Abstract
Objectives
To determine rates of vascular toxicity, acute kidney injury (AKI), chronic kidney disease (CKD) and survival in high-risk cervical cancer patients treated with platinum-based induction chemotherapy followed by thermoradiotherapy.
Methods
Between January 1999 and April 2017, patients with large primary tumors (>6cm) and/or para-aortic lymph node (LN) metastases >1 cm and/or para-iliac LN >2 cm were included. Patient and tumor characteristics, Common Toxicity Criteria v4.03 scores, laboratory tests and treatment data were retrieved from patient records. CT scans were reviewed for the presence of thrombo-embolic events (TEE). The study protocol was approved by the Medical Ethics Review Committee of Erasmus MC, Rotterdam (MEC2017-133).
Results
The 105 included patients had a mean age of 47.9 years (range 22–79) and a median follow-up time of 43 months (IQR 14–72). Median tumor size was 6.0 cm (range 2.6–11.5), 30% had a clinical FIGO stage ≥ IIIB and 42% had enlarged para-aortic LN. Cisplatin-based therapy was started in 86 patients (82%), of whom 30 (35%) switched to carboplatin and 47% of patients completed six cycles of platinum-based chemotherapy. All patients received external beam radiotherapy as planned, 98 patients (93%) underwent brachytherapy as planned or received an external boost, and 95 patients (90%) completed all five planned hyperthermia treatments. During cisplatin chemotherapy, 34 patients experienced AKI (39%). At last follow-up, 35% of patients had chronic renal toxicity (GFR 59 − 15/min/1.73 m2). At presentation, a TEE was present in 10 (10%) and another 23 (22%) patients experienced a TEE (18% venous, 4% arterial) during chemotherapy. Five-year overall survival was 58% (95% CI 47.8–68.6 SE 0.053).
Conclusion
Achieving a five-year overall survival of 58%, platinum-based induction chemotherapy followed by thermoradiotherapy is an effective treatment for advanced-stage high-risk cervical cancer. However, treatment is accompanied by an unacceptably high prevalence of chemotherapy-associated TEE and acute kidney injury, as well as chronic kidney disease. Future studies should investigate the role of carboplatin in reducing toxicity and the effect of thromboprophylaxis in high-risk patients.
1. Introduction
Globally, cervical cancer is the second most commonly diagnosed cancer and third leading cause of cancer death in women [Citation1]. The standard treatment for advanced cervical cancer is external beam radiotherapy and image-guided adaptive brachytherapy [Citation2,Citation3], either with concurrent platinum-based chemotherapy [Citation4,Citation5] or thermoradiotherapy [Citation6]. Historically, high local failure rates in patients with large primary tumors (> 6 cm) and/or bulky lymph node metastases underlined the need to improve treatments. Since 1996, within the Erasmus MC Rotterdam patients with locally advanced cervical cancer with either bulky tumors (i.e., > 6 cm) or lymph node metastases to the common iliac (> 2 cm) or para-aortic lymph nodes (PAO) (> 1 cm), have been treated with weekly platinum-based chemotherapy followed by external beam radiotherapy (EBRT) combined with loco regional hyperthermia (thermoradiotherapy) and brachytherapy to maximize the chance of disease control and cure.
Vascular toxicity and in particular venous events are frequent complications in patients with cancer, and are associated with increased morbidity and mortality [Citation7]. Risk of a thrombo-embolic event in uterine cervical cancer is increased as compared to the general population. The annual incidence of thrombo-embolic events in the general population is about 0.5% [Citation8], compared to a reported incidence of 17% in 48 cervical cancer patients undergoing cisplatin-chemotherapy [Citation9]. In a previous paper, we reported a 40% incidence (17 of 43 patients) of grade 3–4 vascular toxicity in patients treated with platinum-based induction chemotherapy followed by thermoradiotherapy, necessitating further research regarding toxicity [Citation10].
Besides vascular events, acute and chronic renal toxicity is a well-known complication of cisplatin-based chemotherapy. Cisplatin is a highly effective drug in cervical cancer and is considered the standard of care. However, acute kidney injury (AKI) remains the dose-limiting complication of cisplatin, and can lead to a switch to carboplatin or even cessation of chemotherapy treatment. Studies have evaluated the clinical benefits of carboplatin [Citation11,Citation12]. Additionally, in a large retrospective study of 821 patients treated with cisplatin, up to 35% had permanent chronic kidney disease (CKD) stage 2 (eGFR 59–30 ml/min/1.73 m2) [Citation13].
