Abstract
Purpose
To compare the efficacy and safety of focused ultrasound (FUS) therapy and cryotherapy for cervical squamous intraepithelial lesion (SIL).
Methods
In this retrospective study, data pertaining to women treated for cervical SIL with FUS therapy or cryotherapy at the Second Affiliated Hospital of Chongqing Medical University between 21 April 2018 and 31 August 2020 were obtained. The patients were followed up after 3–6 and 6–12 months. The proportions of women with no evidence of disease, recurrent disease, clearance of the human papillomavirus (HPV) and adverse effects or complications were determined.
Results
Of the 250 women with complete data who were included in the study, 144 and 106 received FUS therapy and cryotherapy, respectively. Overall, FUS therapy was observed to be more effective than cryotherapy (91.7 vs. 79.2%, p = 0.005). Statistically significant differences were noted in the treatment efficacy for patients with low-grade SIL (LSIL) (92.3 vs. 80.2%, p = 0.011). However, there were no significant differences in the treatment efficacy for patients with high-grade SIL (HSIL) (88.9 vs. 75.0%, p = 0.390). The recurrence rates in patients with LSIL treated with FUS therapy or cryotherapy showed no significant differences at the 6–12-month follow-up (1.0 vs. 6.0%, p = 0.163). Furthermore, there was no recurrence in patients with HSIL, either in the FUS or cryotherapy group. FUS therapy and cryotherapy resulted in similar HPV clearance at the 3–6-month follow-up (77.1 vs. 64.8%, p = 0.057). No statistically significant differences were observed in the complication rates between the two groups (3.5 vs. 1.9%, p = 0.717).
Conclusion
The results of this study suggest that FUS therapy is superior to cryotherapy in the treatment of cervical LSIL.
Introduction
Cervical squamous intraepithelial lesion (SIL) is a morphological change that occurs in the squamous epithelium of the cervix after human papillomavirus (HPV) infection. SIL can be categorized as low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) according to the severity of the lesions; the latter is a known precursor of cervical cancer. Literature shows that LSIL is the initial histological manifestation of HPV infection; 10% cases of LSIL progress to HSIL [Citation1]. If precancerous lesions are not properly treated, HSIL could progress to cervical cancer within 10–20 years; however, if routine treatment is followed, the risk of malignant transformation can be significantly reduced [Citation2].
Conservative treatment for SIL includes excisional and ablative methods. The current primary treatment for HSIL is cervical conisation, which has high efficacy and involves radical excisional techniques. Nonetheless, compared with ablation, excision in women of various ages who are concerned regarding the potential impact of the treatment on future pregnancy outcomes increases the risk of adverse sequelae, such as preterm birth, low birth weight, NICU admission and perinatal mortality [Citation3,Citation4].
Ablation treatment appears to have an insignificant effect on adverse pregnancy outcomes [Citation4,Citation5]. A Cochrane review comparing the surgical techniques for SIL treatment concluded that no technique was clearly superior in terms of treatment failure or associated morbidity [Citation6]. In some circumstances, ablation is acceptable for patients diagnosed with SIL whose concerns about the effects of treatment on future pregnancy outweigh their concerns about cancer. Ablation treatment includes cryotherapy, laser ablation and thermoablation. Of these, cryotherapy is the only ablation method recommended by the World Health Organization owing to its advantages, such as non-requirement of electricity, ease of use and high efficiency. Although cryotherapy is the standard practice for treating patients with cervical pre-cancer, it exhibits the following disadvantages: risk of causing frostbite, long duration of post-operative vaginal discharge and insufficient treatment depth [Citation7].
