Abstract
Introduction
Uterine fibroids are the most common benign tumors in healthy women. High Intensity Focused Ultrasound (HIFU) is a modern, noninvasive thermal ablation method for treating uterine fibroids. There is increasing evidence that ultrasound guided HIFU (US-HIFU) has no adverse impact on ovarian reserve but little data exists on magnetic resonance guided HIFU (MR-HIFU). There are different options to estimate ovarian reserve, perhaps the most reliable being the measurement of serum Anti-Müllerian hormone (AMH).
Material and methods
Seventy-four (74) premenopausal women with serum AMH 0.1 ug/L or over, aged 24–48 and with fibroids or adenomyosis treated with MR-HIFU were enrolled in our study. AMH levels were analyzed before and 3 months after the MR-HIFU treatment. Correlations between AMH level changes and position of fibroids, fibroid volume, non-perfused volume ratio, and treatment energies were studied.
Results
The median AMH level before the HIFU treatment was 1.20 (range: 0.1–7.75 ug/L) and after the treatment 1.23 (range: 0.1–8.51 ug/L). No significant change was detected (p = .90). The patients were divided in three subgroups depending on the baseline AMH levels. The changes were not significant in any of the subgroups. Neither did the location of the treated fibroid affect the change of AMH levels nor the total energy used during treatment.
Conclusions
MR-HIFU does not compromise the ovarian reserve. Neither the location of the treated fibroid nor the total energy used during MR-HIFU had any effect on the change of AMH levels.
Introduction
Uterine fibroids are the most common benign tumors in healthy women. It is estimated that about 70% of women get a fibroid by the age of 50. Most of the fibroids are asymptomatic but unfortunately the women suffering from symptoms caused by uterine fibroids have a severely compromised quality of life. Common symptoms caused by fibroids are menorrhagia, dysmenorrhea, lower abdominal pain and pelvic pressure. Fibroids can also have an adverse impact on fertility and interfere with pregnancy in multiple ways [Citation1].
There are many different treatment options for symptomatic uterine fibroids. These are medical treatments (hormonal medication, selective progesterone receptor modulators, and medications aiming to reduce bleeding i.e., nonsteroidal anti-inflammatory drugs, tranexamic acid), surgical methods (hysterectomy, laparoscopic myomectomy, open myomectomy and hysteroscopic removal of fibroids) and non-surgical treatment methods (Uterine Artery Embolization, and HIFU) [Citation1].
HIFU is a modern, noninvasive thermal ablation method for treating uterine fibroids. An external ultrasound energy source is used to create thermal ablation inside the body in the tissue to be treated. Either ultrasound (US) or magnetic resonance imaging (MRI) is used for treatment guidance. MRI is superior in providing excellent soft tissue contrast and real-time temperature measuring.
MR-HIFU treatments of uterine fibroids have been performed in Turku University Hospital since May 2016. Since MR-HIFU is considered to be a fertility preserving treatment suitable even for infertility patients, we have considered the safety of this treatment to be of paramount importance. In this context, the impact of the treatment on ovarian reserve has a central role. The close proximity of ovaries to the fibroid and the nature of the treatment (local heating) might cause concern about its effect on ovarian function and reserve.
Alongside its effect on fertility, the ovarian reserve and early onset of menopause are related to a broad spectrum of long-term health risks in women. The negative effects of early menopause include progression of cardiovascular disease, decreased bone density and risk of fracture, negative effect on mental health and cognition. It has been clearly documented that an early menopause increases the risk of frailty among women [Citation2–4].
There are multiple methods for estimating ovarian reserve and function. Follicle stimulating hormone (FSH), antral follicle count (AFC) and Anti-Müllerian hormone (AMH) can be used [Citation5]. AMH is a glycoprotein hormone produced by the granulosa cells of small, growing follicles in the ovary. Serum AMH levels strongly correlate with the number of growing follicles, and therefore AMH has received increasing attention as a marker for ovarian reserve [Citation6,Citation7]. It reaches its highest levels after puberty and decreases over time until it gets undetectable about 5 years before the menopause. AMH levels remain relatively stable during menstrual cycle, which is a major benefit compared to other possible markers [Citation8]. AMH has little intra-cyclic variation although a small variation can be detected, and the plasma level is slightly higher during follicular than luteal phase. Also the inter-cyclical variation is known to be minor [Citation9,Citation10]. There is also some evidence that AMH levels decrease due to the use of oral contraceptives. However, this effect seems to be reversible [Citation7,Citation8,Citation11,Citation12].
