?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
This study evaluated the clinical efficacy of myometrial and endometrial microwave ablation (MEWA) for treating adenomyosis in patients with anemia.
Methods
This retrospective study enrolled 64 patients with adenomyosis who had anemia treated with either MEWA (MEWA group) or myometrial microwave ablation (MMWA group) between May 2019 and May 2021. The uterine volumes, uterine-volume reduction rates, hemoglobin (Hb) levels, cancer antigen 125 (CA125) levels, dysmenorrhea visual analog scale (VAS) scores, uterine fibroblast symptoms and health-related quality of life (UFS-QOL) scores, menstrual flow scores (MFS) before and 3, 6, and 12 months post-treatment, and adverse events and complications in both groups were collected to assess clinical efficacy.
Results
No statistically significant preoperative differences were observed in any measured factors. Postoperatively, there was a significant reduction in uterine volume and CA125 level, an increase in Hb level, and improvement in the UFS-QOL, dysmenorrhea VAS score, and MFS. No differences were observed in postoperative uterine volume, CA125 level, overall response rate, and adverse event rate during the follow-up period until 12 months postoperatively. However, the MEWA group showed a better uterine-volume reduction rate 6 months postoperatively and improvement in Hb level, USF-QOL score, dysmenorrhea VAS score, and MFS postoperatively.
Conclusion
MEWA and MMWA demonstrated high clinical efficacy in treating adenomyosis and anemia. However, MEWA is a more effective therapy that successfully improves anemia, resulting in improved quality of life.
Introduction
Adenomyosis is characterized by the invasion of the endometrial glands or stroma into the myometrium, with subsequent bleeding and fibrous connective tissue proliferation in response to hormones, resulting in focal or diffuse lesions [Citation1]. Based on imaging diagnosis, the global incidence rate of adenomyosis is approximately 40% [Citation2]. Adenomyosis can lead to menorrhagia, prolonged menstruation, dysmenorrhea, subfertility, and uterine enlargement. Among these, menorrhagia and dysmenorrhea are the most common symptoms, with dysmenorrhea accounting for approximately 50–93.4% of the cases, and menorrhagia found in up to 87% of cases [Citation3,Citation4]. Hysterectomy remains the most definitive and effective treatment option for adenomyosis, yet many patients prefer to preserve the uterus to improve postoperative quality of life [Citation5]. Although medications can provide short-term relief, they are unsuitable for long-term treatment because of severe adverse events, including irregular uterine bleeding, muscle cramps, hot flashes, and decreased libido [Citation6]. The patient’s symptoms are adequately improved by a levonorgestrel-releasing intrauterine system (LNG-IUS); however, dislodgement and downward migration may occur in patients with a large uterine volume. Furthermore, a uterine volume >150 ml is considered a risk factor for LNG-IUS treatment failure [Citation7]. This factor has significant implications for patients with adenomyosis because most of them have a large uterine volume. Uterine artery embolization can reduce clinical symptoms; however, it may affect ovarian function and fertility in patients [Citation8]. Recently, real-time ultrasound-guided microwave ablation (MWA) has gained prominence as a treatment option for adenomyosis. It is safe, effective, causes minimal trauma, and promotes rapid recovery [Citation9]. A recent study found that microwave endometrial ablation (MEA) could effectively improve the adenomyosis symptoms, such as menorrhagia and dysmenorrhea [Citation10]. Generally, MWA in patients with adenomyosis is performed with myometrial ablation to eliminate the lesion, whereas MEA is performed with complete endometrial ablation to achieve amenorrhea. However, further investigation is needed to determine whether myometrium and partial endometrial microwave ablation (MEWA) can improve anemia while preserving menstrual function. Therefore, this study aimed to investigate the effectiveness of MEWA in improving the outcomes of patients with adenomyosis and anemia.