As a new and promising therapy, focused ultrasound (FUS) serves as a noninvasive method for tissue ablation. FUS can preserve the integrity of the cervical tissue structure, and there is no scab after treatment. FUS generates heat, mechanical and cavitation effects that denature the diseased tissues, facilitate necrosis and allow the dead tissues to be replaced by surrounding healthy tissues [Citation8]. FUS has been reported to be a safe, effective and feasible therapy for HPV-related cervicitis and cervical SIL [Citation9–11]. However, the application of this technique to SIL needs further investigation. Therefore, this study aimed to compare the efficacy and safety of FUS therapy with classical cryotherapy in the treatment of cervical SIL.
Materials and methods
In this retrospective study, data were obtained from the hospital records of women with cervical SIL who were treated with FUS therapy or cryotherapy between 21 April 2018 and 31 August 2020 and were followed up after 3–6 and 6–12 months at the Second Affiliated Hospital of Chongqing Medical University. Ethical approval was obtained from the ethical review committee of the hospital (No. 201938). All patients provided informed consent to undergo the procedure and for the use of their data in future studies.
According to expert consensus, patients with biopsy-proven LSIL for >2 years or with symptoms such as contact bleeding or leukorrhagia as well as those with biopsy-proven HSIL diagnosed at the hospital were treated with FUS or cryotherapy if the following criteria were met: the squamocolumnar junction and the upper limit of lesions from adequate colposcopy examinations were completely visualized before ablation treatment, lesions involved three quadrants or fewer of the transformation zone and there was no evidence of glandular epithelial lesions or invasive carcinoma. The patients who met the following criteria were excluded: (1) lesions extending into the cervical canal, (2) positive findings on endocervical curettage, (3) lesions extending beyond the cryotip being used, (4) lesions covering >75% of the surface area of the ectocervix calculated on the colposcopy image (the percentage of the lesion area in the cervix can be calculated using a colposcopy image processing software by outlining the ectocervix and lesions on the photograph), (5) previous history of ablation treatment or conisation, (6) presence of acute genital tract infection and (7) positive pregnancy test or lactating.
Prior to the treatment, the histologic findings, treatment procedure and potential adverse effects or complications were explained to each patient and informed consent was obtained. To clearly demarcate the lesion on the cervix, colposcopy was performed before ablation.
The cryotherapy group was treated using the MGC CO2 cryotherapy device (MedGyn Products Inc., IL, USA). An appropriate probe was selected according to the lesions and placed against the entire transformation zone. The scope of the lesions was covered completely and over the lesion edge by 3 mm. The use of the double freeze–thaw–freeze technique (freeze for 3 min, thaw for 5 min and freeze again for 3 min) improved the reliability. To protect the cervix, full thawing and rewarming of the cryoprobe was required before pulling out the probe.
The FUS group was treated using a Model-CZF FUS therapeutic device (Haifu Technology Co., Ltd., Chongqing, China) with a frequency of 9.65 MHz and a power output of 7.7–8.6 W. The device comprised a power generator, therapeutic transducer, central console and circulating degassed water system. Prior to treatment, an ultrasonic coupling agent was used to allow better attachment of the therapeutic probe to the surface of the cervical transition zone and lesions. The commonly used therapeutic techniques were circular and radial scanning at a speed of 2–5 mm/s, and the treatment area included 3–5 mm beyond the edge of the lesion. The total treatment duration for each patient was approximately 180–300 s. The operation was discontinued when the treatment area converged with the color of white or white with red in it, along with inward cervical sag.
After the treatment, the patients were provided instructions on self-care, expected symptoms and follow-up care. They were informed of the possibility of mild fever, pain, bleeding or spotting and excessive vaginal discharge for up to 2–4 weeks. They were advised to report back to the treatment center if they experienced severe pain or bleeding, foul smelling discharge and/or fever. In addition, they were advised to practice abstinence for 2 months and not to use a vaginal douche or tampons for 1 month after the treatment.
All patients were followed up after 3–6 and 6–12 months with thinprep cytologic test (TCT), HPV testing and colposcopy. Punch biopsy samples were obtained from any identified abnormal areas. Once HSIL or minimally invasive carcinoma was detected, diagnostic conisation was performed.
