-130) and Suining Central Hospital (LLSNCH20200065). Written informed consent was obtained from all patients. From January 2021 to February 2022, a total of 110 patients with 255 breast fibroadenomas who were eligible for HIFU treatment were enrolled. Of this, there were 57 patients with 98 breast fibroadenomas in the First Affiliated Hospital with Nanjing Medical University (Center 1) and 53 patients with 155 breast fibroadenomas in Suining Central Hospital (Center 2).
Inclusion criteria were as follows: (1) patients were more than 18-year-old, (2) BI-RADS grade ≤ 3 by ultrasonography, (3) the diagnosis of breast fibroadenoma was confirmed by core needle biopsy, and (4) the size of lesion was between 5–40 mm. Exclusion criteria included: (1) pathological diagnosis of breast cancer, (2) superficial lesions (< 3 mm beneath the skin), (3) pregnant or lactating women, and (4) with a history of high-dose radiation therapy in the ipsilateral breast.
Ultrasound and contrast-enhanced ultrasound
All patients underwent color Doppler ultrasonography (DC80, Mindary, China) before HIFU treatment to measure the longitudinal diameter (a), anteroposterior diameter (b), and transverse diameter (c) of the fibroadenomas and the volume was calculated by the following equation: V = 0.5233 × a × b × c. After the HIFU treatment, the echoic change of the lesions and blood flow distribution would be observed. Contrast-enhanced ultrasound using a micro-bubble agent (SonoVue, Bracco, Milan, Italy) was also performed on every patient before and immediately after HIFU treatment to evaluate therapeutic effects. Non-perfused volume (NPV), representing coagulative necrosis volume, was measured in 3 dimensions and then calculated according to the above-mentioned equation. The NPV ratio was defined as NPV/fibroadenoma volume × 100%. All ultrasound (US) images were assessed independently by two senior radiologists.
The procedure of HIFU ablation
HIFU treatment was performed by the same surgical team in each center who have received HIFU procedure training, using the high-intensity focused ultrasound device (Model JCQ-B, Focused Ultrasound Ablation System for Breast, Chongqing Haifu Medical Technology Co. Ltd., China). This procedure was performed under local anesthesia with injecting 10–20 ml of 1% ropivacaine around the fibroadenoma and into the space between the breast and the pectoralis major. The breast skin above fibroadenomas was marked. Then patients were carefully placed in a prone position on the HIFU table, with target breast placed immersed in the degassed water. Real-time US was used for monitoring the lesion and observing the relationship among the breast lesion and the pectoralis major and the skin. The focus was placed in the deep site of the fibroadenoma, and when the hyperechoic scale change of the target area emerged, the focus would been moved to the margin of this scale change until it spread to the whole fibroadenoma, which was the termination criteria of HIFU treatment (). After treatment, an ice bag was put on the breast skin for 0.5–2 h.
Figure 1. The significant scale changes during HIFU treatment for breast fibroadenoma. A: Pre-HIFU ultrasound showed a hypoechoic breast fibroadenoma. B: Intra-HIFU ultrasound showed a significant hyperechoic scale changes emerging during HIFU (white arrow). C: Post-HIFU ultrasound showed that the significant hyperechoic scale change covered the whole fibroadenoma.
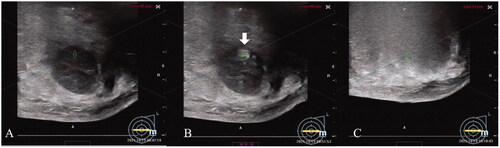
During HIFU treatment, following parameters were recorded: (1) screening time (min), defined as the time for target lesions were located by intra-treatment real-time US; (2) treatment time (min), defined as the first sonication administered till last sonication administered; (3) sonication time (s), defined as the total time of sonication; (4) hyperechoic scale changes emerging time (s), defined as sonication duration from the first sonication output to the hyperechoic scale change emerging; (5) sonication energy (kJ), defined as the total energy given in the treatment; (6) and energy effect factor (EEF), defined as the sonication energy required for ablating 1 mm3 of the tissue, was calculated by the following equation: EEF = sonication energy/NPV (J/mm3).