Materials and methods
Study participants and grouping
Myometrium microwave ablation was defined as MMWA, and myometrium and endometrial microwave ablation was defined as MEWA. This retrospective study enrolled patients with adenomyosis who had anemia and underwent MEWA or MMWA at our institution between May 2019 and May 2021. The Ethics Committee of our institution approved the study (approval number: [2021] Ethics Committee Approval for Scientific Research No. 150). The inclusion criteria were as follows: 1) presenting with adenomyosis-related symptoms, including dysmenorrhea and menorrhagia, and diagnosed with adenomyosis by ultrasound and magnetic resonance imaging (MRI); 2) preoperative hemoglobin (Hb) level <110 g/L and confirmation that anemia was not caused by other systemic diseases; 3) no fertility plans; 4) having a safe transabdominal puncture route; and 5) wishing to preserve their uterus, refusing medication or other treatment options, and willingly consenting to undergo MMWA or MEWA. The exclusion criteria were as follows: 1) pregnancy or lactation; 2) cervical intraepithelial neoplasia grade III or higher or severe atypical endometrial hyperplasia; 3) uncontrolled acute and chronic pelvic inflammation and severe bleeding; 4) severe cardiac, pulmonary, hepatic, renal, and other vital organ dysfunction; and 5) severe coagulation dysfunction. All patients provided written informed consent before the study for the publication of any images or data included in this article.
Preoperative preparation
All patients were required to understand the treatment principles, protocols, expected outcomes, and complications and to sign an informed consent form before undergoing ablation. Anticoagulants or antiplatelet drugs were discontinued 5–7 days preoperatively, and the procedure was performed 3–7 days after the termination of menstrual bleeding.
Preoperative questionnaire surveys were given to collect uterine fibroid symptom and quality of life (UFS-QOL) scores, dysmenorrhea visual analog scale (VAS) scores, and menstrual flow scores (MFS): (1) the UFS-QOL score was based on symptom severity score (SSS) items (items 1 − 8) and health-related quality of life (HRQL) items (items 9 − 37) [Citation11]; (2) the severity of dysmenorrhea was evaluated by measuring VAS scores on a scale of 0 − 10 [Citation12]; (3) menstrual flow was rated 1 − 5 according to the patient’s description, with 1 corresponding to ‘normal;’ 2, ‘slightly increased;’ 3, ‘moderately increased;’ 4, ‘severely increased;’ and 5, ‘extremely severely increased [Citation13].’ Anemia was defined as a Hb level <110 g/L.
Preoperatively, all patients underwent routine blood tests, serum cancer antigen 125 (CA125) tests, electrocardiogram examinations, gynecologic ultrasonography, and MRI examinations. The following equation was used to calculate uterine volume:
where
and,
Operation method
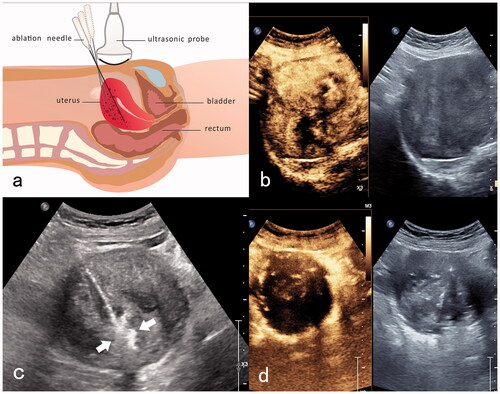
Two experienced surgeons performed all procedures. Contrast-enhanced ultrasonography (CEUS) was performed before ablation to determine the extent of the lesion and reveal its blood supply distribution and relationships with the surrounding tissue structures. Artificial ascites were also created and a wet gauze was inserted into the vagina. In the MMWA group, an ablation instrument (KY-2000A; Nanjing Kangyou Microwave Energy Sources Institute, Nanjing, China) was inserted using a needle into the myometrium lesion under ultrasound guidance. Next, the lesion was ablated from the deep to superficial positions. Ablation was discontinued when hyperechoic signals were generated throughout the lesion or when the ablation needle was 3 − 5 mm from the serosa or endometrium. In addition, CEUS was performed after the dissipation of bubbles in the thermal field to observe the perfusion of the ablated area. Further ablation was immediately performed if the perfusion of the contrast agent was still present ().