In this study, the primary outcome was no evidence of disease at the 3–6-month follow-up. Regardless of the HPV test results, patients with no histologically proven SIL or negative screening results on both cytology and colposcopy results if no biopsy results were available, were considered as no evidence of disease. The secondary outcome was the clearance of HPV at the 3–6-month follow-up, which was characterized by negative screening results for the same HPV type. The tertiary outcome was disease recurrence, defined as the histologic presence of SIL diagnosed at the 6–12-month follow-up in a patient who had no evident disease at the 3–6-month follow-up [Citation12]. As a measure of safety for the procedure, the following complications were observed: mild or severe pain during or after the treatment, severe vaginal bleeding, excessive vaginal discharge, local cervical infection, pelvic inflammatory disease and unintended major surgery.
All data were processed using SPSS version 26 (IBM, Armonk, NY, USA). The distribution of patient baseline characteristics and measures of efficacy and safety were presented as proportions. Fisher’s exact test was employed to compare the baseline patient characteristics and outcomes. Statistical significance was set to p < 0.05.
Results
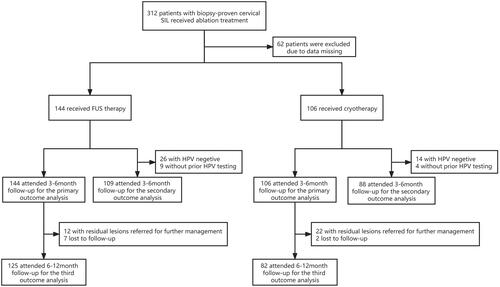
A total of 312 patients with biopsy-proven SIL who received FUS therapy or cryotherapy from 21 April 2018 to 31 August 2020 were evaluated. Of these, 62 patients were excluded because of missing data. Of the 250 patients with valid data, 144 (57.6%) and 106 (42.4%) received FUS therapy and cryotherapy, respectively (). No statistically significant difference was observed between the groups in terms of the baseline demographic and clinical characteristic data ().
Table 1. Treatment group’s baseline demographic, behavioral, and clinical characteristics.
Seven and two patients from the FUS and cryotherapy groups, respectively, were lost to the 6–12-month follow-up because of failure to return to the hospital for further examination. All of these patients had previous biopsy-proven LSIL and a non-evident disease at the 3–6-month follow-up.
Among the FUS group, 132 (91.7%) had no evidence of disease after 3–6 months, 108 (92.3%) in the LSIL group and 24 (88.9%) in the HSIL group respectively. Among the cryotherapy group, 84 (79.2%) had no evidence of disease after 3–6 months, 69 (80.2%) in the LSIL group and 15 (75.0%) in the HSIL group respectively. Overall, FUS therapy was observed to be more effective than cryotherapy (91.7 vs. 79.2%, p = 0.005) (). Statistically significant differences were noted in the efficacy of the treatment for LSIL (92.3 vs. 80.2%, p = 0.011). However, no difference was found in the efficacy of the treatment for HSIL, (88.9 vs. 75.0%, p = 0.390) ().
Table 2. Comparison of the efficacy of FUS therapy and cryotherapy.
Table 3. Comparison of the efficacy of FUS therapy and cryotherapy at different grades of lesions.
The FUS group had a slightly higher, but non-statistically significant, HPV clearance rate than the cryotherapy group (77.1 vs. 64.8%, p = 0.057) (). Furthermore, there were no statistically significant differences in the HPV clearance rate between the two therapies in the treatment of LSIL (73.2 vs. 62.3%, p = 0.154) or HSIL (88.9 vs. 73.7%%, p = 0.210) ().
At the 6–12-month follow-up, one patient with LSIL from the FUS group and four patients with LSIL from the cryotherapy group had recurrent disease (1.0 vs. 6.0%, p = 0.163) (). However, no disease recurrence was observed in patients with HSIL who were treated with either FUS or cryotherapy ().