While the platinum-based induction chemotherapy followed by thermoradiotherapy showed promising data for overall survival (OS) in our specific high-risk group showing a high local tumor load or affected para-aortic lymph nodes, the data on acute and long-term toxicities of treatment are unacceptable [Citation10]. The primary objective of this analysis was therefore to evaluate the incidence of acute renal and vascular toxicity, together with chronic kidney disease, in a larger cohort of patients with advanced cervical cancer undergoing platinum-based induction chemotherapy followed by thermoradiotherapy.
2. Methods
2.1. Patient selection and study design
In this retrospective cohort study we included all women treated between January 1999 and April 2017 with curative intent for uterine cervical cancer with induction chemotherapy followed by radiotherapy and hyperthermia. Patients were selected from our hyperthermia database and the study protocol was approved by Medical Ethics Review Committee of Erasmus MC, Rotterdam (MEC2017-133). All patients underwent external beam RT and hyperthermia at Erasmus MC, however some patients have received the brachytherapy in another center, but with similar protocols and dose regimens.
Patients were eligible if they had a large primary cervical tumor (>6 cm) and/or para-aortic lymph node metastases larger than 1 cm and/or iliac lymph node metastases larger than 2 cm. Also included were patients with recurrent disease after primary surgery who fulfilled the same criteria. Women with previous pelvic radiation were excluded. Staging was defined according to the International Federation of Gynecology and Obstetrics (FIGO) 2008 classification. Stage of disease is only given for women with primary tumors. Scheduled treatment consisted of platinum-based chemotherapy, followed by radiotherapy and hyperthermia. Treatment details can be found in a previous paper [Citation10] and in Supplemental Figure 1.
2.2. Chemotherapy
All patients were treated with platinum-based chemotherapy in a weekly schedule paclitaxel 90 mg/m2/cisplatin 70 mg/m2 or paclitaxel 90 mg/m2/carboplatin AUC 4 according to local hospital policy [Citation10]. See Supplemental Figure 1 and Text.
2.3. Radiotherapy
Two to six weeks after the completion of chemotherapy, patients started combined treatment consisting of external beam radiotherapy (RT), hyperthermia and brachytherapy. External beam RT was delivered by megavoltage 6–10 MV photons to treat the primary tumor, (proximal) vaginal wall, parametria, and draining pelvic lymph nodes. The same technique was applied when the para-aortic region was included up to the level of L2–L3 in case of positive lymph nodes along the common iliac artery. In case of the event of para-aortic lymph node metastasis, two parallel opposed anterior–posterior fields were used to include the draining lymph nodes up to the level of Th10–Th11. A total dose of 46 Gy was delivered in 2 Gy fractions to the pelvic field, when the radiation field comprised the para-aortic region as well, 48.6 Gy was delivered in 1.8 Gy fractions. In case of residual parametrial tumor at the time of first brachytherapy (BCT), patients usually received an additional pelvic sidewall boost, thus increasing the total dose delivered to the pelvic sidewall to 60 Gy, taking into account the dose contributed by BCT. External beam RT was delivered using a 3D conformal technique up to 2011, after which subsequently IMRT (intensity modulated radiotherapy) technique was introduced with a plan of the day protocol to decrease radiation exposure of bladder and bowel. This was again replaced in 2014 for a faster VMAT (volumetric arc therapy) technique [Citation14,Citation15]). BCT was first delivered using 192 Ir (high-dose rate) to a total dose of 17 Gy applied in two fractions, or 18–21 Gy in three fractions; using 137 Cs (medium-dose rate) in a single fraction of 20–24 Gy over 20–24 h or 30 Gy over 60 h (low-dose rate). The BCT technique employed depended on availability in the institute in which the RT was administered and may have changed during the study period (i.e., some institutes switched from low-dose rate to pulse-dose rate and/or high-dose rate). During the study period, most institutes made the transition toward image-guided brachytherapy in 2011–2012 [Citation16]. These technical advances in radiation dose delivery techniques are expected to decrease radiation exposure of bladder and bowel while maintaining or even improving the dose delivered to the target area. Typically, the maximum acceptable total dose in critical normal tissue structures contemplated in treatment planning was 50 Gy for the small bowel, 70 Gy for the rectum and 80 Gy for the bladder. During the radiotherapy treatment period, patients visited their radiation oncologist weekly for evaluation of acute treatment-related toxicity.
