Abstract
Background
The original meta-analysis of hyperthermic intraperitoneal chemotherapy (HIPEC) is already outdated, owing to the latest trial results. This study aimed to clarify the efficacy and adverse events of cytoreductive surgery with HIPEC compared to conventional therapy for advanced and platinum-sensitive recurrent epithelial ovarian cancer (OC).
Methods
In this meta-analysis, phase II/III controlled trials regarding ‘HIPEC’ and ‘ovarian cancer’ were searched for in electronic databases from inception to March 2022.
Results
Twenty-one studies were included in the quantitative synthesis. The pooled hazard ratio [HR] in the HIPEC group for progression-free survival (PFS) (HR = 0.61, 95% confidence interval [CI]: 0.45–0.83, p = .002) and overall survival (OS) (HR = 0.65, 95% CI: 0.51–0.82, p < .001) were improved in the HIPEC group compared with the non-HIPEC group. For primary advanced disease, OS and PFS were significantly increased in patients receiving interval debulking surgery + HIPEC, whereas PFS was not significantly different between primary debulking surgery (PDS) + HIPEC and PDS alone. For platinum-sensitive recurrent disease, no correlation was observed for PFS and OS between the HIPEC and non-HIPEC groups (p < .05). The incidence of procedure-related complications was higher in the HIPEC group than in the non-HIPEC group (odds ratio = 1.93, 95% CI: 1.24–3.01, p < .01). The morbidity of leukopenia, neutropenia, nausea, hypoalbuminemia, and grades III–IV electrolyte disturbance was higher in the HIPEC group than in the non-HIPEC group. However, HIPEC administration reduced the risk of intra-abdominal bleeding and constipation.
Conclusion
HIPEC-based regimens improved the clinical prognosis for primary advanced OC, whereas no significant value was elicited for recurrent OC.
1. Introduction
Ovarian cancer (OC) is the most lethal gynecological cancer, and >95% of cases are epithelial ovarian cancer (EOC). Optimal surgical cytoreduction and platinum-based chemotherapy are significant treatments for primary advanced ovarian cancer (AOC). More than 75% of patients are diagnosed at an advanced stage (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV) because of the absence of screening and specific symptoms when tumors usually have peritoneal cavity and upper abdominal organ metastasis [Citation1]. Under these circumstances, approximately 70 % of patients with advanced diseases develop recurrence within 3 years after surgery and chemotherapy, and the 5-year relative survival rate is <45% [Citation2]. The main reason for the high recurrence rate of AOC is that cancer cells frequently spread from the primary tumor into the peritoneal cavity and are implanted in abdominal organs, which makes complete cytoreductive surgery (CRS) difficult to achieve.
Previous research has established that optimal debulking prolong the overall survival (OS) of AOC and recurrent ovarian cancer (ROC). Harter et al. reported that total resection of ROC (R0 resection) was associated with an OS improvement of approximately 3 months for every 10% improvement in total resection [Citation3]. Therefore, optimal debulking is particularly crucial for improving survival in OC. However, when optimal debulking cannot be achieved, intraperitoneal chemotherapy (IPC) may be a supplemental treatment strategy. Compared with systematic chemotherapy, IPC via direct delivery of anticancer agents to peritoneal seeding lesions not only provides higher peritumoural drug concentrations by eliminating the hepatic first-pass effect but also reduces the systemic toxicity of agents. However, the incidence of IPC-related adverse events (AEs) was not negligible. Catheter-related complications, such as intra-abdominal infections, dislodgement of catheters, and catheter clogging, are particularly serious. Moreover, there are also severe AEs with regard to abdominal pain, fever, and gastrointestinal effects, which have proven difficult for patients to tolerate. The aforementioned AEs would contribute to diminishing the quality of life of patients with OC, and in turn, approximately 60% of the administration of IPC is forced to be discontinued or modified [Citation4]. Consequently, the use of IPC as routine treatment remains controversial. Recently, HIPEC has been administered in patients under anesthesia at the end of cytoreductive/debulking surgery for OC. Not only does this procedure require intraperitoneal catheterization, but patients are also assured to experience painless operative processes. Consequently, the HIPEC procedure is well-tolerated. Intraperitoneal perfusion was performed to increase the tumor drug concentration before the end of the operation when tissue adhesion was not formed [Citation5]. Additionally, mild hyperthermia (temperatures between 40 °C and 44 °C) is cytotoxic to cells by enhancing DNA damage and endoplasmic reticulum stress [Citation2,Citation6]. Besides, it has been demonstrated that combining chemotherapy with regional hyperthermia can sensitize tumor tissues to anticancer agents, resulting in improved OS prolongation [Citation7]. Moreover, the heat shock protein gp96 is produced by thermal effects, which activate CD4+ T and CD8+ T cell proliferation, in turn increasing antitumor immunity [Citation8].