Both treatments were well tolerated by the patients. The main anticipated adverse effects reported in the two groups were mild pain or cramps during or immediately after the treatment and mild-to-moderate vaginal discharge within 1–2 weeks after the treatment, which lasted 2–3 weeks. In this study, seven patients had unexpected adverse effects (complications). In the FUS group, four patients suffered secondary hemorrhage 2 weeks after the treatment and vaginal gauze packing was performed and one patient had local cervical infections necessitating antibiotics. In the cryotherapy group, one patient experienced severe lower abdominal pain immediately after treatment and required medication and one patient experienced nasal obstruction and peri-orbital edema within 1 day after the treatment and required no intervention. No statistically significant differences were observed in the complication rates between the two groups (3.5 vs. 1.9%, p = 0.717) ().
Table 4. Complications by treatment.
Discussion
In 2020, the International Agency for Research on Cancer reported that cervical cancer is the fourth most common cancer amongst women worldwide. To reduce the morbidity and mortality rates of cervical cancer, appropriate management should be provided.
FUS therapy is a new type of highly accurate and noninvasive therapy for lesions [Citation8]. For decades, FUS has been widely applied clinically, especially for the treatment of non-neoplastic epithelial disorders of the vulva and chronic cervicitis [Citation9,Citation13]. Cryotherapy remains a trusted treatment owing to its high reliability. Moreover, it utilizes the double freeze–thaw–freeze technique and is capable of subsequently eradicating the disease via cryonecrosis [Citation6]. Owing to its high efficacy, cost-effectiveness and ease of use, it has been considered an appropriate and sustainable treatment in low- and middle-income countries; it is also a part of the screen-and-treat approach for cervical cancer prevention.
In our study, >80% of the patients were histologically diagnosed with LSIL. The difference in the sample proportion between the LSIL and HSIL groups was due to various reasons. First, as is known, biopsy-proven LSIL is managed through clinical observation; however, patients experiencing contact bleeding or leukorrhagia are more likely to prefer minimally invasive ablation than clinical observation or excision. Second, biopsy-proven HSIL is managed primarily with cervical conisation; ablation is limited to patients who fulfill the criteria for ablation and whose concerns regarding the impact of treatment on future pregnancies outweigh their concerns related to cancer. Third, the incidence of LSIL is higher than that of HSIL. The limited sample size of the HSIL group might reduce the power of the test. Thus, studies with a large sample size for HSIL should be conducted in the future.
The results from this retrospective study indicated a significant difference in the treatments of cervical SIL. FUS therapy was observed to be better than cryotherapy, with the overall cure rate of 91.7% and LSIL cure rate of 92.3% at the 3–6-month follow-up. No significant difference was noted in the recurrence rates at the 6–12-month follow-up between the two treatments. The cure rate of cryotherapy for cervical SIL is lower than that of FUS therapy, but similar to the cure rate reported in most literatures [Citation14,Citation15]. It is difficult to use cryotherapy for conformal therapy as it employs a fixed probe with low flexibility. On the contrary, FUS therapy can be used for conformal therapy by moving the probe. Consequently, FUS therapy is considered more effective and feasible for patients undergoing ablations. In our study, the efficacy of FUS therapy for LSIL was slightly higher than that reported in a previous study by Fu Z [Citation10] that recruited 30 patients with LSIL and achieved a total efficacy rate of 90.0%. This discrepancy could be attributed to the different study methods, the postoperative follow-up periods, the sample size and the different criteria for effectiveness. No significant difference was observed between the two therapies for HSIL, with FUS therapy achieving a cure rate of 88.9% at the 3–6-month follow-up. More studies involving a large sample size are warranted to further investigate the safety, efficacy and feasibility of FUS therapy as a noninvasive treatment for cervical SIL.