2.4. Deep locoregional hyperthermia
Patients were scheduled for five weekly hyperthermia sessions during the period of external beam radiotherapy. The BSD-2000 3 D system (BSD Medical Systems, Salt Lake City, UT, USA) was used for all treatments. For thermometry, Bowman probes were placed intraluminally in the bladder, vagina and rectum with closed-tip catheters. Thermal mapping along the catheters was performed every 5 min with a step size of 1 cm and a maximum map length of 14 cm. Pulse rate and blood pressure were automatically measured before and every 5 min during treatment, and oral temperature was measured at 0, 15, 30, 60 and 90 min. Heating started at a power output of 400 W at 77 MHz. Patients were carefully instructed to report any discomfort due to too high temperatures in normal tissue during treatment. If symptoms developed, treatment settings for power, phase and frequency were adjusted accordingly. If no symptoms developed, 100 W was added to the power output every 5 min. The treatment objective was to achieve intraluminal temperatures of 40–43 °C as homogeneously as possible. For all patients, 90-min sessions were scheduled for each hyperthermia treatment; 30 min to reach the initial target and 60 min of actual treatment time.
2.5. Toxicity
Serum creatinine was used to measure acute kidney toxicity (AKI) and AKI was graded according to Common Toxicity Criteria Adverse Events version 4.03 (CTCAE v4.03. 0: Cr <1.5x baseline; 1: Cr: 1.5–2.0 x baseline; 2: Cr: 2.0–2.9x baseline; Cr: ≥3.0x baseline.
Chronic Kidney Disease (CKD) was defined as eGFR below 60 ml/min/1.73 m2 for more than 3 months. Grading of CKD was defined as: 1: eGFR 89–60; 2: eGFR 59–30; 3: eGFR 29–15; 4: eGFR <15 (or dialysis or renal transplant indicated)(CTCAE v4.03). In electronic patient records normal laboratory values for eGFR are > 60 ml/min, so we therefore classified > 60 as grade 0. CKD was evaluated at three time points: before start of radiotherapy (equal to 10 weeks after start of chemotherapy), at the end of radiotherapy and the latest available value prior to any relapse.
Vascular toxicity was any thrombo-embolic event (TEE) reported as adverse event and confirmed by scheduled or unscheduled imaging, with CT scans reviewed independently.
2.6. Treatment evaluation
The electronic medical record for each patient was comprehensively reviewed, together with all records of treatment data, including any adjustments in chemotherapy treatment. In addition, dose delays and reductions related to renal impairment and the switch from cisplatin to carboplatin were both recorded. Laboratory values including complete blood counts, creatinine and eGFR values were abstracted for all patients.
Secondary endpoints were response rate (RR), progression free survival (PFS) and five-year overall survival (OS). After therapy completion and during follow-up, medical examinations were performed in accordance with the guidelines of the Dutch Association of Comprehensive Cancer Centers [Citation11]. CT scans were made for cancer staging, before start of radiotherapy and in case of suspicion of TEE or tumor progression.
A CT-thorax-abdomen is conducted to detect the presence of lymphadenopathy and distant metastases, and a MRI is used to measure tumor size and local tumor growth. In the present study, all scans were reevaluated by an experienced, independent radiologist (M.D.K.) for uniform reporting and rigorous assessment. The CT scan is scored according to RECIST-1.1 [Citation17]. Responses noted in this study are the best responses achieved at least 1 month during follow-up. All data were anonymized and data collection as well as study management were performed using OpenClinica open source software version 3.12 (OpenClinica LLC and collaborators, Waltham, MA, USA).
2.7. Statistical considerations
Baseline characteristics of study patients were summarized in terms of frequencies and percentages for categorical variables, with means and standard deviation (SD), or median and interquartile range (IQR) for continuous variables.
Laboratory values for toxicity analyses were categorized in toxicity grades. Logistic regression modeling techniques were used to examine individual and multiple relations between patient, tumor and treatment predictor variables, and as well as the binary outcome of the occurrence or nonoccurrence of a TEE.
Overall survival (OS) and progression free survival (PFS) for primary tumors were depicted using Kaplan–Meier analysis, censoring for date of last contact for PFS analysis. OS was defined as the interval between the date on which treatment ended and the date of death or last visit/date last known to be alive. PFS was defined as the interval between the date on which treatment ended and the date of documented disease progression or of last follow-up.
A Cox regression model was used to evaluate the association between clinical variables, OS and local control. Regarding local control, an event was defined as either a partial response, stable disease or as disease progression during follow-up. Survival curves and Cox regression analyses were only conducted for patients with a primary tumor. p Values of less than .05 were considered significant. Data analysis was carried out in SPSS version 26.0 (IBM, Chicago, IL).