Recently, a meta-analysis reported the benefits of HIPEC in prolonging OS and PFS in patients with AOC. However, whether this can improve progression-free survival (PFS) or OS remains controversial. Morbidity has rarely been reported in previous studies. Therefore, we conducted a systematic review and meta-analysis to investigate the prognostic value and AEs of CRS + HIPEC in AOC and platinum-sensitive ROC.
2. Materials and methods
2.1. Search strategy
We performed a comprehensive literature search of electronic databases, including PubMed, Embase, Cochrane, Medline, and Web of Science, from inception to March 2022. The following combination of medical subject heading-terms and entry-terms strategy was used: ‘hyperthermic intraperitoneal chemotherapy’, ‘HIPEC’, ‘ovarian neoplasms’, and ‘ovarian cancer’. All retrieved abstracts were independently screened by two authors, and any disagreements between the reviewers were resolved by consensus through discussion.
2.2. Selection criteria
In this meta-analysis, comparative clinical trials were included, and the language was restricted to English. The following inclusion criteria were applied: (1) the study participants included patients with primary advanced or platinum-sensitive recurrent EOC; (2) the patients received CRS plus HIPEC in the experimental arm; (3) the patients received only CRS without HIPEC in the control arm, and all enrolled patients received appropriate chemotherapy; and (4) PFS and OS were the primary outcomes, and the AEs were the secondary outcomes. The exclusion criteria were as follows: (1) literature reviews and systematic reviews; (2) case reports or series; (3) animal or cell experiments; (4) phase I clinical trials; and (5) studies that included only a HIPEC group for OC. Available data provided the frequency and severity of AEs and sample size. Importantly, treatment-related AEs were defined by the fifth version of the Common Terminology Criteria for Adverse Events of the National Cancer Institute.
2.3. Data extraction and quality assessment
The following data were extracted: first author, year of publication, country, study design, number of study participants, patient characteristics, surgical debulking and systemic chemotherapy strategies, HIPEC regimens, temperature and duration of treatment, and outcomes. The Newcastle-Ottawa Scale was used for the quality assessment of comparative studies. Studies with scores from 0 to 3, 4 to 6, and 7 to 9 were considered to be low, moderate, and high quality, respectively. The Jadad scale was used to assess the quality of randomized controlled trials (RCTs). Studies with scores of 1 or 2 were considered low quality, and those with scores of 3–5 were considered high quality.
2.4. Statistical meta-analysis
Hazard ratios (HRs) and odds ratios (ORs) were used to measure the prognostic value and safety of treatment. The HRs of the publications were obtained using Engauge Digitizer (version 11.3). All data were evaluated using Stata software (version 14.0; StatCorp) and Review Manager (version 5.3; Cochrane Training). Forest plots were generated to display outcomes for each study with their corresponding 95% confidence intervals (CIs) and the overall effects pooled estimate with 95% CIs. Statistical heterogeneity was evaluated using the I2 statistic, and an I2 value <50% indicated that the heterogeneity was not statistically significant; thus, the fixed-effects model was used. When the opposite occurred, a random-effect model was applied. Subgroup analyses were performed according to the study design. We evaluated publication bias for each of the pooled study groups using a funnel plot, and the publication bias of the studies was further appraised using the Egger test.