Figure 1. MMWA for a patient with adenomyosis who had anemia. (a) Diagram of trans-abdominal ultrasound-guided ablation of the myometrial lesion. The ‘mobile-layered ablation’ technique was adopted to move the ablation needle from deep to superficial positions in a fan-shaped ablation zone; (b) preoperative contrast-enhanced ultrasonography showed hyper-enhancing lesions in the arterial phase; (c) ultrasound-guided ablation of myometrial lesions with a thermal field of hyperechoic signals around the ablation needle (white arrow); (d) postoperative contrast-enhanced ultrasonography reveals no perfusion in the myometrial ablation area.
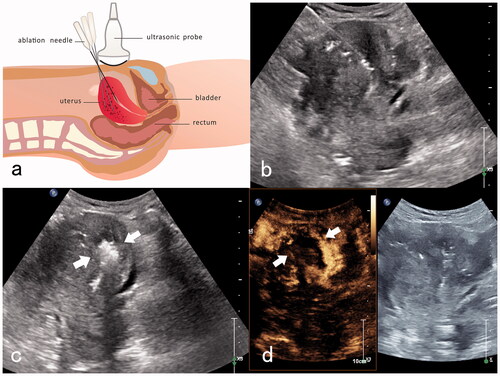
In the MEWA group, myometrial lesions were ablated using a similar procedure as in the MMWA group. Next, with real-time ultrasound guidance, a microwave ablation needle was inserted into the endometrium near the uterine fundus, and the ablation instrument was turned on. The ablation range was monitored in real-time, and ablation was terminated when the hyperechoic range reached approximately one-third of the endometrium near the uterine fundus, leaving the normal lower two-thirds of the endometrium intact. After the dissipation of the thermal field bubbles in the myometrial and endometrial areas, CEUS was performed, followed by additional ablation as required, according to the contrast agent perfusion status (). Adverse events were monitored intra- and postoperatively.
Figure 2. MEWA for an adenomyosis patient with anemia. (a) Diagram of trans-abdominal ultrasound-guided the myometrial and endometrial ablation. An ablation needle was inserted into the endometrium near the uterine fundus, and the ablation area was one-third of the endometrium near the uterine fundus. (b) Transabdominal ultrasound-guided insertion of the ablation needle into the endometrium near the uterine fundus. (c) Hyperechoic signals are detected immediately after the endometrium ablation (white arrow). (d) Post-ablation imaging reveals no perfusion of the upper one-third of the endometrium near the uterine fundus (white arrow).
Monitoring indicators
The patients underwent reexamining at 3, 6, and 12 months postoperatively. They were requested to complete the three questionnaires referenced above and repeat Hb, CA125, and gynecologic ultrasound examinations. Their uterine volume and uterine-volume reduction rate were calculated, with the latter calculated using the following equation:
Intra- and postoperative adverse events and complications were also recorded.
Clinical efficacy assessment criteria
The clinical efficacy was assessed regarding postoperative improvement in dysmenorrhea or menstrual flow. The criteria are summarized as follows [Citation14]: (1) complete remission, absence of dysmenorrhea, and/or return to normal menstrual flow; (2) significant remission, a decrease of >4 in the VAS score, and/or a decrease of >2 in the MFS; (3) partial remission, a decrease of <4 on the VAS or a decrease of <2 in the MFS; (4) no remission, no decrease in both VAS and MFS; and (5) worsening, a higher VAS and/or MFS than that before treatment. Clinical treatment was considered effective when the patient showed complete, significant, or partial remission. Postoperative recurrence was defined as meeting the following criteria: (1) an increase of >4 in the VAS or an increase of >2 in the MFS during the follow-up of patients for whom the treatment had shown clinical efficacy after the procedure, and (2) an increase lasting >3 months.