There is increasing evidence that US-HIFU has no adverse impact on ovarian reserve but not many reports can be found about MR-HIFU [Citation5,Citation6,Citation13]. Despite a strong argument in favor of its safety, we have in our center encountered one case in which the ovarian function was significantly decreased and AMH lowered after MR-HIFU treatment. This case has also undergone an investigation concerning our legal liability for the possible unfairly caused harm to the patient by the national Patient insurance center. Thus, we felt that it was of utmost importance to analyze the effect of the HIFU treatment on the ovarian reserve of all our patients in detail and this was done by analyzing AMH levels. The case report is presented in more details in the Supplementary material.
Materials and methods
Seventy-four (74) premenopausal women with baseline AMH 0.1 μg/L and over were enrolled in our study. The age of the women was 24–48 years. 69 of the women had a symptomatic fibroid and five had adenomyosis evaluated to be suitable for MR-HIFU treatment. Criteria for MR-HIFU treatment were: diameter of fibroid primarily up to 10 cm (one patient with a fibroid diameter of 11 cm was also included due to lack of other treatment options), number of treated fibroids up to three, subcutaneous fat up to 4 cm, suitable fibroid structure on planning MR images (primarily Funaki type 1 or 2 with low to intermediate blood flow levels, but also some Funaki type 3 fibroids with higher blood flow levels were included), suitable anatomical position of the target making it MR-HIFU treatable and suitable positioning of ovaries making it possible to implement the treatment without damaging them. More detailed information about the study population is presented in .
Table 1. More detailed information about the study population.
This study was performed in accordance with the ethical regulations of the Ethics Committee of the Hospital District of Southwest Finland and the National Committee of Medical Research Ethics (T366/2017 25.1.2018) and registered in clinicaltrials.gov NCT03937401. Written informed consent was obtained from all patients.
MR-HIFU system and treatment procedure
All treatment procedures were performed using an extracorporeal clinical tabletop MR-HIFU system (Sonalleve V2, Profound medical Inc., Mississauga Canada). The radiologist planned the treatment by positioning the ellipsoid treatment cells into the targeted fibroid one by one to cover the whole fibroid. Treatment power was selected based on test sonication to achieve best possible temperature rise in the target tissue. During the sonication, heating of the targeted area and possible undesired heating of surrounding tissue were monitored with real-time MR thermometry. After the treatment, a contrast-enhanced T1 weighted image was acquired by injecting the contrast agent (DOTAREM, Guerbet, Aulnay-Sous-Bois, France, 0.1 mmol/kg) to evaluate the non-perfused volume (NPV). NPV was used to calculate the non-perfused volume ratio (NPV%) for each patient, that is, non-perfused fibroid volume/total fibroid volume.
After the treatment, the total treatment energy was calculated from the treatment data by multiplying used power and sonication time for each sonication and summing up the energies for all sonications. To consider attenuation, the total delivered acoustic energy at the focus per treatment was calculated as described in a previous study published in 2021 [Citation14].
Information about the ovarian reserve was gathered as a part of a larger study of the effectiveness and safety of MR-HIFU treatments. All patients were summoned for a control 3 months after MR-HIFU. Using a standardized manner the patients were interviewed about their fibroid symptoms, quality of life as well as recovery from the treatment and current gynecological health and symptoms (including menopausal symptoms). AMH levels were analyzed before the MR-HIFU treatment and 3 months after. For the AMH test, 5 mL of serum was collected and stored in a freezer. The assay was made with Elecsys AMH Plus which analytical imprecision is under 5% (Roche Diagnostics).