3. Results
3.1. Characteristics of the included studies and risk of bias
The literature search and study selection procedure are summarized in . According to the search strategy using ‘HIPEC’ and ‘ovarian cancer’, 3075 hits were searched. Based on the inclusion and exclusion criteria, 21 articles were eligible for the meta-analysis, including 5 RCTs, 15 retrospective trials, and a prospective case-control study (). Ten studies comprising 1604 patients reported survival outcomes and AEs [Citation9–18]. Nine studies comprising 686 patients reported survival outcomes [Citation19–27]. Two studies comprising 20,269 patients reported AEs [Citation28,Citation29]. Thirteen studies were about primary advanced EOC [Citation9–17,Citation19–21,Citation29], and 7 studies were about platinum-sensitive recurrent EOC [Citation18,Citation22–27]. Platinum and/or paclitaxel was the most commonly used HIPEC agent. The duration of HIPEC ranged from 60 to 120 min. Lei and He et al. performed HIPEC procedures on days 1, 3, and 5 after cytoreduction [Citation13,Citation17]. In other studies, HIPEC was administered as a single treatment following the end of the cytoreductive surgical procedure. The details of the 21 publications are listed in . The Newcastle–Ottawa scale score of the 16 comparative studies was not <5, and the Jadad score of the 5 RCTs was not <3, indicating that the overall quality of the included trials was standard. Furthermore, we assessed publication bias in the literature, and the T-values of the Egger test were −1.12 (p = .281) and −0.58 (p = .568) for PFS and OS, respectively (Supplementary Appendix 4 A, B) ().
Figure 1. PRISMA guided flow diagram of study selection for inclusion in this review and meta-analysis.
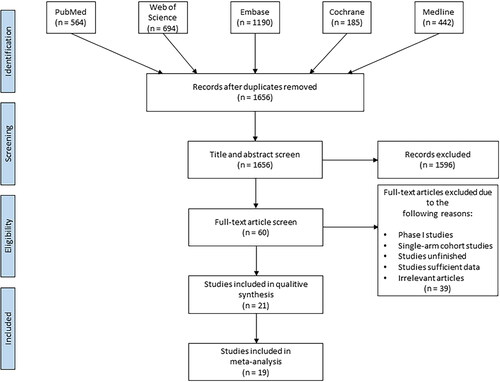
Figure 2. Funnel plot of studies evaluating the publication bias of PFS and OS between HIPEC and No-HIPEC.
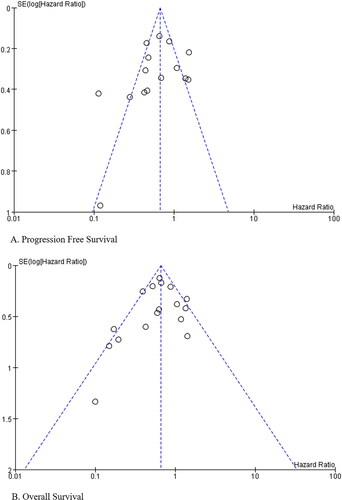
Table 1. The characteristics of the included studies. CRS + HIPEC vs CRS.
3.2. Endpoints
3.2.1. Primary outcome
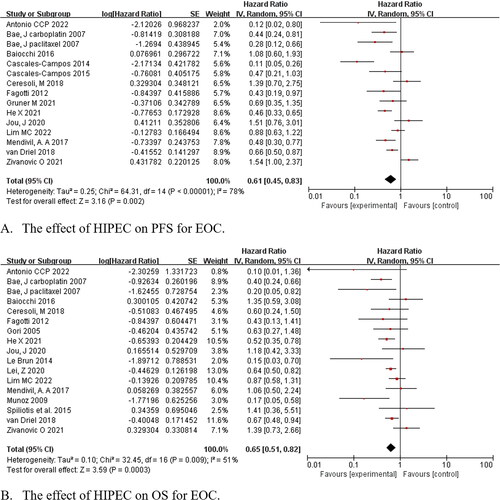
Fourteen studies were eligible to assess the effect of HIPEC on PFS, and HIPEC improved the outcome (HR = 0.61, 95% CI: 0.45–0.83, p = .002). Pooled data from 16 studies demonstrated that there was an improvement in OS in the HIPEC groups compared with the non-HIPEC groups for all populations (HR = 0.65, 95% CI: 0.51–0.82, p < .001) ().