Concerning the prediction models for TEE or AKI during chemotherapy, variables associated with an increased risk of TEE (p < .20) in univariate analysis were included in a multivariate logistic regression model for the stepwise selection process [Citation18,Citation19]. In the multivariate logistic and Cox regression analyses, we only accepted 10 events per variable to avoid overfitting [Citation20]. The reported p values are not adjusted for multiple testing.
3. Results
3.1. Patient and tumor characteristics
Over the study period, 105 patients met the inclusion criteria (see Supplemental Figure 2). Mean age was 47.9 years (range 22–79), median tumor size was 6.0 cm (range 2.6–11.5) and a clinical FIGO stage ≥ IIIB (including hydronephrosis) was diagnosed in 33%. Seven patients (6.7%) received platinum-based induction chemotherapy followed by thermoradiotherapy for their first recurrence after surgery, at a median six months (range: 1–179 months). In the total group, 42% of patients (44/105) had radiologically enlarged para-aortic lymph nodes and the most common histology was squamous cell carcinoma (86%). The median follow-up period was 43 months (IQR 14–72). Patient and tumor characteristics are summarized in .
Table 1. Patient and tumor characteristics of the locally advanced cervical cancer group (N = 105).*
3.2. Chemotherapy
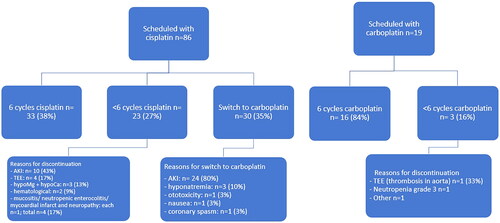
Of the 105 patients, 47% of patients completed the six cycles of platinum-based chemotherapy. Eighty-six patients (82%) started treatment with cisplatin and 19 (18%) with carboplatin (). Chemotherapy was discontinued in 23 patients (27%) receiving cisplatin and in 3 patients (16%) receiving carboplatin. Thirty patients (35%) switched from cisplatin to carboplatin, mainly due to renal toxicity (80%). Other reported toxicities were nausea grade 3 (n = 1), grade 3 hyponatremia (n = 3), grade 2 ototoxicity (n = 1) and coronary spasm (n = 1). Treatment planning and reasons for amendments are visualized in .
3.3. Radiotherapy and hyperthermia
All patients received external beam radiotherapy as planned. Fifty-seven (54%) patients received an External Beam Radiation Therapy (EBRT) schedule of 27 × 1.8 Gy and 46 patients (44%) received the 23 × 2.0 Gy. When the EBRT schedule is applied, it has mainly to do with the extension of the radiation fields. If no lymph node or only the lower iliac lymph node metastasis were present, the para-aortic lymph node chain could be excluded from the radiation field and a dose of 23 × 2 Gy was delivered. If lymph node metastases were present in the higher echelons, the para-aortic lymph node chain had to be included in the radiation field exponentially increasing the dose to vulnerable small bowel structures. In that case, a lower dose per fraction was chosen to decrease bowel toxicity. The time interval between chemotherapy and thermoradiotherapy is approximately mean 4.1 weeks (range: 1–8weeks). Using Fisher’s Exact test: there were no differences observed in toxicity (p = .56) and response (p = .65) between the two radiation schedules. In the case of 27 patients treated since 2009, the current treatment planning system allowed us to reliably estimate kidney dose, which did not exceed above the set threshold. The mean renal dose for both kidneys was 11.8 Gy (range 0.85–17) and the mean dose in 1/3 of both kidneys (D33) was 10.05 Gy (range 0.1–16.9).
Of the 105 patients, 90 (86%) underwent brachytherapy as planned. Eight patients did not receive brachytherapy due to insufficient tumor regression or anatomical changes. An external boost was given in these cases. Brachytherapy was not feasible in seven cases since these patients previously had a primary surgery. Ninety-five patients (90%) completed all five planned hyperthermia treatments. Among the 10 patients who stopped hyperthermia due to severe discomfort or intolerance, 4 patients received three sessions or less and 6 patients received four sessions.
3.4. Response evaluation and survival
Salvage surgery was performed in nine patients (8.6%). Eighty-six patients (82%) had a complete response after completing therapy (). Five-year OS was 58% (95% CI 47.8–68.6, SE 0.053) (Supplemental Figure 3a & Table 3b) and five-year PFS was 61% (95%CI 50.1–71.5, SE 0.055) (Supplemental Figure 4a & Table 4b). Thirty-three patients recurred, of whom 25 within 1 year after therapy. The mean time to recurrence was 10 months (range 1 to 72 months).