Assessment criteria for adverse events and complications
The Society of Interventional Radiology (SIR) classification system was used to evaluate intraoperative adverse events and postoperative follow-up [Citation15]. The SIR classification system categorizes adverse events into the following classes: (1) SIR A: no need for treatment and no adverse consequences; (2) SIR B: needs simple treatment and observation but has no adverse consequences; (3) SIR C: requires a short period of inpatient treatment (<48 h); (4) SIR D: needs primary treatment, an increased level of care, and a prolonged hospital stay (>48 h); (5) SIR E: permanent sequelae; and (6) SIR F: death.
Statistical analysis
Statistical analyses were performed using the international business machine statistical package for social sciences (version 26.0; IBM Corp. Armonk, New York). Measurement data that followed a normal distribution were expressed as X ± S and subjected to a t-test. The rates were compared using the χ2 or Fisher’s exact probability test. In addition, clinical efficacy regarding various monitoring indicators pre- and postoperatively across and within groups were compared using 2 × 2 repeated measure analyses, and differences were considered statistically significant when p < 0.05.
Results
The preoperative clinical baseline of patients in both groups combined
Overall, 64 patients with adenomyosis who had anemia (mean age, 42.6 ± 4.5 years; mean uterine volume, 305.6 ± 144.0 cm³) were included in this study. Thirty-one patients underwent MEWA, whereas 33 patients underwent MMWA. In both groups, no significant preoperative differences were observed in uterine volume, CA125 level, Hb level, UFS-QOL score, VAS score, and MFS ().
Table 1. Comparison of preoperative clinical baseline characteristics between the two groups.
Inter-group comparison of clinical efficacy in terms of various monitoring indicators pre- and postoperatively
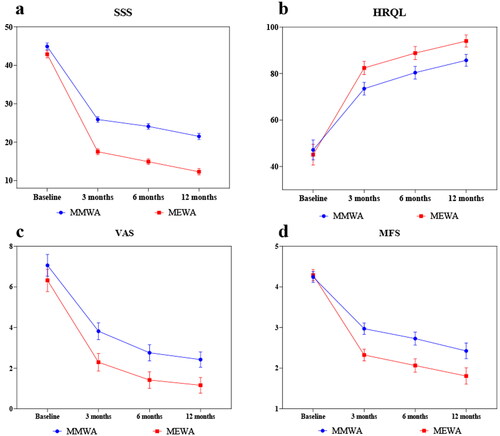
Each observation indicator showed a significant improvement in both groups (p < 0.05), as presented in and . After the operation, the uterine-volume reduction rate, HRQL and Hb level increased, whereas the uterine volume, CA125 level, SSS, MFS, and VAS score decreased in the two groups. The uterine-volume reduction, uterine volume, SSS, and HRQL of the two groups and the Hb and VAS of the MEWA group gradually increased or decreased within 1 year after surgery. Furthermore, the MFS of the two groups and the HB and VAS of the MMWA group increased or decreased within 6 months postoperatively and then tended to be stable. CA125 levels decreased 3 months postoperatively and subsequently stabilized.
Table 2. Clinical efficacy regarding various monitoring indicators pre- and postoperatively in the MMWA group.
Table 3. Clinical efficacy regarding various monitoring indicators pre- and postoperatively in the MEWA group.
Inter-group comparison of clinical efficacy
During the 12-month postoperative follow-up period, seven of the 33 patients in the MMWA group showed complete remission (21.1%), 20 showed significant remission (60.6%), three showed partial remission (9.1%), two showed no significant remission (6.1%), and one showed recurrence (3.0%), with an overall response rate (ORR) of 90.9%. In comparison, 10 of 31 patients in the MEWA group indicated complete remission (32.3%), 18 showed significant remission (58.1%), three demonstrated partial remission (9.6%), and none showed recurrence or worsening, with an ORR of 100%. The ORR in both groups was 95.3%.