Persistent infection with high-risk HPV is an established cause of cervical SIL and cervical cancer [Citation16]. Moreover, the persistence of HPV infection at follow-up is a significant predictor of residual or recurrent disease after the therapy [Citation17,Citation18]. Our study demonstrated that the total clearance of HPV after FUS therapy and after cryotherapy did not differ significantly (77.1 vs. 64.8% at the 3–6-month follow-up). The clearance rates of the two therapies were similar to those reported in previous studies [Citation9,Citation10,Citation19–21]. Furthermore, the clearance rates of HPV amongst the different grades of lesions did not differ significantly between the two treatment groups at follow-up. A slightly higher, but not statistically significant, clearance rate was observed in the FUS therapy group. The underlying mechanism remains unclear, but it might be related to the following factors: First, from the physical perspective, FUS therapy offers the advantage of tissue penetration of ultrasound; it accurately focuses the energy wave on the lesions and causes instantaneous high temperature, cavitation, sonication and other physical effects to eradicate the HPV-infected lesions [Citation10,Citation16,Citation19]. Second, from the molecular biological perspective, previous studies have found a decrease in p16 and Ki-67 levels and an increase in Fas levels in diseased cervical tissue after FUS therapy. FUS can regulate cell proliferation to control cell apoptosis [Citation22–24]. The apoptosis and elimination of lesional cells are beneficial for HPV elimination. Third, dying HPV-infected cells may trigger an intense immune reaction, thus serving as a ‘vaccine’ to eliminate HPV. Our previous study has demonstrated that OI_NPs, when combined with near-infrared light and ultrasound, significantly induce DAMP expression and generate long-term vaccination [Citation25].
The present study showed that the incidence rates of complications were not significantly different between the FUS and cryotherapy groups. For both therapies, mild lower abdominal discomfort is the main symptom of perioperative pain, and the procedures are well tolerated by the patients. Compared with cryotherapy, FUS therapy causes mild to moderate vaginal discharge that lasts 2–3 weeks without intervention. The occurrence of severe secondary hemorrhage or local cervical infection following FUS therapy is rare. Minimal complications have been reported as unexpected adverse effects, which are consistent with those documented in previous FUS research [Citation9,Citation10].
The present study has some limitations. First, subgroup analysis was conducted according to the SIL grade before the treatment. The efficacy rate in HSIL seemed similar between the groups, without statistically significant differences. However, in this study, <20% of the patients were histologically diagnosed with HSIL. The difference in the sample size between the two groups could reduce the power of the test to some extent; thus, further studies involving more patients with HSIL are warranted to evaluate the difference. Second, women with cervical SIL receiving FUS therapy or cryotherapy were assessed for outcomes only 3–6 and 6–12 months after the treatment. A long-term follow-up is required in future studies. Third, this study only used data from one hospital, which might impact the representativeness of our samples. Caution should be exercised when applying the results of this study to the general population. Multi-center, prospective randomized clinical trials are necessary to compare the efficacy and safety aspects of FUS therapy and cryotherapy in the treatment of cervical SIL.
In conclusion, FUS was observed to be a more suitable and appropriate technique for the treatment of cervical SIL than cryotherapy owing to its reliability, simplicity, acceptability and safety.
Acknowledgments
We are grateful to Michael Saad (Wayne State University School of Medicine, Detroit, MI, USA) for language embellishment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Moscicki AB, Ma Y, Wibbelsman C, et al. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet Gynecol. 2010;116(6):1373–1380.
- Vink MA, Bogaards JA, van Kemenade FJ, et al. Clinical progression of high-grade cervical intraepithelial neoplasia: estimating the time to preclinical cervical cancer from doubly censored national registry data. Am J Epidemiol. 2013;178(7):1161–1169.
- Bjørge T, Skare GB, Bjørge L, et al. Adverse pregnancy outcomes after treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2016;128(6):1265–1273.
