Abstract
Background
We comprehensively evaluate the efficacy and safety of US-guided radiofrequency ablation (RFA) in the treatment of papillary thyroid microcarcinoma (PTMC) via a systematic review and meta-analysis.
Methods
We searched the PubMed, Embase and Cochrane Library databases for studies published during the time between the establishment of the database through October 2021. We included a 10 non-randomized controlled trial (non-RCT) that reported the application of US-guided RFA in PTMC. The sample size of patients totaled 1279. We evaluated the ablation efficacy by analyzing the volume reduction rate (VRR), complete disappearance rate (CDR) and recurrence rate of PTMC treated by RFA. We analyzed all data using STATA version 15.1 (Stata Corporation, College Station, TX).
Results
Our pooled results proved RFA treatment significantly reduces the volume of tumors (Weighted Mean Difference [WMD] = −103.20, 95% CI: −111.93 – −94.48, p = 0.000). We also found the VRR at 12 months after RFA was 93.27% (95% CI: 84.68–101.86), and the CDR at 12 months after RFA was 64% (95% CI: 39–89%). Additionally, pooled results showed the incidence of mPTC residue in ablation area, newly discovered mPTC and lymph node metastases after RFA treatment were respectively 0.3% (95% CI: −0.1–0.7%), 2.5% (95% CI: 1.1–3.9%) and 1.0% (95% CI: 0.2–1.9%), and the incidence of complications after RFA treatment was 1.8% (95% CI: 0.7–3.2%).
Conclusions
US-guided RFA is effective and safe for treating PTMC. It could be an excellent alternative to the existing treatment options.
1. Introduction
Thyroid nodules (most of which are benign) are a common disease of the endocrine system, and malignant nodules account for 5–15% of all cases. Although the recommendations of the Korean guidelines for thyroid cancer screening published in 2015 do not routinely recommended thyroid ultrasonography for healthy subjects [Citation1], according to the ATA guidelines [Citation2], thyroid nodules can be classified into five categories using thyroid ultrasound (US): benign pattern (0% risk), very low suspicion pattern (<3% risk), low suspicion pattern (5–10% risk), intermediate suspicion pattern (10–20% risk) and high suspicion pattern (>70–90% risk). The most common type of malignant thyroid tumor is papillary thyroid carcinoma (PTC), which accounts for 85% of thyroid cancers [Citation3]. In PTC, if the diameter of the tumor is less than or equal to 1 cm, then it can be defined as papillary thyroid microcarcinoma (PTMC), which has a better prognosis of low mortality and recurrence rate [Citation4]. The current standard treatment for PTMC is surgery (e.g., thyroid lobectomy) and preventive or therapeutic lymph node dissection [Citation5,Citation6]. However, a variety of postoperative complications can occur, including hypothyroidism and injury of the parathyroid glands, which can affect patients’ quality of life and physical health [Citation7,Citation8]. Therefore, we must find treatments with fewer complications that give patients better quality of life and better cosmetic results.
In recent years, radiofrequency ablation (RFA) guided by US has achieved satisfactory results in the treatment of thyroid diseases [Citation9–11]. In addition, US-guided RFA can avoid the complications of general anesthesia, achieve a lower trauma rate and speed recovery [Citation12]. The 2021 CIRSE/ETA clinical practice guidelines proposed active surveillance (AS) and US-guided minimally invasive treatment (MIT) for occasional cases of PTMC [Citation13]. We conducted a systematic review and meta-analysis by compiling studies that reported the application of US-guided RFA in PTMC to explore the efficacy and safety of US-guided RFA in the treatment of PTMC.
2. Methods
2.1. Literature inclusion and exclusion criteria
The inclusion criteria:
Study object: Patients with PTMC
Intervention measures: US-RFA
Outcome indicators: Volume of tumors, the volume reduction (VRR), the complete disappearance rate (CDR), the recurrence rate and the incidence of complications.
Study design: Non-randomized controlled trial (non-RCT).
The exclusion criteria:
Studies that reported the application of other ablation methods in PTMC; The sample size is less than 10; Repeated publications; Studies without full text or with the inability to conduct data extraction; Studies using animal experiments; Reviews and systematic reviews.