Table 2. Radiological response after three or six cycles of neo-adjuvant chemotherapy (NAC) and at the end of treatment, using RECIST criteria.
3.5. Toxicity analyses
Complete chemotherapy toxicity data were available for 104 patients (99%). AKI was reported in 46 patients (44%); 28 events (27%) were grade 1, 9 were grade 2 (8.6%) and 9 grade 3 (8.6%) (). Other grade 3–4 toxicities included myocardial infarction (n = 1), cerebrovascular event (n = 1), thrombotic stenosis of the aorta (n = 2), thrombosis of the arm (n = 1), symptomatic cerebral venous sinus thrombosis (n = 1), hematological toxicity (n = 8), hypomagnesaemia (n = 3), hypocalcemia (n = 2), pneumonia (n = 1), mucositis (n = 1), nausea/vomiting (n = 1), neutropenic enterocolitis (n = 1) and neuropathy (n = 1). Seven patients experienced more than one grade 3–4 adverse events (see ).
Table 3. Acute and chronic toxicity scored with CTCAE.a
After treatment 38 patients had grade 2 CKD. Twenty-nine patients (28%) had hydronephrosis before start of treatment and 13 of these had an indication for percutaneous nephrostomy. Thirty-seven (35%) patients had grade 2 CKD one month after treatment (see ). At last follow-up, two patients had developed end-stage renal failure. Patients treated with carboplatin plus paclitaxel-based chemotherapy as NAC experienced temporary haematologic toxicity; however, acute nephrotoxicity was not observed in these patients.
3.6. Vascular toxicity
At presentation and before start of chemotherapy 10 patients had a TEE, including pulmonary embolisms (n = 2), deep venous thromboses (n = 7) and a thrombus of the renal vein (n = 1). During chemotherapy, 19/105 patients (18%) experienced a TEE (20 events in total, as one patient suffered two events). The most frequent TEE was pulmonary embolism (n = 9), of which four were incidental findings on a routine CT scan. In addition, two other events were discovered incidentally (a thrombosis of the renal vein and a deep venous thrombosis, Supplemental Table 5). Four patients developed an arterial TEE during chemotherapy (3.8%), consisting of one myocardial infarction, one cerebrovascular accident and an aortic thrombosis in two patients. After completing platinum-based induction chemotherapy followed by thermoradiotherapy, one patient experienced thrombosis of the inferior vena cava.
All patients with a venous TEE were treated with low-molecular-weight heparin. The patients with arterial TEE were treated with platelet aggregation inhibitors. None of the patients had a recurrent thrombotic event during follow-up.
3.7. Regression analyses
The multivariate logistic regression analyses did not show significance in kidney injury or in the prediction of TEE during chemotherapy (data not shown). In addition, none of the variables had an influence on OS and PFS in the Cox regression analyses.
4. Discussion
In this retrospective study, we investigated vascular toxicity, acute kidney injury and chronic kidney disease in high-risk patients with locally advanced cervical cancer who received platinum-based induction chemotherapy followed by thermoradiotherapy. The chemotherapy regimen is based on previous phase I/II data in patients with ovarian cancer who received neo-adjuvant therapy [Citation21]. With a five-year overall survival (OS) rate of 58%, this therapy seems to be relatively effective. However, this was achieved at the cost of chemotherapy-associated vascular toxicity in 22%, acute kidney injury (AKI) in 70% and chronic kidney disease (CKD) in 35% of the patients. These results were in contrast to previous phase I/II studies with weekly paclitaxel and cisplatin or carboplatin [Citation21,Citation22], and were neither reported in the phase III studies with neo-adjuvant chemotherapy for locally advanced cervical cancer [Citation23,Citation24].
Cisplatin-induced acute kidney injury (AKI) remains a clinical problem for patients with advanced cervical cancer. In the present study, approximately 40% of the patients who received cisplatin experienced an AKI event during chemotherapy, resulting in an adjustment of schedule or type of chemotherapy (switch to carboplatin) or even cessation of chemotherapy. As a result, only 38% of the patients completed the scheduled cisplatin therapy. Several risk factors for cisplatin-induced AKI in diverse cancer types have been reported in the literature, including older age, female gender, a history of smoking [Citation25], hypertension, diabetes [Citation26] and cumulative doses of cisplatin [Citation27]. In our study, multivariate logistic regression analyses did not reveal risk factors (data not shown). This may be due to the relatively small number of patients included, and some data on smoking, hypertension and diabetes was missing.