The two groups showed no significant differences in postoperative uterine volume, CA125 levels, ORR, or adverse event rates (p > 0.05). However, the MEWA group showed a better uterine-volume reduction rate at 6 months postoperatively and a significantly higher Hb level than the MMWA group at 3, 6, and 12 months postoperatively (p < 0.05). Postoperatively, more improvements were observed in the MEWA group in UFS-QOL, VAS, and MFS than in the MMWA group (p < 0.05). A comparison of clinical efficacy between the MMWA and MEWA groups regarding various monitoring indicators pre- and postoperatively is shown in and and .
Figure 3. (a–d) Changes in Hb, CA125, uterine volume, uterine-volume reduction in MMWA and MEWA groups.
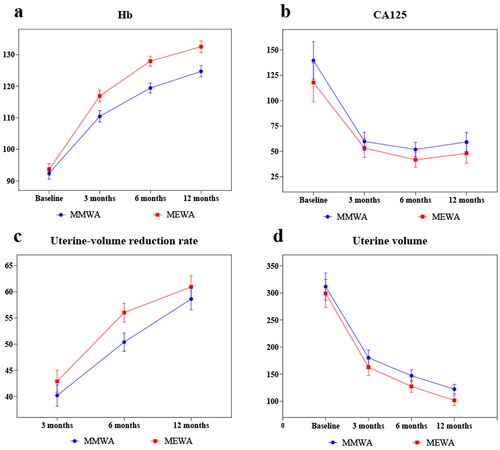
Table 4. Comparison of clinical efficacy between the two groups.
Adverse events and complications
Postoperative adverse events and complications included abnormal vaginal discharge, pain, fever, nausea, vomiting, hypomenorrhea, transient amenorrhea, and pelvic infections. However, no severe complications were reported, such as uterine perforation, bowel, or bladder injury. The incidence of adverse events was 68.8%, 12.5%, and 4.7% in SIR A, B, and C, respectively. Both SIR C were pelvic infections with long vaginal discharge (more than 20 days). The patient experienced postoperative pain, which resolved spontaneously within 24 h. In addition, postoperative vaginal fluid discharge mostly appeared pale pink or brown and lasted 0–60 days. The vaginal discharge in the MWA group disappeared within 1 week, whereas the vaginal discharge in the MEWA group lasted longer, mostly 2–4 weeks. The patients experienced low fever, which resolved spontaneously after 1 − 2 days of monitoring with oral medication. Up to 1 year postoperatively, five patients in the MEWA group had transient postoperative amenorrhea, which was resolved a few months later. Moreover, one patient developed intrauterine adhesions and consequent amenorrhea postoperatively, which was managed with lysis under hysteroscopy. In the two groups, 10 cases of hypomenorrhea were recorded.
Discussion
This results of this study revealed that MMWA had a positive clinical effect on patients with adenomyosis and anemia. Recently, MEA has been effective in improving adenomyosis-associated menorrhagia and reducing recurrence [Citation10]; however, no studies exist on the efficacy of the combined application of the two treatment methods. This study found that MEWA improved the symptoms and signs of adenomyosis in patients with anemia to a greater extent, allowed better improvement of anemia, and significantly improved patients’ quality of life.