3.2.2. Subgroup analysis
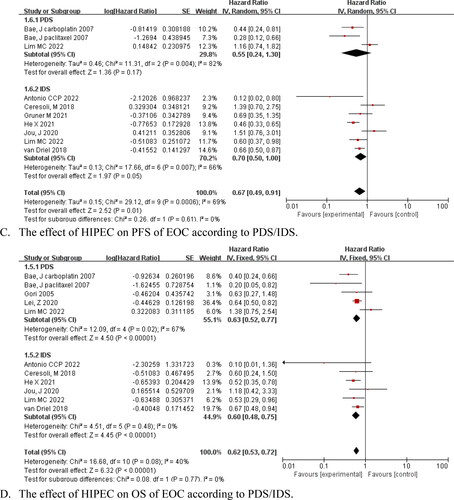
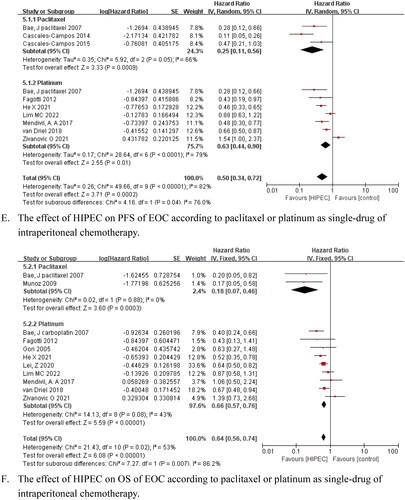
The subgroup analysis indicated that HIPEC improved PFS and OS for patients with advanced primary disease, PFS (HR = 0.58, 95% CI: 0.41–0.81, p = .001), and OS (HR = 0.63, 95% CI: 0.52–0.77, p < .001) for patients with recurrence; additionally, PFS and OS were not prolonged in these subgroups (HR = 0.82, 95% CI: 0.44–1.52, p = .52; HR = 0.62 and 95% CI: 0.28–1.37, p = .24, respectively) ()). A significant increase in PFS and OS was observed for interval debulking surgery (IDS)+HIPEC followed by systemic chemotherapy (HR = 0.70, 95% CI: 0.50–1.00, p = .05 and HR = 0.60, 95% CI: 0.48–0.75, p < .001, respectively). Moreover, primary debulking surgery (PDS) + HIPEC after adjuvant chemotherapies also prolonged OS (HR = 0.63, 95% CI: 0.52–0.77, p < .001), whereas IDS failed to improve PFS (HR = 0.55, 95% CI: 0.24–1.30, p = .17) ()). Based on the HIPEC chemotherapy agents, our pooled data suggested that PFS and OS benefited from paclitaxel or platinum as single drug therapy for AOC and ROC (HR = 0.25, 95% CI: 0.11–0.56, p < .001; HR = 0.18, 95% CI: 0.07–0.46, p < .001; HR = 0.63, 95% CI: 0.44–0.90, p = .01; and HR = 0.66, 95% CI: 0.57–0.76, p < .001, respectively) ()).
Figure 4. Forest plots showing the effect of HIPEC on survival of EOC after subgroup analysis.