Chronic kidney disease (CKD) was prevalent in survivors subsequent to platinum-based induction chemotherapy followed by thermoradiotherapy. After a median follow-up time of 43 months, 33% of the survivors had permanent chronic kidney disease of whom one underwent a kidney transplant. This confirms data reported in a large retrospective study in which up to 32% had a permanent chronic kidney disease of grade 2 or more (eGFR 59–30 ml/min/1.73 m2) [Citation13]. Less severe renal toxicity is also unfavorable since a decreased GFR is associated with increased risks of cardiovascular disease and all-cause mortality [Citation28]. Current protocols do not offer guidance on long-term health issues in these survivors, but improved treatment will result in a larger group of patients that require monitoring of renal function and education concerning the risk and prevention of late treatment effects.
Vascular toxicity was also prevalent among the patients in this study, and 10% were diagnosed with a venous thrombo-embolic event (TEE) at presentation. This number is higher than expected and might be related to specific characteristics of the cancer [Citation29]. Subsequently, a very high incidence of vascular toxicity (18% venous, 4% arterial TEE) was observed during chemotherapy, although this was in line with levels of vascular toxicity in cisplatin treated patients in earlier studies [Citation30,Citation31]. Several explanations can be offered for the high frequency of thrombo-embolic events (TEEs) during chemotherapy. Firstly, patients with a malignancy are hypercoagulable and are therefore at increased risk for developing thromboses [Citation32]. Hypercoagylation may be the result of alterations or injury to coagulation factors, anticoagulant proteins or endothelial cells [Citation9]. In addition, chemotherapy can act as an extra trigger, contributing to an increased incidence of thrombotic events by causing vascular endothelial injury or by producing changes in the clotting cascade [Citation33]. Cisplatin-induced renal toxicity might also play a contributing role, as decreased renal function increases TEE risk [Citation34]. TEE is a serious condition in the oncology setting, as it represents the leading cause of death in ambulatory patients undergoing chemotherapy [Citation35]. Large randomized studies of the use of low-molecular-weight heparin (LMWH) in a heterogeneous cancer population receiving chemotherapy showed a significant reduction in TEE. This was without impact on OS and with a favorable toxicity profile [Citation36,Citation37]. A recent meta-analysis of almost 12,000 cancer patients, undergoing either surgery or chemotherapy, found that prophylactic LMWH resulted in a significant decrease in TEEs, and pooled analyses did not show an increase in major bleeding events [Citation38]. It is important to realize that the present study population included patients with large, locally advanced cervical cancers of whom 42% had pathological para-aortic (PAO) lymph nodes at presentation, making this a very unfavorable risk group for recurrence-free survival. Historical five-year OS rates in literature are low for patients with locally advanced cervical cancer with or without positive PAO lymph nodes. In the EMBRACE-I multicenter prospective cohort study, 98 patients with pathological PAO lymph nodes had a five-year OS of 61% with standard chemoradiation and brachytherapy, while 190 patients with a FIGO IIIB status had an OS of 59% [Citation39]. Furthermore, 28% of patients in our study had hydronephrosis at presentation, which is also an indication of advanced-stage disease in cervical cancer patients. In literature, the presence of PAO lymph nodes and hydronephrosis reportedly have a negative effect on the survival of patients with locally advanced cervical cancer [Citation40].
The particular patient group in our study is characterized by more extensive locally advanced disease, therefor it was also difficult to find comparable cohorts which could help to reliably determine the efficacy of the platinum-based induction chemotherapy followed by thermoradiotherapy. Currently, there is no national or international consensus on the standard treatment approach. Historically patients were treated locoregionally with EBRT alone with mediocre success. Since 1999 chemoradiation including simultaneous administration of platinum-based chemotherapy has been widely adopted as standard of care [Citation41]. Radiotherapy with hyperthermia has been shown to be an equivalent locoregional treatment, especially for larger size tumors [Citation6,Citation42,Citation43], although less thoroughly investigated than the addition of platinum-based chemotherapy. With the large-scale adoption of combined simultaneous chemoradiation in 1999, there were some concerns on the toxicity of extended field radiotherapy (including the common iliac and/or para-aortic lymph nodes) or bulky tumor load combined with platinum-based chemotherapy). Till now, no prognostic advantage is found for neo-adjuvant chemotherapy before chemoradioation or before surgery [Citation23,Citation24]. Additionally, adjuvant chemotherapy following chemoradiation also did not show difference in overall survival or progression-free survival [Citation44]. Therefore, we chose to aim for downstaging of the local tumor and the elimination of possible micrometastatic disease by administering neo-adjuvant chemotherapy, sequentially followed by extended field radiotherapy with hyperthermia as a radiosensitizer. Hyperthermia is expected to improve the clinical effectiveness of radiotherapy alone by improving oxygenation, inhibiting DNA repair, stimulating the immune response to tumor cells and even direct cell death at temperatures ≥ 42 °C [Citation45].