2.2. Search strategy
In this meta-analysis, we searched PubMed, Embase and Cochrane Library databases from the official establishment of the above three databases until October 2021. The search terms were: ‘papillary thyroid microcarcinoma’ OR ‘Carcinoma, Papillary’ OR ‘Thyroid Neoplasms’ OR ‘PTMC’ AND ‘radiofrequency ablation’ OR ‘radio frequency ablation’ OR ‘radio-frequency ablation’.
2.3. Literature screening and data extraction
Two researchers independently carried out literature search, screening and information extraction. When a question or dispute arose, we made a decision after discussion or negotiation. The data extraction included the author(s); articles’ publication year; country; research type; number of patients; and outcome indicators, including the volume of tumors, VRR, CDR, tumor progression rate and the incidence of complications. Tumor progression was defined as cytologically or histologically confirmed recurrence at the ablation zone (local recurrence) [Citation14], newly found mPTC elsewhere in the thyroid or cervical lymph node metastasis or distant metastasis.
2.4. Literature quality assessment
Two independent researchers assessed the quality of evidence for each study using the methodological index for non-randomized studies (MINORS) scale [Citation15]. There were 12 items in total, each with a score of 0–2 and a total score of 24. Studies were classified as moderate quality from 9 to 16 and as high quality from 17 to 24.
2.5. Data synthesis and statistical analysis
We analyzed all data using STATA version 15.1 (College Station, TX). We evaluated the efficacy of ablation by analyzing the VRR, CDR and recurrence rate of PTMC treated by RFA and then evaluated the safety by analyzing the incidence of complications. We used I2 and Q tests to evaluate heterogeneity. If the heterogeneity test was p ≥ 0.1 and I2 ≤ 50%, then there was homogeneity among studies, and the fixed effects model was used for combined analysis; if p < 0.1 and I2 > 50%, then there was heterogeneity, and a sensitivity analysis was conducted to find its source. If the heterogeneity was still large, then we used a random effects model or gave up the combination of results and used descriptive analysis. We used a funnel plot and Egger’s test to assess the publication bias.
3. Results
3.1. Results of the literature search
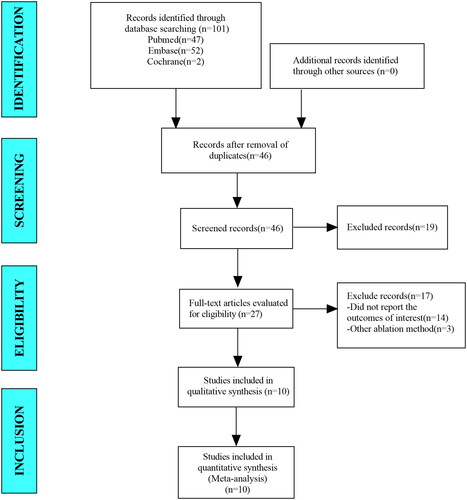
In this meta-analysis, we retrieved a total of 101 studies from the databases including PubMed, Embase and Cochrane Library. After eliminating duplicate studies, 46 studies remained. After browsing titles and abstracts, we obtained 27 studies. After browsing full-text studies, we obtained 27 studies and excluded any that did not report the outcomes of interest and other ablation methods. Finally, we included 10 articles in the meta-analysis ().
3.2. Baseline characteristics and quality assessment of the included studies
In total, we included 10 non-RCT studies in this meta-analysis; eight studies were retrospective studies and two studies were prospective. The patient sample size totaled 1279. MINORS scores were all above 16 points. Three studies received a score of 16, one study scored 17, four studies scored 18 and two studies scored 19, thus indicating the literature included is of moderate or high quality ().
Table 1. Baseline characteristics and quality assessment of the included studies.
3.3. Results of the meta-analysis
3.3.1. Efficacy
3.3.1.1. Volume of tumors
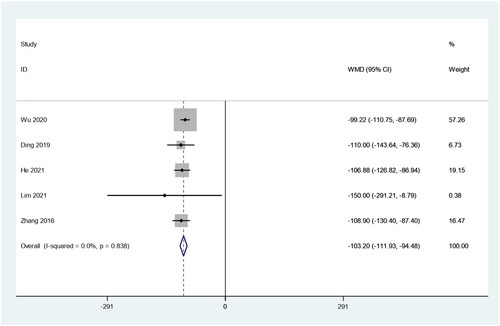
There were five studies in which 447 patients were enrolled that reported changes in tumor volume before and after RFA treatment. Because there was no heterogeneity (I2 = 0.0%, p = 0.838), we conducted a meta-analysis using a fixed effects model. The pooled results showed that the tumor volume after RFA treatment was about 100 mm3 lower than preablation, and the difference was statistically significant (weighted mean difference [WMD] = −103.20, 95% CI: −111.93 – −94.48, p = 0.000; ).