3.2.3. Secondary outcome
To assess the safety of HIPEC, we examined AEs, and no differences were observed for grades III and IV AEs overall (141/335 and 124/291, respectively; p = .36). The incidence of procedure-related complications and individual AEs were as follows: the incidence of procedure-related complications was statistically higher in the HIPEC group (28/180) than in the non-HIPEC group (1483/20,042) (OR = 1.93, 95% CI: 1.24–3.01, p < .01), and the risk of intraoperative complications was higher in the HIPEC group than in the non-HIPEC group (OR = 1.93, 95% CI: 1.24–3.01, p < .01). Charo et al. reported a higher risk of procedure-related complications associated with HIPEC (p < .01). Ghirardi et al. found that grades I–IV postoperative complications were statistically higher in the HIPEC group than in the non-HIPEC group (36/52 and 25/51, respectively, p = .04). Jou et al. reported a higher risk of grades III and IV postoperative complications (13/20 and 2/48, respectively, p < .01) and grades III and IV abdominal infection (5/20 and 0/48, respectively, p = .02) in the HIPEC group than in the control group. However, the pooled analyses showed that the HIPEC group was associated with a lower risk of any grade of inter-abdominal bleeding compared to the non-HIPEC group (OR = 0.31, 95% CI: 0.11–0.86, p = .02). There were no significant differences between the HIPEC and non-HIPEC groups in ostomy creation, pleural effusion, thromboembolic, ileus, or blood transfusion. HIPEC appeared to have a positive effect in decreasing the risk of constipation (OR = 0.68, 95% CI: 0.47–0.99, p = .04). The morbidities of nausea, leukopenia, neutropenia, grades III–IV electrolyte imbalance, and hypoalbuminemia were associated with the HIPEC group, with ORs of 1.50 (95% CI: 0.02–2.20, p = .04), 1.72 (95% CI: 1.19–2.47, p < .01), 1.62 (95% CI: 1.18–2.21, p < .01), 3.01 (95% CI: 1.79–5.07, p < .01), and 4.67 (95% CI: 2.21–9.87, p < .01, respectively). The risk of cardiotoxicity, pulmonary events (hypoxia, respiratory distress, dyspnea, and acute respiratory distress syndrome), gastrointestinal events (vomiting and diarrhea), anemia, thrombocytopenia, abdominal pain, and neurotoxicity was compared between the groups ().
Table 2. Meta-analysis estimates of adverse events between HIPEC and No-HIPEC.
4. Discussion
This study focused on the prognosis of CRS + HIPEC versus CRS in patients with AOC and platinum-sensitive ROC. Considering that patient tolerability is a critical factor for HIPEC application in routine clinical practice, we also analyzed the morbidity of CRS + HIPEC compared with the control. Consequently, we are confident that this meta-analysis will provide useful clinical evidence for the treatment of AOC and platinum-sensitive ROC.
Our meta-analysis indicated that HIPEC prolonged OS and PFS for AOC compared to non-HIPEC. Optimal cytoreduction, especially complete surgical debulking, is considered the most important factor for improving the prognosis of patients with AOC [Citation30]. Our results revealed that HIPEC, as an additional therapy, effectively improved OS and PFS by partially eliminating the macroscopic and microscopic residual disease. The results of our meta-analysis are consistent with those of previous studies [Citation31–34]. Therefore, it is reasonable to believe that HIPEC following CRS is a meaningful treatment strategy for AOC. However, Ceresoli et al. and Jou et al. did not find any advantages of HIPEC for AOC compared to non-HIPEC [Citation11,Citation12]. The reason for their statistically insignificant result was probably due to the small sample size of patients. There are some other reasons for the lack of statistically significant differences between the HIPEC and control groups in some trials. First, the patients in the HIPEC group might have a higher disease burden than those in the control group. GOG182 showed that the baseline character of disease burden remains an independent prognostic indicator despite complete cytoreduction [Citation35]. In addition, patients with a higher disease burden might be confronted with a higher surgical complexity, which might lead to shorter survival times. A recent study indicated that compared with low surgical complexity scores (SCS), intermediate/high SCSs for debulking operations were associated with worse PFS and OS despite achieving no residual disease [Citation36]. In summary, it can be concluded that HIPEC is significant for extending the survival of patients with AOC under conditions of equal tumor burden and surgical complexity compared with CRS alone. In the subgroup analysis, the pooled outcomes verified the prominent advantage of IDS + HIPEC in patients with AOC in our meta-analysis. Van Driel, Lim, and Antonio reported identical superiority in their RCTs [Citation14,Citation15,Citation21]. Nevertheless, there are inadequate RCTs to confirm that PDS + HIPEC performs well in AOC.