4.1. Strengths and limitations
Important strengths of this study included the large number of subjects treated with a well-described platinum-based induction chemotherapy followed by thermoradiotherapy for advanced cervical cancer, supported by a long follow-up time and substantial toxicity data. To the best of our knowledge, long-term toxicity data concerning this therapy have not been previously published. Preliminary findings on both vascular and renal adverse events pushed us to consider replacing cisplatin induction by carboplatin. However, given the retrospective design, this study has several important limitations. As the patient group is heterogeneous, including patients with various highly unfavorable prognostic features, it is difficult to compare our survival outcomes with previous studies. Another limitation was that, in order to collect toxicity data, we could only select patients who received chemotherapy and radiotherapy in our clinic. Finally, our study was limited by selection bias: patients were selected from the hyperthermia database. Patients who started but did not respond to induction chemotherapy were therefore not included in the study, even though they were treated over the same time period and may have had similar tumor characteristics.
Newer techniques, in particular image-guided brachytherapy, achieve better local control and may improve outcomes in women with advanced cervical cancer [Citation37]. Patients with similar profiles to our study group are likely to benefit from MRI-guided and interstitial brachytherapy, which was not available during the entire study period.
5. Conclusion
With a five-year overall survival rate of 58%, platinum-based induction chemotherapy followed by thermoradiotherapy appears relatively effective in the treatment of advanced-stage, high-risk cervical cancer. However, this is accompanied by an unacceptably high prevalence of vascular toxicity, acute kidney injury as well as chronic kidney disease. Future studies should investigate the potential role of carboplatin reducing toxicity and the benefits of thromboprophylaxis in this high-risk patients groups.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Review Committee of Erasmus MC, Rotterdam (MEC2017-133).
Patient consent
Patient consent was waived due to the retrospective nature of the study.
Author contributions
X. S. Gao, I. A. Boere, M. J. H. A. Kruip, H. C. van Doorn: study conception and design; X. S. Gao: data acquisition and statistical analysis; I. A. Boere, M. Franckena, H. C. van Doorn, H. J. v Beekhuizen: treatment of patients; X. S. Gao, I. A. Boere, M. J. H. A. Kruip, H. C. van Doorn: interpretation of the data; X. S. Gao, I. A. Boere, M. J. H. A. Kruip, H. C. van Doorn: drafting of the manuscript; X. S. Gao, I. A. Boere, H. J. van Beekhuizen, M. D. Kulawska, M. J. H. A. Kruip, M. Franckena, R. Nout, H. C. van Doorn: critical revision of the manuscript for important intellectual content and final approval of the manuscript.
Supplemental Material
Download PDF (321.9 KB)Acknowledgments
We would like to thank G. Papageorgiou for his feedback regarding statistical aspects. Manuscriptedit service (www.manuscriptedit.com), edited this manuscript for language. Funding for this editing service was provided by the author.
Disclosure statement
MJHAK has received an unrestricted grant (outside this work) from Sobi; payment to the Erasmus Medical Center and speakers fee from Sobi, Roche and Bristol-Myers Squibb, payment to the institute. RN reports having received funding for research other than the current work from the Dutch Cancer Society, Dutch Research Council, Elekta, Varian and Accuray. XG, IB, HB, MF, MDK and HD have nothing to declare.
Data availability statement
The data supporting the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
- Gaffney DK, Erickson-Wittmann BA, Jhingran A, et al. ACR appropriateness criteria(R) on advanced cervical cancer expert panel on radiation Oncology-Gynecology. Int J Radiat Oncol Biol Phys. 2011;81(3):609–614.
- Hellebust TP, Kirisits C, Berger D, et al. Recommendations from gynaecological (GYN) GEC-ESTRO working group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96(2):153–160.
- Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;(3):CD002225.