Safety assessment
Incidences of any adverse events during and immediately after HIFU treatment, including discomfort on skin, breast pain, and intra-treatment Visual Analogue Scale (VAS) scores were recorded. Patients were carefully monitored for delayed skin burn, fever or pectoralis major injury within 72 h after HIFU treatment.
Learning curve
The learning curve was accessed by the cumulative sum (CUSUM). The deviation between the observed value and the target value for each sample were calculated, and then CUSUM were calculated by summing method [Citation16]. First of all, patients were arranged chronologically by treatment date, then the CUSUM value of the first case was the difference between the value for the first case and the mean for all cases. The CUSUM value of the second case was the CUSUM of the first case added to the difference between the value for the second case and the mean for all cases. This repetitive process continued until CUSUM for the last case was calculated as zero. A learning stage was deemed to be completed when an inflection was observed in the CUSUM plot. The trend line of HIFU treatment time was plotted according to the Sinusoidal model of surgical learning curve, and the oscillating sine curve Y = sin X - π/2, in which Y represented HIFU treatment time, and X represented the number of treatments, was used to fit the moving average.
Statistical analysis
Data were analyzed using SPSS 24.0 software (SPSS, IBM Company, USA). Data with normal distribution and variance homogeneity were analyzed by an independent sample T-test and represented by mean ± standard deviation; data with skew distribution or variance heterogeneity were analyzed by the Wilcoxon rank-sum test and represented by median and 25th (Q1) and 75th (Q3) quantiles. P-value < 0.05 was defined as statistical significance. Categorical variables were presented as frequencies and percentages and compared using the chi-square test or Fisher’s exact test.
Results
Learning curve analysis according to HIFU treatment time
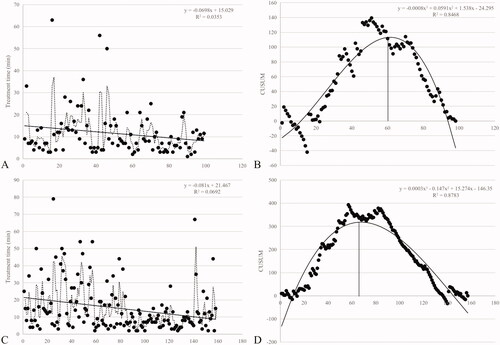
All the 110 patients with 255 lesions successfully completed HIFU treatment. As shown in the scatter plots of HIFU treatment time ()), There were downward trends of treatment time with increasing cases in both centers. The fitted curves were plotted in the CUSUM of HIFU treatment time learning curves in the centers ()). The formulas of scatter plot polynomial fitting curves were Y = –0.0008x3 + 0.0591x2 + 1.538x–24.295 in Center 1, and Y = 0.0003x3–0.147x2 + 15.274x–146.35 in Center 2, respectively, in which Y represented HIFU treatment time and X represented the number of treatments. It was demonstrated that the fitting effects of both curves were good, with the correlation coefficient R2 = 0.8468 in Center 1 and R2 = 0.8783 in Center 2. The CUSUM showed that after treating around 60–65 lesions, a change in performance of HIFU treatment time occurred. Since the inflection point of the learning curve were the 60th treatment in Center 1 and 65th treatment in Center 2, the learning curves were divided into two groups: initial phase with the first 60 treatments and consolidation phase with the following 38 treatments in Center 1; initial phase with first 65 treatments and consolidation phase with the following 92treatment in Center 2.
Figure 2. The trend of the treatment time and CUSUM in the two centers. A: The trend of the treatment time in HIFU treatment of breast fibroadenoma in Center 1. B: The learning curve (CUSUM chart) of HIFU treatment time in Center 1 (60 was the vertex of the curve). C: The trend of the treatment time in HIFU treatment of breast fibroadenoma in Center 2. D: The learning curve (CUSUM chart) of HIFU treatment time in Center 2 (65 was the vertex of the curve).
Baseline characteristics of patients and lesions
Baseline characteristics of patients were shown in . There were no significant differences between the two groups in both centers (p > 0.05). As shown in , there was no significant differences in baseline characteristics of lesions between the two groups in Center 1 and Center 2 (p > 0.05), except for the distance from deep margin of fibroadenoma to the pectoralis major between groups in Center 1 (p = 0.029).
Table 1. Baseline characteristics of patients in two centers.
Table 2. Baseline characteristics of lesions in two centers.
