Abstract
Background
Although endobiliary radiofrequency ablation (RFA) may be an option for the treatment of ingrowth occlusion after self-expandable metal stent (SEMS) deployment, its utility remains uncertain. This study aimed to clarify its utility and safety using bovine liver.
Methods
A prototype multifunctional RFA catheter and conventional uncovered SEMS were employed in this experimental study. We devised three model types: the ingrowth-ablation, ingrowth-ablation with stent-wire contact (created such that the electrodes were in contact with the metal stent-wire), and standard-ablation models (control). The study outcome was the ablation depth associated with RFA, which was compared among the three models.
Results
Thirty-six ablation procedures were conducted (12 for each of the 3 models). In the unipolar mode, the median ablation depth with the stent-wire contact model (1.0 mm) was significantly lower than that of the ingrowth-ablation (2.0 mm, p = 0.005) and standard-ablation models (2.3 mm, p = 0.004). There was no significant difference between the ingrowth-ablation and standard-ablation models (p = 0.563). In the bipolar mode, the median ablation depth with the stent-wire contact model (1.0 mm) was also significantly lower than that of the ingrowth-ablation (2.1 mm, p = 0.008) and standard-ablation models (2.0 mm, p = 0.011), and there was no significant difference between the ingrowth-ablation and standard-ablation models (p = 0.807). Scorching around the stent-wire was not observed in any specimen.
Conclusions
In this ex vivo study, endobiliary RFA for ingrowth occlusion can be considered a useful modality, but the ablation effect is diminished when the electrode comes into contact with the stent-wire.
Introduction
Self-expandable metal stent (SEMS) deployment is recommended for the treatment of unresectable malignant biliary strictures, owing to its durability and longer stent patency period [Citation1]. However, stent occlusion eventually occurs in nearly half of cases even after SEMS placement, necessitating reintervention [Citation2]. Appropriate reintervention methods have not yet been established, and a method that is both simple and yields robust long-term results is needed.
Endobiliary radiofrequency ablation (RFA) is a promising adjuvant treatment modality that has emerged in recent years for the treatment for malignant biliary stricture [Citation3–4]. Studies have suggested that RFA may be able to extend the duration of biliary stent patency, and others have indicated that it can prolong survival in patients with extrahepatic cholangiocarcinoma [Citation5–7]. Endobiliary RFA functions by causing coagulative necrosis of the tissue in the stricture. Based on this mechanism of action, the management of ingrowth occlusion after SEMS placement can be considered as another indication for RFA [Citation8–11]. However, evidence for its application in this area is extremely scarce. Moreover, several procedure-related concerns exist, namely, whether RFA can be safely performed in the presence of a metallic stent, and whether it can yield a similar and appropriate effect without being affected by the stent’s metal component. This study aimed to clarify the utility and safety of endobiliary RFA for ingrowth occlusion after SEMS placement in the bovine liver.
Methods
Instruments
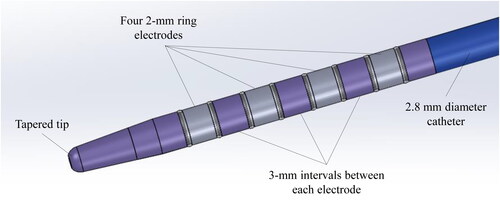
This study utilized a prototype multifunctional RFA catheter (Japan Lifeline Co., Ltd., Tokyo, Japan), which supports the use of unipolar, bipolar, and multipolar RFA. The catheter measures 2.8 mm in diameter and 2000 mm in length, which is compatible with a guidewire measuring 0.025/0.035 inch. Four 2-mm ring electrodes are placed at 3-mm intervals each, at the tip of the catheter (). The desired electrode can be set as the active electrode in the unipolar, bipolar, or multipolar mode. RFA energy is delivered by a modified ARFA-GEN200 generator (Japan Lifeline Co., Ltd.).