Statistical analysis
Statistical analysis was performed using JMP Pro statistical software version 16.2.0 (SAS Institute Inc.). A p-value less than .05 was considered statistically significant. The normal distribution of each dataset was analyzed with the Shapiro-Wilk W test. The correlation between normally distributed parameters was analyzed by Pearson product-moment correlation and non-normally distributed parameters were analyzed by Spearman’s rank correlation analysis. Group means of normally distributed datasets were compared using Tukey-Kramer honestly significant difference (HSD) test for all pairs and non-normally distributed datasets were compared using the Steel-Dwass method for all pairs.
Results
The median AMH level before the HIFU treatment was 1.20 (range: 0.1–7.75 μg/L) and after the treatment 1.23 (range: 0.1–8.51 μg/L). No significant change was detected (p = .90). These results are shown in . None of the women reported any symptoms indicating loss of ovarian function.
Figure 1. (a–d) Box Whisker plots (median and interquartile range) presenting AMH values before the treatment and 3 months after. (a) All AMH values before the treatment and 3 months after. No significant change was seen. (p = .9). (b) AMH values in the low AMH group (0.1–1 ug/L) before the treatment and 3 months after. No significant change was seen. (p = .26). (c) AMH values in the intermediate AMH group (1–2.5ug/L) before the treatment and 3 months after. No significant change was seen (p = 1.0). (d) AMH values in the high AMH group (>2.5 ug/L) before the treatment and 3 months after. No significant change was seen (p = .98).
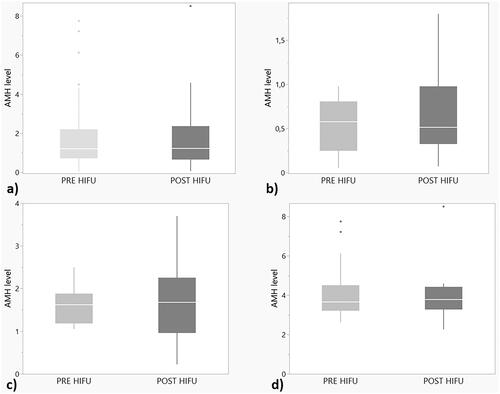
In order to analyze possible effects in women with different initial ovarian reserve, the patients were divided into three different subgroups depending on the baseline AMH levels.
Group 1 (28 women) had the AMH 0.1–1 μg/L before the MR-HIFU treatment. The median AMH level was 0.58 (range: 0.1–0.98 μg/L) before and 0.52 (range: 0.1–1.8 μg/L) after the treatment. The change was not significant (p = .26), .
Group 2 (31 women) had AMH levels 1–2.5 μg/L. The median AMH was 1.62 (range: 1.05––2.5 μg/L) in the beginning and 1.68 (range: 0.22–3.7 μg/L) after treatment, . No significant change was detected (p = 1.00).
Group 3 (15 women) had the highest AMH levels at the beginning, above 2.5 μg/L. The pretreatment median AMH level was 3.65 (range: 2.63–7.75 μg/L) and post treatment was 3.78 (range: 2.26–8.51 μg/L). The change was not significant (p = 0.98), .
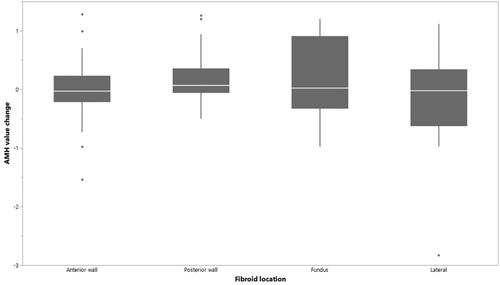
We also studied the AMH level change for different fibroid location: anterior wall, posterior wall, fundus and lateral. This was done because in one case (presented later), where the ovarian reserve was diminished after treatment, the ovaries were situated closely behind the uterus. There were no significant differences between the mean AMH level changes and different fibroid locations, .
Figure 2. The change of AMH and fibroid location. There were no significant differences between the mean AMH level changes and different fibroid locations.
The correlations between the AMH level changes, the total treatment energies, the total delivered acoustic energy at the focus and NPV ratios (%) are presented in . There were no statistically significant correlations between these parameters.
Table 2. The correlations between the AMH level changes, the total treatment energies, the total delivered acoustic energy at the focus and NPV ratios (%).