Evaluation of HIFU treatment
As shown in and , the screening time, treatment time, sonication time and hyperechoic scale change emerging time of both centers were significantly shortened in consolidation phase than in initial phase (p < 0.05). There were no significant differences in sonication energy, NPV ratio and EEF in Center 1 (p > 0.05, ). In Center 2, the median NPV ratio was 92.47 (70.02, 110.17) % in consolidation phase, which was significantly higher than in initial phase (77.33 (52.79, 100) %, p = 0.010, ). The median EEF was 17.48 (8.29, 35.03) J/mm3 in initial phase and 11.03 (6.16, 17.83) J/mm3 in consolidation phase. There was a significant difference between the two groups in Center 2 (p = 0.022, ).
Figure 3. The box plot of screening time, treatment time, sonication time and hyperechoic scale change emerging time between groups in the two centers. A: Screening time. B: Treatment time. C: Sonication time. D: Hyperechoic scale change emerging time. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
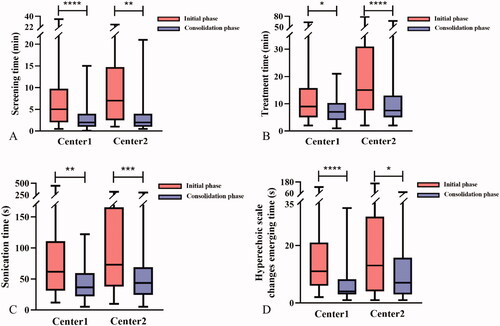
Figure 4. The box plot of sonication energy, NPV ratio, EEF and intra-treatment VAS scores between groups in the two centers. A: Sonication energy. B: NPV ratio. C: EEF. D: Intra-treatment VAS scores. *p < 0.05; **p < 0.01.
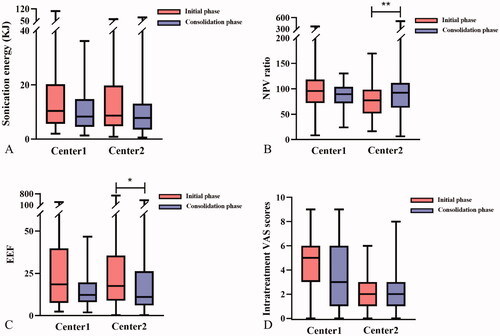
Table 3. HIFU treatment results for fibroadenoma in two centers.
Safety evaluation during and after HIFU treatment
All the patients tolerated HIFU treatment well under local anesthesia. In this study, the common complaint during HIFU ablation was the pain of treatment area, which was usually transient and mild. There was no difference in VAS scores between the two groups in both centers (, ). No skin burn was found immediately after HIFU treatment. No delayed skin injury, fever or pectoralis major injury was found in both groups within 72 h after treatment.
Discussion
With the main advantages of in situ ablation, noninvasiveness and well cosmetic results, HIFU treatment has been increasingly applied and accepted for breast fibroadenoma patients. A number of studies have shown that HIFU treatment of breast fibroadenoma was feasible and safe. Peek et al. showed that HIFU treatment was effective for pain management as most of fibroadenoma patients with pretreatment pain were pain-free at 12-month follow-up [Citation17]. Hahn et al. found a successful reduction in fibroadenomas volume and an obvious relief of the pretreatment pain in 63% patients by HIFU treatment after 12 months [Citation18]. Their study also showed that all patients were satisfied with the cosmetic results. Kovatcheva and his colleague reported the long-term efficacy of HIFU that after 24 months, fibroadenoma volume reduction rate was 77.32% with one-session HIFU treatment and 90.47% with two-session HIFU treatment [Citation19].
Since HIFU, as a new technology for the treatment of breast fibroadenoma, has now become a promising noninvasive method for breast fibroadenoma, doctor training is a key way to increase the clinical application of this technology. The learning curve analysis is an essential tool to improve skill training. CUSUM analysis, as an excellent statistical index, has been used in a number of surgical and non-surgical fields to quantitatively assess the learning curve of surgeons and other specialists, and to give real-time feedback and provide continuous technical quality assurance to themselves [Citation20,Citation21]. In the past few years, many studies have analyzed the learning curve for different surgical techniques, such as laparoscopic pancreatoduodenectomy (LPD), laparoscopic liver resection (LLR), laparoscopic hysterectomy (LH) and so on [Citation16,Citation22,Citation23]. The CUSUM chart was a precise representation of the temporal relationship between the chronological number of cases performed and a surgeon’s ability in a specific surgical task, which had especially important implications for novel techniques [Citation14,Citation21]. It was shown by some studies that operative time was shortened and the incidence of adverse events and relapses decreased after the learning stage of CUSUM analysis [Citation16,Citation21–24].