3.3.1.2. VRR
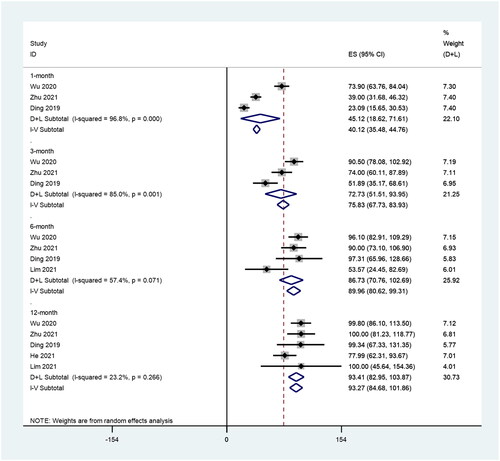
We further analyzed the VRR during different time periods, including 1, 3, 6 and 12 months after RFA. There were three, three, four and five studies included with 350, 350, 363 and 468 patients enrolled that reported the VRR post-RFA treatment of PTMC, respectively. There was significant heterogeneity at 1 (I2 = 96.8%, p = 0.000), 3 (I2 = 85.0%, p = 0.001) and 6 (I2 = 57.4%, p = 0.071) months after RFA, whereas there was no significant heterogeneity at 12 months (I2 = 23.2%, p = 0.266) after RFA. The pooled results showed that the VRRs at 1, 3, 6 and 12 months after RFA were 45.12% (95% CI: 18.62–71.61), 72.73% (95% CI: 51.51–93.95), 86.73% (95% CI: 70.76–120.69) and 93.27% (95% CI: 84.68–101.86; ).
3.3.1.3. CDR
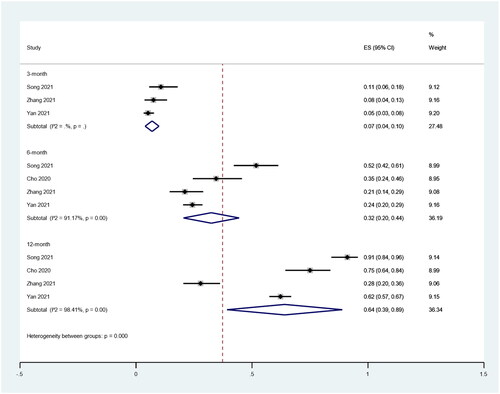
We also analyzed the CDR at different time periods, including 3, 6 and 12 months after RFA. There were three, four and four studies included in which 669, 753 and 753 patients were enrolled that reported the CDR after RFA treatment of PTMC, respectively. There was significant heterogeneity at 6 (I2 = 91.17%, p < 0.1) and 12 (I2 = 98.41%, p < 0.1) months after RFA. The pooled results showed the CDRs at 3, 6 and 12 months after RFA were 7% (95% CI: 4–10%), 32% (95% CI: 20–44%) and 64% (95% CI: 39–89%; ).
3.3.1.4. Tumor progression rate
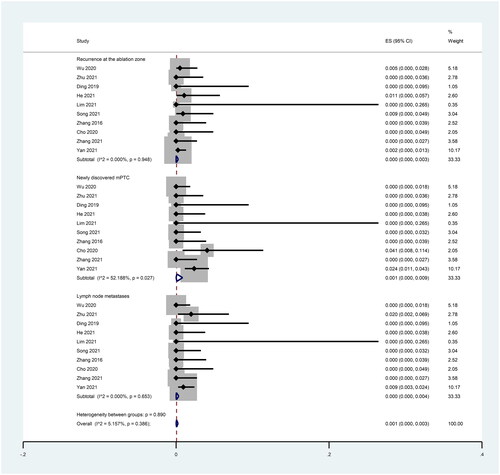
We explored the incidence of each of the three types of recurrence separately. Ten studies reported mPTC recurrence in the ablation area, and the pooled results showed that the incidence of mPTC residue in the ablation area after RFA treatment was 0.0% (95% CI: 0.0–0.3%; I2 = 0.00%, p = 0.948; ).