In this study, 12 months postoperatively, patients with adenomyosis who had anemia in the MMWA group had an ORR of 95.3%. Most of the adverse events were classified as SIR A or B; SIR C was indicated for pelvic infection, and the vaginal discharge time was > 20 days. Prolonged vaginal discharge time may be associated with pelvic infection. These findings indicate that MMWA has relatively high clinical efficacy and safety for patients with adenomyosis who had anemia. Furthermore, in follow-up assessments, patients with adenomyosis who had anemia showed a significant reduction in VAS, UFS-QOL, and MFS, an increase in Hb level, a decrease in CA125 level, and a decrease in uterine volume 3 months after MMWA. These improvements persisted for 12 months after surgery. Thus, MMWA not only resulted in significant symptom improvement, anemia improvement, and improved quality of life within a short period in patients with adenomyosis who had anemia, but also allowed the improvement to be sustained for 1 year postoperatively. The mechanism underlying the MMWA-induced improvement in adenomyosis symptoms and signs in patients with anemia can be summarized as follows: (1) invagination of the endometrial basalis because of the activation of the uterine tissue injury and repair (TIAR) mechanism is one of the primary pathogenesis of adenomyosis [Citation16]. Due to the instantaneous high temperature, MMWA has a local thermal effect, including irreversible coagulative necrosis of target tissues and transmural injury of the vascular walls, which impedes the TIAR, thereby reducing the invasion of endometrial tissues into the myometrium and relieving dysmenorrhea; (2) the local heat generated by MMWA inhibits the expression of cyclooxygenase 2, estrogen receptor (ER), and progesterone receptor (PR); weakens the proliferating and repairing effect of estrogen on the endometrium; reduces prolactin secretion; and relieves excessive uterine contraction, thus reducing bleeding and improving dysmenorrhea [Citation17,Citation18]; (3) in addition, the uterine volume is significantly reduced, and the pressure on the surrounding veins is alleviated, which allows the uterine muscle to contract effectively, thereby improving symptoms, such as dysmenorrhea and menorrhagia.
In this study, no significant postoperative differences were observed between the two groups in uterine volume, CA125 level, ORR, or adverse event rate (p > 0.05). However, the postoperative Hb level was higher in the MEWA group than in the MMWA group (p < 0.05). In contrast, the uterine-volume reduction rate at 6 months postoperatively and the UFS-QOL, VAS, and MFS postoperatively were always better in the MEWA group than in the MMWA group (p < 0.05). Therefore, MEWA helps patients with adenomyosis and anemia by increasing Hb levels, improving anemia, and improving the quality of life. The high clinical efficacy of MEWA may be attributed to the following findings: (1) Menorrhagia and dysmenorrhea are associated with increased uterine volume, endometrial vascularization, prostaglandin, and estrogen levels [Citation19]. MEWA may reduce uterine volume, hormone receptor expression, and vascularized endometrial area to a greater extent, thus improving dysmenorrhea and anemia [Citation17,Citation18,Citation20]. (2) Adenomyosis symptoms are closely related to the junction zone (JZ). The contractile activity of the uterus during non-pregnancy originates from the JZ [Citation21], which is important in regulating menstrual volume [Citation22]. The JZ-regulated abnormal uterine contraction mode cannot effectively close ruptured blood vessels, resulting in abnormal uterine bleeding and increased uterine cavity pressure to aggravate dysmenorrhea [Citation23]. MEWA can destroy an abnormal JZ, weaken abnormal uterine contractions, and improve symptoms.
Endometrial histopathology of patients with adenomyosis after MEA treatment revealed significantly higher endometrial tubal metaplasia (TM) than in normal menstrual cycles. ER and PR expression were significantly low after MEA, implying that increased TM and a lack of expression of ER and PR in the endometrium following MEA may impact recurrence [Citation19]. However, in this study, the endometrial ablation range of the MEWA intima was only one-third of the endometrium, which not only reduced the degree of TM in the intima, avoided the lack of ER and PR expression, and reduced the possibility of recurrent bleeding, but also had the advantage of avoiding patient anxiety that would arise from amenorrhea due to excessive ablation of the endometrium. Since the endometrium at the uterine fundus was more likely to invade the myometrium [Citation24], one-third of the endometrium near the uterine fundus was selected for ablation in this study.
This study had certain limitations. First, the sample size included in this study was small, and this was a single-center study. Second, because of the study’s short follow-up period, it was impossible to determine the long-term efficacy of MEWA in treating patients with adenomyosis and anemia. Furthermore, the participants in this study were patients without fertility needs, and the effect of MEWA on fertility was not discussed. Therefore, the effects of MEWA on sex hormones and endometrial function will be discussed in future studies. A prospective multicenter study with a larger sample size will be conducted to confirm the long-term efficacy of MEWA compared with other treatments for adenomyosis.