Discussion
MR-HIFU treatments of uterine fibroids and uterine adenomyosis have been performed in Turku University Hospital since 2016. During this time, we have had one patient in which the loss of ovarian function was observed after MR-HIFU treatment.
Only women with significant ovarian reserve reflected in baseline AMH 0.1 or over were included in the analysis presented in this article, even though, we have, of course, treated also women with lower AMH levels. A case of decreased AMH level and typical climacteric symptoms evolving shortly after the HIFU treatment, however with a baseline AMH below 0.1 μg/L is presented in the Supplemental material. Having encountered this patient case, it seemed important to investigate the effect of MR-HIFU treatment on the AMH levels. In this prospective cohort study, we have demonstrated that MR-HIFU does not compromise the ovarian reserve. Our results are in line with earlier studies about the safety of US-HIFU on ovarian reserve and no significant change in the AMH levels was found [Citation5,Citation6,Citation13,Citation15]. Despite these results, it is also important to keep in mind that MR-HIFU is a treatment with potential risk of irreversibly damaging the ovaries.
AMH was chosen as a marker for ovarian reserve since it is widely used to detect the loss of ovarian reserve following surgery, chemotherapy and radiotherapy. These treatments are known to potentially affect ovarian function and reserve and cause premature menopause [Citation10]. We measured serum AMH levels just before MR-HIFU and 3 months after the treatment. The time point of 3 months was chosen based on data from studies on the effect of ovarian surgery on ovarian reserve, that were available in 2016 when starting these treatments in Turku. There are many studies concluding that AMH levels drop immediately after cystectomy for benign ovarian cysts. It is suggested to be caused by the removal of healthy ovarian tissue containing the granulosa cells of small growing follicles, during the stripping of the ovarian wall [Citation10,Citation16–20]. It has been shown that recovery of the AMH levels takes place after surgery. Possible explanations about the mechanisms behind this are reperfusion of ovarian tissue and regathering and recruiting small antral follicles that then start releasing AMH [Citation17–21].
MR-HIFU is a uterine sparing treatment for symptomatic uterine fibroids. It can be used to treat fibroid symptoms in women still hoping to conceive or even for infertility patients. It can be also offered to women wanting to avoid hysterectomy or to treat fibroid symptoms near menopause. For younger patients hoping for future pregnancy, it is off course crucial that the fibroid treatment has no negative effect on ovarian function and reserve. However, it is also important to consider the effects of treatments on the ovarian reserve as the age of reaching menopause has an impact on women’s health in general. A systematic review and meta-analysis published in JAMA Cardiology in 2016 by Muka et al. showed that there is a higher risk of coronary heart disease, cardiovascular disease mortality and overall mortality in women who experience premature onset of menopause (onset at under 45 years of age) [Citation2].
Uterine artery embolization (UAE), another uterine sparing treatment for fibroid symptoms, appears to have a negative effect on ovarian reserve. In a retrospective cohort study published in 2014, Arthur et al. showed that AMH levels and antral follicle count (AFC) were significantly lower in women who underwent UAE compared to those with myomectomy at 12 months after treatment [Citation21]. In a randomized controlled trial published in 2007 Hehenkamp et al. found that after UAE, the AMH levels were significantly decreased and remained so for the entire follow up period of 24 months [Citation22].
Studies of the effect of hysterectomy on ovarian reserve have more complex and controversial conclusions. In 2012 Atebekoglu et al. published that the serum AMH levels in 22 women undergoing total abdominal hysterectomy (TAH) were decreased 4 months after surgery although the decrease was not statistically significant [Citation23]. In 2010 Lee et al. showed that the AMH and ovarian arterial blood flow showed no difference at 1 week, 1 month or 3 months after laparoscopically assisted vaginal hysterectomy (LAVH) or TAH [Citation16]. In 2013 Wang et al. published a study of the effect of hysterectomy and myomectomy on AMH levels. After both operations, the AMH levels were decreased at two days after surgery compared to the preoperative levels. At three months, in the myomectomy group the AMH levels had recovered being at the preoperative stage whereas in the hysterectomy group the AMH level stayed significantly lower at three months. These studies are interesting concerning the ovarian reserve, but of course hysterectomy should not be compared with MR-HIFU when the interest is in preserving the fertility of the patient.