- Vale CL, Tierney JF, Davidson SE, et al. Substantial improvement in UK cervical cancer survival with chemoradiotherapy: results of a royal college of radiologists’ audit. Clin Oncol (R Coll Radiol). 2010;22(7):590–601.
- Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev. 2010;(1):CD006377.
- Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850.
- Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg. 2003;25(1):1–5.
- Jacobson GM, Kamath RS, Smith BJ, et al. Thromboembolic events in patients treated with definitive chemotherapy and radiation therapy for invasive cervical cancer. Gynecol Oncol. 2005;96(2):470–474.
- Heijkoop ST, Franckena M, Thomeer MG, et al. Neoadjuvant chemotherapy followed by radiotherapy and concurrent hyperthermia in patients with advanced-stage cervical cancer: a retrospective study. Int J Hyperthermia. 2012;28(6):554–561.
- Heijkoop ST, Langerak TR, Quint S, et al. Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT. Int J Radiat Oncol Biol Phys. 2014;90(3):673–679.
- Kroesen M, Mulder HT, van Holthe JML, et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother Oncol. 2019;140:150–158.
- Pötter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83(2):148–155.
- Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33(19):2129–2135.
- Lorusso D, Petrelli F, Coinu A, et al. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol Oncol. 2014;133(1):117–123.
- Latcha S, Jaimes EA, Patil S, et al. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;11(7):1173–1179.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–5528.
- Steyerberg EW, Eijkemans MJC, Harrell FE, et al. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710–718.
- de Jongh FE, de Wit R, Verweij J, et al. Dose-dense cisplatin/paclitaxel. a well-tolerated and highly effective chemotherapeutic regimen in patients with advanced ovarian cancer. Eur J Cancer. 2002;38(15):2005–2013.
- Torfs S, Cadron I, Amant F, et al. Evaluation of paclitaxel/carboplatin in a dose dense or weekly regimen in 66 patients with recurrent or primary metastatic cervical cancer. Eur J Cancer. 2012;48(9):1332–1340.
- Gemma K, Stefano G, Ignace V, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage Ib2-IIb cervical cancer, EORTC 55994. J Clin Oncol. 2019;37(15_suppl):5503.
- Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36(16):1548–1555.
- Roura E, Castellsagué X, Pawlita M, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer. 2014;135(2):453–466.
- Mizuno T, Ishikawa K, Sato W, et al. The risk factors of severe acute kidney injury induced by cisplatin. Oncology. 2013;85(6):364–369.
- Stewart DJ, Dulberg CS, Mikhael NZ, et al. Association of cisplatin nephrotoxicity with patient characteristics and cisplatin administration methods. Cancer Chemother Pharmacol. 1997;40(4):293–308.
- Astor BC, Hallan SI, Miller ER 3rd, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226–1234.
- Cohen A, Lim CS, Davies AH. Venous thromboembolism in gynecological malignancy. Int J Gynecol Cancer. 2017;27(9):1970–1978.
- Seng S, Liu Z, Chiu SK, et al. Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30(35):4416–4426.
- Proverbs-Singh T, Chiu SK, Liu Z, et al. Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(23):1837–1840.
- Prandoni P, Piccioli A, Girolami A. Cancer and venous thromboembolism: an overview. Haematologica. 1999;84(5):437–445.
- Soultati A, Mountzios G, Avgerinou C, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38(5):473–483.
- Monreal M, Falgá C, Valle R, et al. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE registry. Am J Med. 2006;119(12):1073–1079.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634.
- Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10(10):943–949.
- Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601–609.
- Liu M, Wang G, Li Y, et al. Efficacy and safety of thromboprophylaxis in cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920907540.
- Pötter R, Tanderup K, Schmid MP, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22(4):538–547.
- Pergialiotis V, Bellos I, Thomakos N, et al. Survival outcomes of patients with cervical cancer and accompanying hydronephrosis: a systematic review of the literature. Oncol Rev. 2019;13(1):387.
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–1153.
- Franckena M, Stalpers LJ, Koper PC, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the dutch deep hyperthermia trial. Int J Radiat Oncol Biol Phys. 2008;70(4):1176–1182.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Mileshkin L, Moore KN, Barnes E, et al., editors. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: the randomized phase III OUTBACK Trial (ANZGOG 0902, RTOG 1174, NRG 0274). 2021 ASCO Annual Meeting; 2021 Jun 4–8, Online Meeting. J Clin Oncol 39, 2021 (suppl 15; abstr LBA3). Available from: https://meetinglibrary.asco.org/record/196619/abstract.
- van den Tempel N, Horsman MR, Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia. 2016;32(4):446–454.