In conclusion, MMWA and MEWA are effective and safe treatment options for patients with adenomyosis who have anemia. They may be preferred for patients who want to preserve their uterus but cannot achieve this goal when treated with other options. Notably, MEWA improves the symptoms of patients with adenomyosis who have anemia to a greater extent and improves anemia better than MMWA, resulting in a significantly improved quality of life.
Acknowledgments
The authors thank Taylor and Francis for the English language editing. The authors thank the anonymous reviewers for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Munro MG. Classification and reporting systems for adenomyosis. J Minim Invasive Gynecol. 2020;27(2):296–308.
- Tellum T, Nygaard S, Lieng M. Noninvasive diagnosis of adenomyosis: a structured review and Meta-analysis of diagnostic accuracy in imaging. J Minim Invasive Gynecol. 2020;27(2):408–418.e3.
- Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril. 2018;109(3):380–388.e1.
- Nelsen LM, Lenderking WR, Pokrzywinski R, et al. Experience of symptoms and disease impact in patients with adenomyosis. Patient. 2018;11(3):319–328.
- Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018;51:119–137.
- Pontis A, D'Alterio MN, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. 2016;32(9):696–700.
- Lee KH, Kim JK, Lee MA, et al. Relationship between uterine volume and discontinuation of treatment with levonorgestrel-releasing intrauterine devices in patients with adenomyosis. Arch Gynecol Obstet. 2016;294(3):561–566.
- Alvi FA, Glaser LM, Chaudhari A, et al. New paradigms in the conservative surgical and interventional management of adenomyosis. Curr Opin Obstet Gynecol. 2017;29(4):240–248.
- Lin XL, Hai N, Zhang J, et al. Comparison between microwave ablation and radiofrequency ablation for treating symptomatic uterine adenomyosis. Int J Hyperthermia. 2020;37(1):151–156.
- Philip CA, Le Mitouard M, Maillet L, et al. Evaluation of NovaSure® global endometrial ablation in symptomatic adenomyosis: a longitudinal study with a 36 month follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;227:46–51.
- Spies JB, Coyne K, Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Karcioglu O, Topacoglu H, Dikme O, et al. A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 2018;36(4):707–714.
- Li X, Zhu X, He S, et al. High-intensity focused ultrasound in the management of adenomyosis: long-term results from a single center. Int J Hyperthermia. 2021;38(1):241–247.
- Xu C, Tang Y, Zhao Y, et al. Use of contrast-enhanced ultrasound in evaluating the efficacy and application value of microwave ablation for adenomyosis. J. Cancer Res. Ther. 2020;16(2):365–371.
- Cardella JF, Kundu S, Miller DL, Society of Interventional Radiology, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl):S189–S191.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–379.
- Luo X, Shen Y, Song WX, et al. Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int. J. Gynaecol. Obstet. 2007;99(1):9–13.
- Yang Y, Zhang J, Han ZY, et al. Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. [Sci Rep]. Sci. Rep. 2015;5:10034.
- Nakayama K, Razia S, Ishibashi T, et al. Pathological findings in the endometrium after microwave endometrial ablation. [Sci Rep. 2020;10(1):20766.
- Kimura F, Hanada T, Nakamura A, et al. Case of adenomyosis causing the activation of the coagulation system after a complete loss of endometrium following microwave endometrial ablation. J Obstet Gynaecol Res. 2021;47(9):3385–3391.
- Brosens JJ, Barker FG, de Souza NM. Myometrial zonal differentiation and uterine junctional zone hyperplasia in the nonpregnant uterus. Hum. Reprod. Update. 1998;4(5):496–502.
- Fusi L, Cloke B, Brosens JJ. The uterine junctional zone. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):479–491.
- Xie T, Xu X, Yang Y, et al. The Role of Abnormal Uterine Junction Zone in the Occurrence and Development of Adenomyosis. Reprod Sci. 2021 Sep 13. Doi: 10.1007/s43032-021-00684-2.
- Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–538.