In our study, the learning curve for HIFU treatment of breast fibroadenoma was also shown by using CUSUM analysis. It was found that after 60–65 treatments, the training stage of the learning curve was finished. Zhang X et al. found that after gaining experiences of performing 40 cases, doctors could complete the learning curve of HIFU treatment of uterine fibroid, with the treatment time and sonication time significantly reduced [Citation25]. This demonstrated that the learning curve was applicable to HIFU technique in different diseases. In our study, we also found that the screening time, treatment time, sonication time, and hyperechoic scale change emerging time were significantly shorter in consolidation phase than that in initial phase (). It indicated that the efficiency of HIFU treatment for breast fibroadenoma had been greatly improved after doctors completing the learning curve in the training stage. For thermal ablation technique, NPV ratio was an important indicator for determining whether the treatment was successful or not [Citation26]. Currently, there is no uniform standard for NPV ratio of breast fibroadenoma by HIFU treatment. A recent learning curve study reported that NPV ratio ≥ 80% was a criteria worthy for HIFU treatment of uterine fibroids, especially for the inexperienced doctors [Citation27]. In our study, we set NPV ratio of at least 80% as the standard to develop the training plan. It was found that the results in initial phase were different in the two centers, with 95.89% in Center 1 and 77.33% in Center 2, but in consolidation phase of both centers, the median NPV ratio achieved this standard. It can be taken from this result that although the treatment effects might not be so stable when doctors started learning HIFU treatment of breast fibroadenoma, this new technique could be well mastered by doctors after completing the learning curve. A deep understanding of the learning curve for HIFU treating breast fibroadenoma is key to develop and implement the practical training plan.
A reduction trend of sonication energy and EEF was observed in consolidation phases in both centers (). It has been shown both in vitro and in vivo that the accumulation of sonication energy was related to the increasing risk of adverse events [Citation28]. Thus, under the premise of ensuring treatment effectiveness by achieving the successful NPV ratio, the reduction of sonication energy and EEF in consolidation phase may indicate that doctors had an improved understanding of HIFU after learning this technique, which may reduce the potential risk of adverse events. In Center 1, a reduction trend of VAS scores in consolidation phase was also found, which might be the result of the distance from deep margin of fibroadenoma to the pectoralis major being significantly longer in consolidation phase than in initial phase () and the reduction trend of sonication energy. Because the pectoralis major was in the far field of the acoustic pathway of HIFU treatment, the increased distance from the deep site of the fibroadenoma and the decreased sonication energy may lead to less stimulation of energy to the intercostal nerves. No reduction trend of VAS scores was observed in Center 2, which was most likely due to no difference in the distance from deep margin of fibroadenoma to the pectoralis major between two groups of Center 2. In consolidation phase of the two centers, the median VAS scores were 3.00 and 2.00, respectively. What’s more, there was no adverse events observed. The low VAS score and no adverse events indicated that once completing the learning curve, the doctors could perform HIFU procedure not only efficiently, but also safely, making patients well tolerating the treatment.
There were some limitations of this study. First, this study focused on the learning curve in HIFU treatment of breast fibroadenoma, while the long-term outcomes had not been reported, which should be evaluated in future. Secondly, all the enrolled lesions were diagnosed with fibroadenoma. Whether HIFU treatment was equally effective in other kinds of benign breast tumors, such as phyllodes tumors, remained unknown and required further investigation.
Conclusion
In conclusion, to the best of our knowledge, this study was the first study to report the learning curve of HIFU treatment for breast fibroadenoma by CUSUM analysis. The results demonstrated that treatment efficacy was significantly improved after performing HIFU treatment of 60–65 fibroadenomas. CUSUM analysis is a promising method for the assessment of HIFU training. A better and deepen understanding of learning curve by CUSUM analysis may help to develop and establish a standard training plan of HIFU treatment for breast fibroadenoma, which is conducive for the wide clinical application of this noninvasive technique.