- Kyrgiou M, Athanasiou A, Kalliala IEJ, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev. 2017;11(11):CD012847.
- Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG. 2011;118(9):1031–1041.
- Martin-Hirsch PP, Paraskevaidis E, Bryant A, et al. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2013;4(12):CD001318.
- Santesso N, Schünemann H, Blumenthal P, World health Organization Steering Committee for Recommendations on Use of Cryotherapy for Cervical Cancer Prevention, et al. World Health Organization guidelines: use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2012;118(2):97–102.
- van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–258.
- Li CZ, Wang ZB, Yang X, et al. Feasibility of focused ultrasound therapy for recurrent cervicitis with high-risk human papillomavirus infection. Ultrasound Obstet Gynecol. 2009;34(5):590–594.
- Fu Z, Fan Y, Wu C, et al. Clinical efficacy and mechanism for focused ultrasound (FUS) in the management of cervical intraepithelial neoplasia 1 (CIN1). Int J Hyperthermia. 2020;37(1):339–345.
- Zeng H, Liu M, Xiao L, et al. Effectiveness and immune responses of focused ultrasound ablation for cervical intraepithelial neoplasia. Int J Hyperthermia. 2022;39(1):539–546.
- El-Nashar SA, Shazly SA, Hopkins MR, et al. Loop electrosurgical excision procedure instead of Cold-Knife conization for cervical intraepithelial neoplasia in women with unsatisfactory colposcopic examinations: a systematic review and meta-Analysis. J Low Genit Tract Dis. 2017;21(2):129–136.
- Krapf JM, Mitchell L, Holton MA, et al. Vulvar lichen sclerosus: current perspectives. Int J Womens Health. 2020;12:11–20.
- de Fouw M, Oosting RM, Rutgrink A, et al. A systematic review and meta-analysis of thermal coagulation compared with cryotherapy to treat precancerous cervical lesions in low- and middle-income countries. Int J Gynaecol Obstet. 2019;147(1):4–18.
- Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the effectiveness of cryotherapy in the treatment of cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2013;120(3):218–223.
- de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13.
- Pirtea L, Grigoraş D, Matusz P, et al. Age and HPV type as risk factors for HPV persistence after loop excision in patients with high grade cervical lesions: an observational study. BMC Surg. 2016;16(1):70.
- Kim YT, Lee JM, Hur SY, et al. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer. 2010;126(8):1903–1909.
- Hoffman SR, Le T, Lockhart A, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): a systematic review. Int J Cancer. 2017;141(1):8–23.
- Chumworathayi B, Thinkhamrop J, Blumenthal PD, et al. Cryotherapy for HPV clearance in women with biopsy-confirmed cervical low-grade squamous intraepithelial lesions. Int J Gynaecol Obstet. 2010;108(2):119–122.
- Duan L, Du H, Belinson JL, et al. Thermocoagulation versus cryotherapy for the treatment of cervical precancers. J Obstet Gynaecol Res. 2021;47(1):279–286.
- Jaisamrarn U, Castellsagué X, Garland SM, HPV PATRICIA Study Group, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLOS One. 2013;8(11):e79260.
- Calil LN, Edelweiss MI, Meurer L, et al. p16 INK4a and Ki67 expression in normal, dysplastic and neoplastic uterine cervical epithelium and human papillomavirus (HPV) infection. Pathol Res Pract. 2014;210(8):482–487.
- Reuschenbach M, Seiz M, von Knebel Doeberitz C, et al. Evaluation of cervical cone biopsies for coexpression of p16INK4a and Ki-67 in epithelial cells. Int J Cancer. 2012;130(2):388–394.
- Xie W, Zhu S, Yang B, et al. The destruction of Laser-Induced Phase-Transition nanoparticles triggered by Low-Intensity ultrasound: an innovative modality to enhance the immunological treatment of ovarian cancer cells. Int J Nanomedicine. 2019;14:9377–9393.