Figure 1. Prototype multifunctional RFA catheter. Four 2-mm ring electrodes are placed at the tip of the catheter at 3-mm intervals each. The catheter measures 2.8 mm in diameter and 2000 mm in length, compatible with a 0.025/0.035-inch guidewire. The intended electrode can be set as the active electrode in the unipolar, bipolar, or multipolar modes.
Experimental procedure
Freshly resected bovine liver specimens were used for this ex vivo experiment. Since no live animals were used, the requirement for ethics approval was waived. First, several blocks measuring 50 × 30 × 30 mm3 were sectioned from the liver specimens. Subsequently, three model types were created: the ingrowth-ablation model, the ingrowth-ablation with stent-wire contact model, and the standard-ablation model.
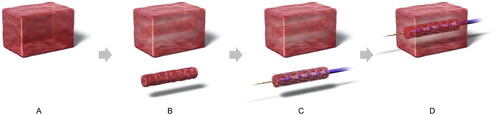
The ingrowth-ablation model was created as follows. A columnar portion of tissue measuring 10 mm in diameter was hollowed out from the center of the block using a cork borer. A 10 mm × 40 mm uncovered SEMS (BileRush Selective; Piolax Medical Devices, Kanagawa, Japan) was filled with this columnar tissue. Subsequently, 0.035-inch guidewire was passed through the center using an inserter, and the RFA catheter was inserted over the guidewire and positioned at the center of the stent. Finally, this assembly was refitted into the hollowed-out portion of the tissue block ().
Figure 2. Ingrowth-ablation model. Tissue blocks measuring 50 × 30 × 30 mm3 were sectioned from the bovine livers (A), and a 10-mm diameter columnar structure was hollowed out from the center of the block using a cork borer (B). A 10 mm × 40 mm uncovered metal stent was filled with this columnar tissue, and 0.035-inch guidewire was passed through the center of it, followed by insertion of the ablation catheter over the guidewire (C). Finally, this assembly was refitted into the hollowed-out tunnel in the block (D).
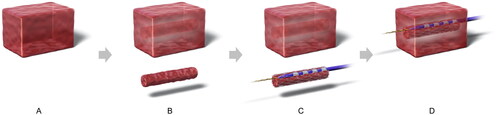
The ingrowth-ablation with stent-wire contact model was created as follows. After fitting the columnar structure tissue into the stent, the guidewire and RFA catheter were inserted into the chink between the stent and the columnar tissue (such that the electrodes were in contact with the stent-metal wire), which was then was refitted into the block (). In the standard-ablation model (i.e. control), the guidewire and RFA catheter were passed through the center of the block without hollowing out the tissue and without the stent.
Figure 3. Ingrowth-ablation with stent-wire contact model. Tissue blocks measuring 50 × 30 × 30 mm3 were sectioned from the bovine livers (A), and a 10-mm diameter columnar structure was hollowed out from the center of the block using a cork borer (B). The guidewire and ablation catheter were inserted into the chink between the stent and the columnar tissue structure (creating a situation where the electrodes are in contact with the stent-metal wire) (C), which was refitted into the hollowed-out block (D).
A total of 36 samples were created, including 12 of each of the three model types. All RFA procedures were performed using two active electrodes (i.e. the second and third ones from the tip). Ablation was performed 6 times with the unipolar mode and 6 times with the bipolar mode for each of the three models. Each RFA application was administered at 9 W of power for 90 s, which was in accordance with the previously reported setting for the bile duct stricture [Citation4]. After ablation, the block was sectioned longitudinally using a pathology knife to expose the stent. The stent was removed subsequently, and the internal ingrowth tissue was cut along the long axis. The ablation range was evaluated and measured macroscopically. The ablated region was fixed in formalin, embedded in paraffin, and sectioned for pathological analysis, which entailed staining with hematoxylin and eosin, to confirm ablation effect microscopically.
Outcomes
The study outcome included the ablation depth associated with RFA. The outcome of each model was compared. The ablation depth was defined as the length of tissue affected by ablation along the direction of the minor axis from the catheter. The presence/absence of any scorching around the stent wires was also evaluated thoroughly.
Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR) and compared using the Mann–Whitney U-test. P-values ˂0.05 were considered statistically significant. All statistical analyses were conducted using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [Citation12].
Results
The results of the experimental procedures are presented in . All RFA applications during the experimental procedure were technically successful. The ablated area was macroscopically recognized as yellowish-white changes, which were histologically recognized as coagulation necrosis.
Table 1. Depth of radiofrequency ablation in three types of models.
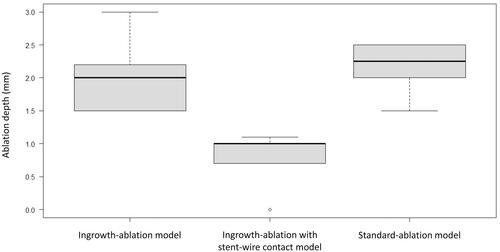
The median depth of the ablation area for experiments in the unipolar mode was 2.0 mm (IQR, 1.6–2.2), 1.0 mm (IQR, 0.8–1.0), and 2.3 mm (IQR, 2.0–2.5) for the ingrowth-ablation, ingrowth-ablation with stent-wire contact, and standard-ablation models, respectively. The depth in the stent-wire contact model was significantly lesser than that in the ingrowth-ablation (p = 0.005) and standard-ablation models (p = 0.004), (). No significant difference was observed between the ingrowth-ablation and standard-ablation models (p = 0.563) (). One application in the stent-wire contact model did not produce any ablation effect.
Figure 4. After ablation, the block was cut longitudinally using a pathology knife to expose the stent. A sufficient ablation effect within the stent can be confirmed in the ingrowth-ablation model (A), while the tissue is hardly ablated in the ingrowth-ablation with stent-wire contact model (B).
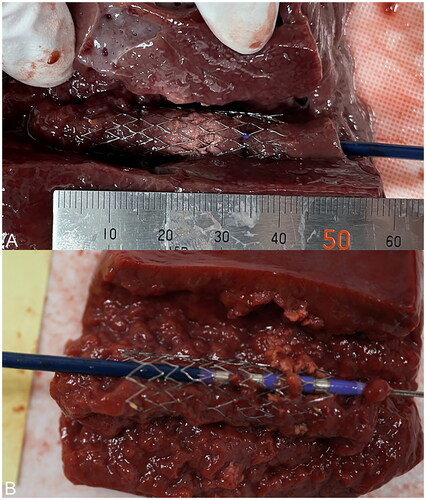
Figure 5. For experiments in the unipolar mode, the median ablation depth of the stent-wire contact model was significantly lower than that of the ingrowth-ablation model (p = 0.005) and the standard-ablation model (p = 0.004). There was no significant difference between the ingrowth-ablation and standard-ablation models (p = 0.563).
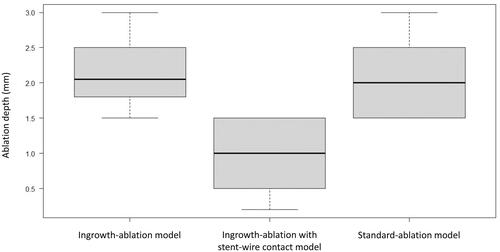
The median depth of the ablation area in experiments with the bipolar mode was 2.1 mm (IQR, 1.9–2.4), 1.0 mm (IQR, 0.6–1.4), and 2.0 mm (IQR, 1.6–2.4) with the ingrowth-ablation, ingrowth-ablation with stent-wire contact, and standard-ablation models, respectively. Similar to the results with the unipolar mode, the depth of the stent-wire contact model was significantly lower than that with the ingrowth-ablation (p = 0.008) and standard-ablation models (p = 0.011), and there was no significant difference between the ingrowth-ablation and standard-ablation models (p = 0.807) (). No scorching was observed around the stent-wire in any procedure.
Figure 6. For experiments in the bipolar mode, the median ablation depth of the stent-wire contact model was significantly lower than that of the ingrowth-ablation model (p = 0.008) and the standard-ablation model (p = 0.011), while there was no significant difference between the ingrowth-ablation and standard-ablation models (p = 0.807).