Ten studies reported the incidence of newly discovered mPTC, and the pooled results showed that the incidence of newly discovered mPTC after RFA treatment was 0.1% (95% CI: 0.0–0.9%; I2 = 53.19%, p = 0.027; ).
Ten studies reported the incidence of lymph node metastasis, and the pooled results showed that the incidence of lymph node metastasis after RFA treatment was 0.0% (95% CI: 0.0–0.4%; I2 = 0.00%, p = 0.653; ).
3.3.2. Safety
There were nine studies in which 1081 patients were enrolled that reported the incidence of complications after RFA treatment of PTMC. Because there was significant heterogeneity (I2 = 74.642%, p = 0.000), we conducted a meta-analysis using a random effects model. The pooled results showed the incidence of complications after RFA treatment was 1.2% (95% CI: 0.0–3.4%; ).
Figure 6. Incidence of complications including total, minor and major complications after RFA treatment of PTMC.
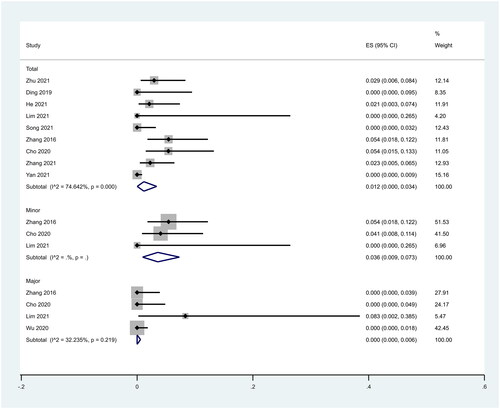
There were three studies that reported minor complications after RFA treatment of PTMC. The pooled results showed that the incidence of minor complications after RFA treatment was 3.6% (95% CI: 0.9–7.3%; ). Four studies reported major complications after RFA treatment of PTMC. Because there was no significant heterogeneity (I2 = 32.235%, p = 0.219), we conducted a meta-analysis using a fixed effects model. The pooled results showed that the incidence of major complications after RFA treatment was 0.0% (95% CI: 0.0–0.5%; ).
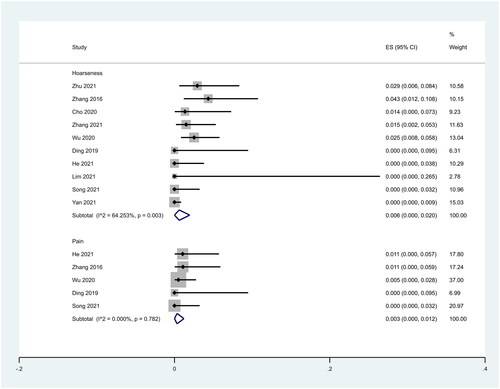
In addition, we analyzed the incidence of different complications. We found that complications were concentrated to hoarseness and pain. Ten studies reported hoarseness. The pooled results showed that the incidence of hoarseness after RFA treatment was 0.6% (95% CI: 0.0–2.0%; ). Five studies reported the incidence of pain. The pooled results showed that the incidence of pain after RFA treatment was 0.3% (95% CI: 0.0–1.2%; ).
3.4. Sensitivity analysis
We conducted a sensitivity analysis by eliminating each included study individually and performing a summary analysis of the remaining studies. Our research results found none of the studies had an excessive effect on the results of the meta-analysis, which suggests the results of this meta-analysis are stable and reliable. Figures S1–S3 show the results of the sensitivity analysis.