Regarding platinum-sensitive ROC, no difference was observed between the HIPEC and non-HIPEC groups for PFS and OS in our meta-analysis. The latest RCT performed by Zivanovic et al. had the maximum weight among all studies. When we removed this study, OS was prolonged in the HIPEC group compared to the control group, which was consistent with the finding of other meta-analyses [Citation31–34]. Zivanovic et al. reported negative PFS and OS of carboplatin-based HIPEC regimens during secondary CRS for platinum-sensitive ROC [Citation27]. Nevertheless, some confounding factors resulted in negative outcomes, e.g., residual disease after surgery was smaller in the control group than in the HIPEC group. The treatment for patients with ROC is not standardized currently, such as the omission of maintenance treatment, shorter platinum-free interval, and inclusion of higher-risk patients. These reasons may have resulted in the negative outcomes of this trial. According to the National Comprehensive Cancer Network guidelines (NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022), patients with ROC are recommended to undergo testing for biomarker mutations, such as the breast cancer susceptibility gene, homologous recombination deficiency, mismatch repair, and microsatellite instability [Citation37]. Biomarker testing is used to determine whether OC has any targetable changes to guide treatment. Currently, bevacizumab and poly (adenosine diphosphate-ribose) polymerase inhibitors are considered maintenance treatments following standard chemotherapy for platinum-sensitive ROC [Citation38–41]. Besides, there are two ongoing trials (ClinicalTrials.gov study IDs: NCT01376752 and 01539785) assessing the effect of HIPEC on the first relapse of OC, which would further provide evidence of the effect of HIPEC in patients with ROC [Citation42,Citation43].
In this study, we reported a higher risk of procedure-related complications in the HIPEC group than in the non-HIPEC group. Jou et al. reported that the frequencies of morbidities of postoperative complications and abdominal infection were higher in the HIPEC group than in the control group in their study [Citation12]. Aggressive upper abdominal procedures, long operation durations, and surgical complexity are the main causes of these complications. These are leading factors for infectious morbidities, thromboembolic events, anastomotic leakages, and intraoperative hypothermia, rather than HIPEC administration. Lim et al. reported that the frequency of morbidities of fistula, perforation, or leakage of the bowel was higher in the HIPEC group for FIGO stages III–IV disease than for other FIGO stages [Citation15]. HIPEC could be administered safely after maximal CRS, and the initiation of adjuvant chemotherapy was not delayed. In contrast, we found a lower incidence of abdominal bleeding in the HIPEC group because surgeons could discover potential bleeding points while performing abdominal perfusion. Consequently, selecting patients with suitable indications to undergo HIPEC is pivotal for improving survival prognosis and reducing surgical complications. Regarding AEs, we found that leukopenia was apparent due to the use of HIPEC, whereas refractory leukopenia was uncommon. Electrolyte imbalance have been reported in several trials, which may have resulted from fluid resorption during peritoneal perfusion [Citation13]. The approaches to avoid this situation were as follows: control the flow rate, perfusion pressure, and infusion time. However, these factors affect drug absorption and tissue penetration, thus affecting the efficacy of HIPEC. Therefore, further clinical trials are required to verify the optimal administration of HIPEC. Unexpectedly, we discovered that morbidity associated with constipation was lower in the HIPEC group than in the non-HIPEC group. Opioid pain relievers, forbidden diets, and water and narcotic drug suppression are likely to cause intestinal mucosa desiccation, which in turn leads to constipation. During peritoneal perfusion, the intestinal mucosa can absorb perfusion fluid and stay moist due to constipation. Although nephrotoxicity has rarely been reported in previous studies, the RCT performed by Lim et al. reported a higher risk of acute kidney injury and increased creatinine levels in the HIPEC group than in the non-HIPEC group [Citation15]. The incidence of these AEs decreased significantly after 21 patients received STS. Additionally, STS can effectively decrease the incidence of ototoxicity secondary to platinum medicines [Citation44].