Discussion
This study demonstrated that RFA can be performed effectively for the management of ingrowth occlusion after SEMS placement. The presence of the SEMS had almost no impact on the ablation range, but the ablation effect was greatly diminished if the electrode was in close contact with the metal stent-wire.
Endobiliary RFA can cause coagulative necrosis in the stricture around the electrode, leading to a reduction in ingrowth and hyperplasia formation [Citation13–15]. Theoretically, this mechanism of action may also be effective for ingrowth occlusion after SEMS placement. It is expected that the stent can simply be recanalized by sweeping the necrotic ingrowth tissue after ablation, and long-term patency can be obtained without the need for further stent placement. Although only a few studies have investigated RFA for ingrowth occlusion, the median/mean patency period is reportedly 107 to 234 days without additional stent deployment [Citation16–19], which is highly desirable in a method of reintervention. However, the principal concern with this treatment modality is the impact of the presence of a metallic stent on RFA, especially the assurance of safety. In vivo and in vitro studies conducted by Yoon et al. [Citation20] found that the presence of a SEMS attenuated the effect of bipolar endobiliary RFA and that the tissue outside the SEMS was not likely to be affected. However, the reason underlying these observations has not been clarified comprehensively, and the result when the electrodes come into contact with the stent-wire, which is the most concerning aspect, is unclear.
In the present study, the ablation range was not affected by the presence of stents in the unipolar nor the bipolar setting. In other words, the ablation effect was not attenuated in the stent portion, and the difference between the absence and presence of the stent was insignificant. However, the ablation effect was substantially weaker when the electrode came into contact with the metal stent-wire. This may be attributed to the decrease in current density, which may be responsible for low clinical success rates. Kadayifci et al. [Citation17] reported that the ingrowth in the stricture was ablated successfully in only 56% of cases, where successful ablation was defined as eradication of the stricture to over 80% of the diameter of the SEMS. Conversely, from a safety perspective, it may be acceptable as long as ablation is performed under the standard setting for the bile duct stricture that has been reported in previous studies. However, caution should be exercised when the electrodes are in intimate contact with the stent. If temperature is used as an index and ablation is continued until it rises, it is assumed that the area around the electrode stent will be strongly ablated.
It is not possible to determine if the electrode is in contact with the wire when using the currently available RFA system, since visualization during the ablation procedure is only possible under fluoroscopic imaging. A system that can determine the absence/presence of contact between the RFA electrode and stent-wire is anticipated in the future. If contact between the electrodes and stent-wires can be identified during the procedure, the position of the catheter can be moved before and during ablation. Alternatively, a system that is unaffected by contact can be expected to yield a stable and robust therapeutic effect on ingrowth occlusion.
The results of this study should be interpreted in the context of its limitations, which arise from its ex vivo experimental design that used bovine liver specimens. Although we attempted to simulate clinical conditions, such as the use of an actual biliary SEMS, RFA in the experimental setting differs from that in the actual clinical setting. Differences exist between the bovine liver and ingrowth tissue; thus, the clinical efficacy and safety of the procedure are still uncertain. Therefore, the findings of this study must be validated by further clinical studies.
In conclusion, this ex vivo study showed that endobiliary RFA can be a useful option for ingrowth occlusion, but the ablation effect is diminished when the electrode comes into contact with the stent-wire. The contact between the electrode and the wire cannot be completely eliminated in the case of RFA for ingrowth, making this a problem that needs to be overcome if endobiliary RFA is to be used as a standard retreatment method in the future.
Author contributions
TI: conception and design, data acquisition, analysis, and interpretation, and drafting and revision of the manuscript. HK: data analysis and interpretation and revision of the manuscript. MI: data acquisition and revision of the manuscript. MY: data interpretation and revision of the manuscript
Acknowledgments
The authors thank Japan Lifeline Co., Ltd. for providing the prototype multifunctional radiofrequency ablation system and for assisting us with the experiments in this study.