The major strength of our prospective study is that we studied the possible changes in AMH levels also in subgroups of different base level AMH. There were no changes in AMH levels regardless of whether the baseline AMH was high (>2.5 μg/L), intermediate or already on lower side of the spectrum (0.1–1.0 μg/L). This is crucial since changes in baseline low levels of AMH can be difficult to detect and results can be unreliable. In the FIRSTT study published in 2019 by Laughlin-Tommaso et al. compared the two fibroid treating methods, MR-HIFU and UAE and also the effect on ovaries was studied by screening AMH levels. Compared to the FIRSTT study population, we had a higher AMH level median at the beginning. In the FIRSTT study the median was 0.3 ng/mL (0.3 μg/L), whereas in our population the median was 1.2 μg/L. In this scope, our study gives new information about the effect of MR-HIFU on ovaries with higher AMH levels. In the subgroup with highest baseline AMH (over 2.5 μg/L), the median baseline AMH was 3.65 μg/L. It could be speculated that the risk of ovaries getting affected by the HIFU would be higher in ovaries with higher AMH levels as they usually are bigger in size and therefore often closer to the treatment field. In our study we have not been able to detect such an effect [Citation24].
On the other hand, there is some evidence that an aging ovary with already declining ovarian reserve is more sensitive to damage than a younger ovary with more reserve [Citation25]. As mentioned before, UAE has been reported to have a negative effect on ovarian reserve and this effect seems to be more apparent in women over 40 years old [Citation26]. It has also been reported that UAE predisposes women to earlier menopause [Citation22]. We found no negative effect of MR-HIFU even in the subgroup of women, whose ovarian reserve was already compromised. There is no long-term accumulation of the heat or changes in the circulation of uterine or ovarian vessels with MR-HIFU. Whereas, in contrast, obliteration of the uterine arteries and possible drifting of the embolizing particles to ovarian vessels may cause long term changes in the circulation of the ovaries and thus have subtle effects on their function that manifest over long time.
Our study also reveals that the location of the treated fibroid has no correlation on the change of the AMH levels and neither the total treatment energy. These are important new results ensuring that treating fibroids with MR-HIFU has no adverse impact on ovarian reserve. We studied the correlation of the AMH and the location of the fibroid and the correlation between the AMH and the total treatment energy since usually a bigger fibroid requires more treatment energy in total. With higher total energy, the risk of causing non-target tissue damage might increase significantly. For this reason why we found it more relevant to investigate the correlation between AMH and total treatment energy tha the size of the treated fibroid.
Although the location of the fibroid has no effect alone, it is very important to pay attention to the location of the ovaries when planning the fibroid treatment window and placing the treatment cells. The patient case presented in the supplement is an example of the possible adverse effect of MR-HIFU treatment on ovarian function. In a patient with a very low AMH level and thus poor ovarian reserve, even a minute effect on ovarian function may initiate amenorrhea and menopausal symptoms. This highlights the importance of appropriate patient counseling. Perhaps the ovarian reserve should routinely be evaluated with AMH measurement prior to the HIFU treatment so that the random occurrence of menopause and associated symptoms would not unduly be blamed on the treatment.
A limitation of our study is that information about the menstrual cycle was not recorded. On the other hand it is known that inter and intra-cyclic variation of AHM is low [Citation27]. The strength is that AMH seems to be a stable marker compared to other markers. Another limitation is that no later AMH samples were gathered. In the case of a longer follow up, there should have been a control group enrolled in this study. Also, one limitation is the nature of the HIFU treatment which includes the acquisition of MR images of the pelvis, which provide detailed information on fibroid as well as on location of the ovaries. Patients with unfavorable location of ovaries that might lie in the treatment field, are therefore excluded in the selection process and, at least in our center, not treated with HIFU.
Based on our results and previous studies, it seems that MR-HIFU, in general, has no adverse impact on ovarian reserve nor function. However, we have encountered in our center one case, in which MR-HIFU may have been an associated factor to the unfortunate premature failure of ovarian function and our patient was postmenopausal 3 months after the treatment. After this case we have become highly meticulous in addressing the position of the ovaries in the MR images and an individual assessment of the effect of HIFU treatment on the ovaries is made for each patient.