Disclosure statement
Zhibiao Wang is a senior consultant to Chongqing Haifu Medical Technology Co., Ltd. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med. 1998;13(9):640–645.
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116(33-34):565–574.
- Cerrato F, Labow BI. Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg. 2013;27(1):23–25.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275–285.
- El-Wakeel H, Umpleby HC. Systematic review of fibroadenoma as a risk factor for breast cancer. Breast. 2003;12(5):302–307.
- Yu-Ting W, Shou-Tung C, Chih-Jung C, et al. Breast cancer arising within fibroadenoma: collective analysis of case reports in the literature and hints on treatment policy. World J Surgl Oncol. 2014;12:335.
- Salati SA. Breast fibroadenomas: a review in the light of current literature. Pol Przegl Chir. 2020;93(1):40–48.
- Sperber F, Blank A, Metser U, et al. Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg. 2003;138(7):796–800.
- Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg. 2014;3(2):142–148.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4(3):294–302.
- Izadifar Z, Izadifar Z, Chapman D, et al. An introduction to high intensity focused ultrasound: systematic review on principles, devices, and clinical applications. JCM. 2020;9(2):460.
- Wu F, Wang ZB, Cao YD, et al. A randomised clinical trial of highintensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89(12):2227–2233.
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound. 2015;3(1):1.
- Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296(18):1044–1045.
- Gao Y, Kruger U, Intes X, et al. A machine learning approach to predict surgical learning curves. Surgery. 2020;167(2):321–327.
- Morató O, Poves I, Burdío F, et al. Evaluation of the learning curve for laparoscopic pancreatoduodenectomy by CUSUM analyses. Cohort study. Int J Surg. 2020;80:61–67.
- Peek MCL, Ahmed M, Scudder J, HIFU-F Collaborators, et al. High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial). Int J Hyperthermia. 2018;34(7):1002–1009.
- Hahn M, Fugunt R, Schoenfisch B, et al. High intensity focused ultrasound (HIFU) for the treatment of symptomatic breast fibroadenoma. Int J Hyperthermia. 2018;35(1):463–470.
- Kovatcheva R, Zaletel K, Vlahov J, et al. Long-term efficacy of ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma. J Ther Ultrasound. 2017;5:1.
- Biau DJ, Resche-Rigon M, Godiris-Petit G, et al. Quality control of surgical and interventional procedures: a review of the CUSUM. Qual Saf Health Care. 2007;16(3):203–207.
- Hu Y, Jolissaint JS, Ramirez A, et al. Cumulative sum: a proficiency metric for basic endoscopic training. J Surg Res. 2014;192(1):62–67.
- Sultana A, Nightingale P, Marudanayagam R, et al. Evaluating the learning curve for laparoscopic liver resection: a comparative study between standard and learning curve CUSUM. HPB (Oxford). 2019;21(11):1505–1512.
- Brummer TH, Seppälä TT, Härkki PS. National learning curve for laparoscopic hysterectomy and trends in hysterectomy in Finland 2000-2005. Hum Reprod. 2008;23(4):840–845.
- Co M, Chen C, Lee C, et al. Prospective clinical trial on the learning curve of high-intensity-focused ultrasound for the treatment of breast fibroadenoma. Surg Today. 2022;52(7):1048–1053.
- Zhang X, Chen J, Hu L, et al. Analysis of the initial learning curve in the treatment of uterine fibroids with high intensity focused ultrasound. Chin J Interv Imaging Ther. 2015;12(03):147–151.
- Zhang C, Liang M, Xia T, et al. Dosimetric analysis of ultrasound-guided high intensity focused ultrasound ablation for breast fibroadenomas: a retrospective study. Int J Hyperthermia. 2022;39(1):743–750.
- Gong X, Zhang X, Liu D, et al. Evaluation of physician experience in achieving non-perfused volume ratio of high-intensity focused ultrasound ablation for uterine fibroids: a multicentre study. J Int Med Res. 2022;50(5):3000605221102087.
- Zhou P, Wang HB, Fu M, et al. Acute biological effects of HIFU on the tumor cells in vitro. J Clin Ultrasound Med. 1999;3(1):162–164.