There are some limitations to this study, including a lack of high-quality trials with available evidence to support our outcomes. Most of the studies were single-center retrospective trials. In addition, the heterogeneity of patient characteristics, HIPEC regimen, debulking surgery, and chemotherapy agents may have led to obvious heterogeneous outcomes in studies and affected the results of the pooled analysis. Further studies are needed to address how best to incorporate HIPEC with optimization of the intraoperative random assignment process to exert the therapeutic effect of HIPEC. This directly influences the efficiency of HIPEC in OC. Specifically, prospective RCTs are needed to assess the prognostic value of HIPEC in patients with OC.
5. Conclusions
The results of our meta-analysis provide encouraging evidence for the use of HIPEC in patients with primary advanced OC. However, current retrospective trials and RCTs lack the compelling proof to confirm the efficacy of HIPEC for patients with ROC. Although a high risk of procedure-related complications has been reported, they are mainly caused by surgical complexity rather than HIPEC. Nevertheless, individual AEs must receive careful attention, especially electrolyte imbalance, leukopenia, and nephrotoxicity. Therefore, high-quality prospective randomized trials are needed to further clarify the efficacy and safety of HIPEC.
Supplemental Material
Download PDF (227.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s)
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296.
- Kong Q, Wei H, Zhang J, et al. Comparison of the survival outcomes of laparoscopy versus laparotomy in treatment of early-stage ovarian cancer: a systematic review and meta-analysis. J Ovarian Res. 2021;14(1):45.
- Harter P, Du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702–1710.
- Tsuyoshi H, Inoue D, Kurokawa T, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J Obstet Gynaecol Res. 2020;46(9):1661–1671.
- Kyrgiou M, Salanti G, Pavlidis N, et al. Survival benefits with diverse chemotherapy regimens for ovarian cancer: meta-analysis of multiple treatments. J Natl Cancer Inst. 2006;98(22):1655–1663.
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–570.
- Kinner-Bibeau LB, Sedlacek AL, Messmer MN, et al. HSPs drive dichotomous T-cell immune responses via DNA methylome remodelling in antigen presenting cells. Nat Commun. 2017;8:15648.
- Bae JH, Lee JM, Ryu KS, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106(1):193–200.
- Cascales-Campos PA, Gil J, Gil E, et al. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann Surg Oncol. 2014;21(7):2383–2389.
- Ceresoli M, Verrengia A, Montori G, et al. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: a propensity score based case-control study. J Gynecol Oncol. 2018;29(3):e53.
- Jou J, Zimmer Z, Charo L, et al. HIPEC after neoadjuvant chemotherapy and interval debulking is associated with development of platinum-refractory or -resistant disease. Gynecol Oncol. 2021;161(1):25–33.
- Lei Z, Wang Y, Wang J, et al. Evaluation of cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for stage III epithelial ovarian cancer. JAMA Netw Open. 2020;3(8):e2013940.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Lim MC, Chang SJ, Park B, et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374–383.
- Gruner M, Chambers LM, Yao M, et al. Anastomotic leak following interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in women with advanced epithelial ovarian cancer. Gynecol Oncol. 2021;162(3):645–651.
- He X, Wei L, Li R, et al. Dense hyperthermic intraperitoneal chemotherapy with cisplatin in patients with stage III serous epithelial ovarian cancer: a retrospective study. BMC Cancer. 2021;21(1):738.
- Cascales-Campos PA, Gil J, Feliciangeli E, et al. The role of hyperthermic intraperitoneal chemotherapy using paclitaxel in platinum-sensitive recurrent epithelial ovarian cancer patients with microscopic residual disease after cytoreduction. Ann Surg Oncol. 2015;22(3):987–993.
- Gori J, Castaño R, Toziano M, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer. 2005;15(2):233–239.