This study provides strong evidence in favor of the safety of MR-HIFU in regard to ovarian reserve. Based on these results, we consider it safe to recommend this treatment to women wishing future pregnancies. However, careful patient selection, treatment planning and monitoring are crucial in obtaining this safety.
Supplemental Material
Download PDF (337.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yan L, Huang H, Lin J, et al. High-intensity focused ultrasound treatment for symptomatic uterine fibroids: a systematic review and meta-analysis. Int J Hyperthermia. 2022;39(1):230–238.
- Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time Since onset of menopause With cardiovascular outcomes, intermediate vascular traits, and All-Cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767–776.
- Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106(7):1588–1599.
- Ruan H, Hu J, Zhao J, et al. Menopause and frailty: a scoping review. Menopause. 2020;27(10):1185–1195.
- Cheung VYT, Lam TPW, Jenkins CR, et al. Ovarian reserve After Ultrasound-Guided High-Intensity focused ultrasound for uterine fibroids: preliminary experience. J Obstet Gynaecol Can. 2016;38(4):357–361.
- Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. Int J Obstet Gy. 2017;124:18–22.
- Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361.
- Broer SL, Broekmans FJM, Laven JSE, et al. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. In: Human reproduction update, Vol. 20. Oxford: Oxford University Press; 2014. p. 688–701.
- Van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25(1):221–227.
- Wong QHY, Anderson RA. The role of antimullerian hormone in assessing ovarian damage from chemotherapy, radiotherapy and surgery. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):391–398.
- Landersoe SK, Forman JL, Birch Petersen K, et al. Ovarian reserve markers in women using various hormonal contraceptives. Eur J Contracept Reprod Health Care. 2020;25(1):65–71.
- Iwase A, Osuka S, Goto M, et al. Clinical application of serum anti-Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998–1006.
- Ji J, Liu J, Chen Y, et al. Analysis of high intensity focused ultrasound in treatment of uterine fibroids on ovarian function and pregnancy outcome. J Clin Ultrasound. 2021;50(2):202–208.
- Sainio T, Saunavaara J, Komar G, et al. Feasibility of apparent diffusion coefficient in predicting the technical outcome of MR-guided high-intensity focused ultrasound treatment of uterine fibroids – a comparison with the funaki classification. Int J Hyperthermia. 2021;38(1):85–94.
- Wang W, Jiang J, Chen Y, et al. The effect of ultrasound-guided high-intensity focused ultrasound treatment for cesarean scar pregnancy on ovarian reserve. Int J Hyperthermia. 2021;38(1):1409–1414.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Ding Y, Yuan Y, Ding J, et al. Comprehensive assessment of the impact of laparoscopic Ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Li H, Yan B, Wang Y, et al. The optimal time of ovarian reserve recovery After laparoscopic unilateral ovarian Non-Endometriotic cystectomy. Front Endocrinol. 2021;12:671225.
- Sireesha M, Chitra T, Subbaiah M, et al. Effect of laparoscopic Ovarian cystectomy on Ovarian reserve in benign ovarian cysts. J Hum Reprod Sci. 2021;14(1):56–60.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Arthur R, Kachura J, Liu G, et al. Laparoscopic myomectomy versus uterine artery embolization: long-term impact on markers of ovarian reserve. J Obstet Gynaecol Can. 2014;36(3):240–247.
- Hehenkamp WJK, Volkers NA, Broekmans FJM, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996–2005.
- Atabekoğlu C, Taşkin S, Kahraman K, et al. The effect of total abdominal hysterectomy on serum anti-Müllerian hormone levels: a pilot study. Climacteric. 2012;15(4):393–397.
- Laughlin-Tommaso S, Barnard EP, AbdElmagied AM, et al. FIRSTT study: randomized controlled trial of uterine artery embolization vs focused ultrasound surgery. Am J Obstet Gynecol. 2019;220(2):174.e1–174.e13.
- Dillon KE, Sammel MD, Prewitt M, et al. Pretreatment antimüllerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99(2):477–483.
- Kim CW, Shim HS, Jang H, et al. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016;206:172–176.
- La Marca A, Giulini S, Tirelli A, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22(3):766–771.