3.5. Publication bias
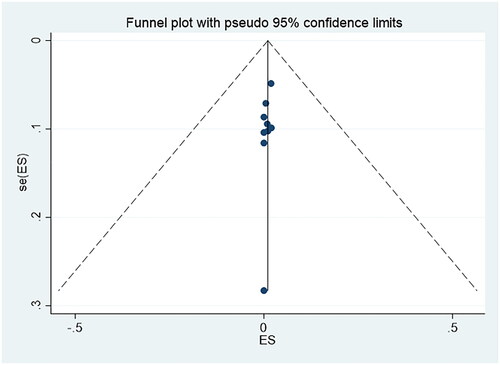
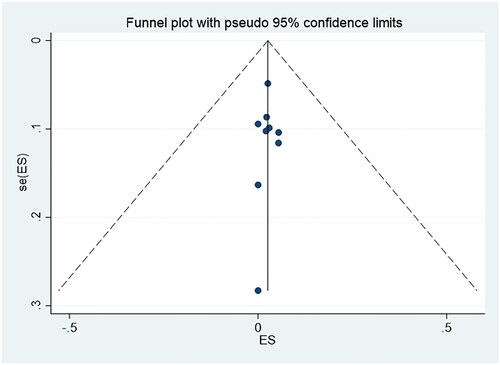
and present the funnel plots. The two funnel plots were symmetrical, and the p values of Egger’s tests were 0.114 and 0.808, respectively, which indicated there was no obvious publication bias in this study.
4. Discussion
Our study results indicate RFA is a safe and effective method to treat PTMC. PTC is the most common malignant tumor of the thyroid, accounting for 85% of all thyroid cancers [Citation26]. With the widespread use of neck US and fine-needle aspiration biopsy techniques, the PTMC diagnosis rate has increased in recent years [Citation27]. Although surgery and AS are currently the primary management methods, the trauma and complications of surgery can seriously affect patients’ quality of life. Conversely, AS can make many patients feel anxious [Citation28–31]. As one of the MITs, US-guided RFA has been used in the treatment of thyroid nodules, PTC and recurrent thyroid cancers [Citation16,Citation32]. In this meta-analysis, we examined the efficacy and safety of US-guided RFA treatment by pooling 10 studies that reported the application of US-guided RFA in PTMC, including 1279 patients, to provide reliable evidence for clinical treatment. It is worth noting that the study by Dijk et al. [Citation33] included studies with a sample size greater than 5. However, we only included studies with a sample size greater than 10, as the objectivity of the results may be challenged due to the fact that the population sample size is too small [Citation34].
Our pooled results showed RFA treatment significantly reduces the volume of tumors, with a VRR and CDR of 93.27% (95% CI: 84.68–101.86) and 64% (95% CI: 39–89%) at 12 months after RFA, respectively. This indicates RFA is a reliable method for treating PTMC. All included studies involved US, which may be related to US’s ability to accurately assess the extension of the ablation area and the treatment effect [Citation35].
Our pooled results also showed the recurrence rate after RFA treatment was 0.5% (95% CI: 0.1–1.1%), which demonstrates that US-guided RFA after treatment of PTMC is effective. Additionally, physicians can use US to locate the actual recurrence area in the enlarged nodule during follow-up examinations [Citation36].
In addition, during the study, we discovered the incidence of complications after RFA treatment was 1.2% (95% CI: 0.0–3.4%), which proves US-guided RFA is a soft method in the treatment of PTMC. More importantly, compared with surgery, there are nearly no reports of thyroid and parathyroid injury after RFA.
Nonetheless, this study has limitations. First, there was heterogeneity in the exploration of the CDR, but the description of the ablation power and energy per unit volume in the studies was not detailed, which may be the cause of the heterogeneity. Second, the follow-up time for most studies’ cases was not long, and only one study included follow-ups for more than five years. PTMC, a disease with slow clinical progress, requires longer follow-up times to determine RFA’s efficacy. In the future, researchers should conduct meta-analyses on the results of long-term follow-ups. Third, most of this research was retrospective. In the future, more large-scale RCTs are needed.
5. Conclusion
Overall, US-guided RFA is effective and safe for treating PTMC. It could be an excellent alternative to the existing treatment options.
Supplemental Material
Download PDF (332.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yi K, Kim S, Kim DH, et al. The Korean guideline for thyroid cancer screening. J Korean Med Assoc. 2015;58(4):302.
- Silver Karcioglu A, Iwata AJ, Pusztaszeri M, et al. The American thyroid association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022;130(3):174–180.
- Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5(1):51–56.
- Gubbiotti MA, Livolsi V, Montone K, et al. Papillary thyroid microcarcinomas: does subtyping predict aggressive clinical behavior? Hum Pathol. 2021;114:28–35.