Disclosure statement
Tadahisa Inoue received honoraria from Japan Lifeline Co., Ltd. And Boston Scientific Japan. The other authors have no financial relationships to disclose relevant to this publication.
Additional information
Funding
References
- Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results:October society of gastrointestinal endoscopy (ESGE) clinical Guideline – Updated October 2017. Endoscopy. 2018;50(9):910–930.
- Inoue T, Naitoh I, Suzuki Y, et al. Multi-center study of endoscopic revision after side-by-side metal stent placement for malignant hilar biliary obstruction. Dig Endosc. 2021;33(5):807–814.
- Sofi AA, Khan MA, Das A, et al. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87(4):944–951.e1.
- Inoue T, Yoneda M. Updated evidence on the clinical impact of endoscopic radiofrequency ablation in the treatment of malignant biliary obstruction. Dig Endosc. 2022;34(2):345–358.
- Yang J, Wang J, Zhou H, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50(8):751–760.
- Gao DJ, Yang JF, Ma SR, et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: a multicenter randomized controlled trial. Gastrointest Endosc. 2021;94(1):91–100.e2.
- Inoue T, Ibusuki M, Kitano R, et al. Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy. 2020;52(7):595–599.
- Pai M, Valek V, Tomas A, et al. Percutaneous intraductal radiofrequency ablation for clearance of occluded metal stent in malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2014;37(1):235–240.
- Nayar MK, Oppong KW, Bekkali NLH, et al. Novel temperature-controlled RFA probe for treatment of blocked metal biliary stents in patients with pancreaticobiliary cancers: initial experience. Endosc Int Open. 2018;6(5):E513–E517.
- Inoue T, Kitano R, Yoneda M. Endobiliary radiofrequency ablation for ingrowth occlusion after bilateral metal stent placement in patients with malignant hilar biliary obstruction. Endosc Int Open. 2021;9(6):E907–E908.
- So H, Oh CH, Song TJ, et al. Feasibility and safety of endoluminal radiofrequency ablation as a rescue treatment for bilateral metal stent obstruction due to tumor ingrowth in the hilum: a pilot study. J Clin Med. 2021;10(5):952.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Atar M, Kadayifci A, Daglilar E, et al. Ex vivo human bile duct radiofrequency ablation with a bipolar catheter. Surg Endosc. 2018;32(6):2808–2813.
- Kim EJ, Chung DH, Kim YJ, et al. Endobiliary radiofrequency ablation for distal extrahepatic cholangiocarcinoma: a clinicopathological study. PloS One. 2018;13(11):e0206694.
- Cho JH, Jeong S, Kim EJ, et al. Long-term results of temperature-controlled endobiliary radiofrequency ablation in a normal swine model. Gastrointest Endosc. 2018;87(4):1147–1150.
- Duan XH, Wang YL, Han XW, et al. Intraductal radiofrequency ablation followed by locoregional tumor treatments for treating occluded biliary stents in Non-Resectable malignant biliary obstruction: a Single-Institution experience. PloS One. 2015;10(8):e0134857.
- Kadayifci A, Atar M, Forcione DG, et al. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy. 2016;48(12):1096–1101.
- Xia N, Gong J, Lu J, et al. Percutaneous intraductal radiofrequency ablation for treatment of biliary stent occlusion: a preliminary result. World J Gastroenterol. 2017;23(10):1851–1856.
- Inoue T, Ibusuki M, Kitano R, et al. Endoscopic radiofrequency ablation for ingrowth occlusion following bilateral metal stenting for malignant hilar biliary obstruction: a prospective pilot study. ]. Gastrointest Endosc. 2022;S0016. 2022 Oct 8;5107(22):02023–02025. Published online ahead of print
- Yoon WJ, Kim YT, Daglilar ES, et al. Evaluation of bipolar radiofrequency ablation for occluded self-expandable metal stents in the bile duct: in vivo and in vitro study. Endoscopy. 2015;47(12):1167–1170.