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: a 3 year experience. Cancer Chemother Pharmacol. 2017;80(2):405–410.
- Antonio CCP, Alida GG, Elena GG, et al. Cytoreductive surgery with or without HIPEC after neoadjuvant chemotherapy in ovarian cancer: a phase 3 clinical trial. Ann Surg Oncol. 2022;29(4):2617–2625.
- Baiocchi G, Ferreira FO, Mantoan H, et al. Hyperthermic intraperitoneal chemotherapy after secondary cytoreduction in epithelial ovarian cancer: a single-center comparative analysis. Ann Surg Oncol. 2016;23(4):1294–1301.
- Fagotti A, Costantini B, Petrillo M, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up. Gynecol Oncol. 2012;127(3):502–505.
- Le Brun JF, Campion L, Berton-Rigaud D, et al. Survival benefit of hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer: a multi-institutional case control study. Ann Surg Oncol. 2014;21(11):3621–3627.
- Muñoz-Casares FC, Rufián S, Rubio MJ, et al. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin Transl Oncol. 2009;11(11):753–759.
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–1575.
- Zivanovic O, Chi DS, Zhou Q, et al. Secondary cytoreduction and carboplatin hyperthermic intraperitoneal chemotherapy for platinum-sensitive recurrent ovarian cancer: an MSK team ovary phase II study. J Clin Oncol. 2021;39(23):2594–2604.
- Charo LM, Jou J, Binder P, et al. Current status of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer in the United States. Gynecol Oncol. 2020;159(3):681–686.
- Ghirardi V, Ronsini C, Trozzi R, et al. Hyperthermic intraperitoneal chemotherapy in interval debulking surgery for advanced epithelial ovarian cancer: a single-center, real-life experience. Cancer. 2020;126(24):5256–5262.
- Du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d‘Investigateurs Nationaux Pour les Etudes des Cancers de l‘Ovaire (GINECO). Cancer. 2009;115(6):1234–1244.
- Wang Y, Ren F, Chen P, et al. Effects of CytoReductive surgery plus hyperthermic IntraPEritoneal chemotherapy (HIPEC) versus CytoReductive surgery for ovarian cancer patients: a systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(3):301–309.
- Zhang G, Zhu Y, Liu C, et al. The prognosis impact of hyperthermic intraperitoneal chemotherapy (HIPEC) plus cytoreductive surgery (CRS) in advanced ovarian cancer: the meta-analysis. J Ovarian Res. 2019;12(1):33.
- Wu Q, Wu Q, Xu J, et al. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: a meta-analysis. Int J Hyperthermia. 2019;36(1):562–572.
- Kim SI, Cho J, Lee EJ, et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: a systematic review and meta-analysis. Medicine. 2019;98(50):e18355.
- Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33(8):937–943.
- Aletti GD, Dowdy SC, Podratz KC, et al. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197(6):676.e1–676.e7.
- Armstrong DK, Alvarez RD, Backes FJ, et al. NCCN guidelines® insights: ovarian cancer, version 3.2022. J Natl Compr Canc Netw. 2022;20(9):972–980.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–2045.
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. (Erratum in: Lancet Oncol. 2017 Sep;18(9):e510).
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG oncology/gynecologic oncology group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–791.
- U S National Library of Medicine. Hyperthermic intra-peritoneal chemotherapy (HIPEC) in relapse ovarian cancer treatment (CHIPOR): ClinicalTrials.gov identifier: NCT01376752. 2011. Available from: www.clinicaltrials.gov/ct2/show/NCT01376752
- U S National Library of Medicine. Hyperthermic Intra-peritoneal chemotherapy (HIPEC) in ovarian cancer recurrence (HORSE): ClinicalTrials.gov identifier: NCT01539785. 2012. Available from: www.clinicaltrials.gov/ct2/show/NCT01539785
- Chen C, Huang C, Lin HH, et al. Association of sodium thiosulfate with risk of ototoxic effects from Platinum-Based chemotherapy: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(8):e2118895.