- Elliott MS, Gao K, Gupta R, et al. Management of incidental and non-incidental papillary thyroid microcarcinoma. J Laryngol Otol. 2013;127(S2):S17–S23.
- Patel SG, Carty SE, Lee AJ. Molecular testing for thyroid nodules including its interpretation and use in clinical practice. Ann Surg Oncol. 2021;28(13):8884–8891.
- Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years’ follow-up. Thyroid. 2020;30(3):408–417.
- Koshkina A, Fazelzad R, Sugitani I, et al. Sawka, association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: a systematic review and Meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(6):552–560.
- Ha EJ, Baek JH, Lee JH, et al. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid. 2013;23(3):289–293.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a Mayo clinic case series. Mayo Clin Proc. 2018;93(8):1018–1025.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36(1):359–367.
- Mauri G, Hegedüs L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197.
- Wei Y, Niu WQ, Zhao ZL, et al. Microwave ablation versus surgical resection for solitary T1N0M0 papillary thyroid carcinoma. Radiology. 2022;304(3):704–713. )
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of Ultrasound-Guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.
- Wu R, Luo Y, Tang J, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: a retrospective analysis of 198 patients. Int J Hyperthermia. 2020;37(1):168–174.
- Cho SJ, Baek SM, Lim HK, et al. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. 2020;30(12):1745–1751.
- Zhu Y, Che Y, Gao S, et al. Long-term follow-up results of PTMC treated by ultrasound-guided radiofrequency ablation: a retrospective study. Int J Hyperthermia. 2021;38(1):1225–1232.
- He H, Song Q, Lan Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma in patients aged 55 years or older: a retrospective study. Int J Hyperthermia. 2021;38(1):604–610.
- Lim LS, Lin WC, Chiang PL, et al. One year follow-up of US-Guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: the first experience in Taiwan. J Formos Med Assoc. 2021;121:1406–1413.
- Song Q, Gao H, Tian X, et al. Evaluation of ultrasound-guided radiofrequency ablation as a treatment option for papillary thyroid microcarcinoma in the isthmus: a retrospective study. Front Endocrinol (Lausanne). 2020;11:599471.
- Zhang C, Yin J, Hu C, et al. Comparison of ultrasound guided percutaneous radiofrequency ablation and open thyroidectomy in the treatment of low-risk papillary thyroid microcarcinoma: a propensity score matching study. Clin Hemorheol Microcirc. 2022;80(2):73–81.
- Yan L, Zhang M, Song Q, et al. Ultrasound-Guided radiofrequency ablation versus thyroid lobectomy for low-risk papillary thyroid microcarcinoma: a propensity-matched cohort study of 884 patients. Thyroid. 2021;31(11):1662–1672.
- Geisbush TR, Dymon Z, Gabriel MS, et al. A multimodal and pathological analysis of a renal cell carcinoma metastasis to the thyroid gland 11 years post nephrectomy. J Radiol Case Rep. 2019;13:1–9.
- Gharib H, Papini E, Garber JR, et al. A.A.A.T.F.o.T. nodules, American Association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22:622–639.
- Haugen BR, Alexander EK, Bible KC, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Farkas EA, King TA, Bolton JS, et al. A comparison of total thyroidectomy and lobectomy in the treatment of dominant thyroid nodules. Am Surg. 2002;68(8):678–682.
- Wormald R, Sheahan P, Rowley S, et al. Hemithyroidectomy for benign thyroid disease: who needs follow-up for hypothyroidism? Clin Otolaryngol. 2008;33(6):587–591.
- McHenry CR, Slusarczyk SJ. Hypothyroidisim following hemithyroidectomy: incidence, risk factors, and management. Surgery. 2000;128(6):994–998.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- van Dijk SPJ, Coerts HI, Gunput STG, et al. Assessment of radiofrequency ablation for papillary microcarcinoma of the thyroid: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2022;148(4):317–325.
- Yan Q, Prasla S, Carlisle DC, et al. Deep venous arterialization for chronic limb threatening ischemia in atherosclerosis patients - A meta-analysis. Ann Vasc Surg. 2022;81:1–21.
- Solbiati L, Ierace T, Tonolini M, et al. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51:S19–S23.
- Kim JH, Baek JH, Lim HK, et al. Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography. 2019;38(2):125–134.