?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
In this study, C118P, a novel vascular disrupting agent (VDA), was evaluated for its ability in improving the ablative effect of high-intensity focused ultrasound (HIFU) on uterine fibroids by reducing blood perfusion.
Methods
Eighteen female rabbits were infused with isotonic sodium chloride solution (ISCS), C118P or oxytocin for 30 min, and an HIFU ablation of the leg muscles was performed within the last 2 min. Blood pressure, heart rate and laser speckle flow imaging (LSFI) of the auricular blood vessels were recorded during perfusion. Ears with vessels, uterus and muscle ablation sites were collected and sliced for hematoxylin–eosin (HE) staining to compare vascular size, as well as nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) staining to observe necrosis after ablation.
Results
Analyses revealed that the perfusion of C118P or oxytocin steadily reduced blood perfusion in the ears to approximately half by the end of the perfusion, constricted the blood vessels of the ears and uterus, and improved HIFU ablation in the muscle tissues. C118P increased blood pressure and decreased heart rate. The degree of contraction of the auricular and uterine blood vessels was positively correlated.
Conclusion
This study confirmed that C118P could reduce blood perfusion in various tissues and had a better synergistic effect with HIFU ablation of muscle (the same tissue type as fibroids) than did oxytocin. C118P could therefore possibly replace oxytocin in facilitating HIFU ablation of uterine fibroids; however, electrocardiographic monitoring is required.
1. Introduction
High-intensity focused ultrasound (HIFU) is a noninvasive technique that penetrates biological tissues with ultrasound, generating instantaneous focal high temperatures and inducing coagulation necrosis in deeper tissues [Citation1]. It is often used in the treatment of benign tumors such as uterine fibroids [Citation2]. HIFU is noninvasive, real-time, highly precise, deep-tissue penetrating and broadly applicable [Citation3]. Despite these advantages, abundant blood supply to tissues can dissipate the focal heat produced, weakening the ablative efficacy; therefore, methods to reduce blood perfusion can improve the ablative efficacy [Citation4].
Currently, oxytocin, misoprostol/diclofenac [Citation5] and pituitrin [Citation6] are commonly used clinically in combination with HIFU ablation for the treatment of adenomyosis or uterine fibroids. Though they provide improvement in efficacy, doctors are still not satisfied with the level of improvement, as the HIFU power also needs to be increased to achieve favorable outcomes, resulting in pain and skin burns [Citation7,Citation8]. Novel drugs that can effectively reduce blood perfusion and consequently assist HIFU ablation are yet to be developed.
Vascular disrupting agents (VDAs) take advantage of structural defects in tumor vessels and preferentially destroy the endothelial cytoskeleton (endothelial cells are modified from a flat to a spheroid shape) in tumors but not in the healthy vessels; this leads to narrowing and even obstruction of the vascular lumen, and ultimately to ischemia and necrosis of the tumor [Citation9]. Although VDAs preferentially act on the blood vessels of tumors, they also temporarily contract normal blood vessels [Citation10]. Due to this, they may be favorable replacements for oxytocin or other drugs in improving HIFU ablation of uterine fibroids. The most studied VDA with the best efficacy is combretastatin A-4 phosphate (CA4P), which can inhibit tubulin polymerization [Citation11]. This damages the tubulin cytoskeleton in vascular endothelial cells, changing their flat structure and causing lumen narrowing and reduced blood perfusion in the healthy vessels or lumen occlusion and blood-flow occlusion in tumor vessels. CA4P phase III clinical trials were discontinued following its patent expiry, but an analogue (C118P) was created and used as a replacement. As a novel microtubule inhibitor, its mechanism of action has not been proven; however, it should be the same as CA4P. C118P was first studied for its ability to disrupt microscopic protein polymerization, thereby inhibiting mitosis in cancer cells [Citation12,Citation13]. Moreover, the biological half-life of C118P in vivo is longer than that of CA4P, suggesting a stronger efficacy [Citation12]. However, it has only been reported to block the blood supply of VX2 tumors and reduce blood perfusion in rabbits [Citation14]. As a VDA, it is speculated that C118P also constricts blood vessels in benign tissues, suggesting its probable use in improving the efficacy of HIFU treatment.
Animal studies of the HIFU treatment of uterine fibroids is difficult. First, although the animals used for studying uterine fibroids include guinea pigs, rats, mice [Citation15], miniature pigs [Citation16], rhesus macaques [Citation17] and other experimental animals, HIFU equipment for small animals has not been fully developed. Second, uterine fibroids are benign tumors. Animal models of malignant tumors can be established in a short time by tumor cell transplantation, but animal models for benign tumors cannot be established in this manner. Moreover, bladder filling is required for ultrasound localization of the uterus, even if uterine fibroids are successfully developed; although humans can voluntarily perform urine retention, animals cannot. To circumvent these challenges, this study evaluated the efficacy of HIFU in treating uterine fibroids based on its ablative efficacy in other muscle tissues. Herein, the leg muscle tissues of rabbits (instead of uterine fibroids) were selected as ablation sites, and changes in uterine blood vessels and peripheral blood perfusion were evaluated to determine the feasibility of C118P-assisted HIFU for the treatment of uterine fibroids.
2. Materials and methods
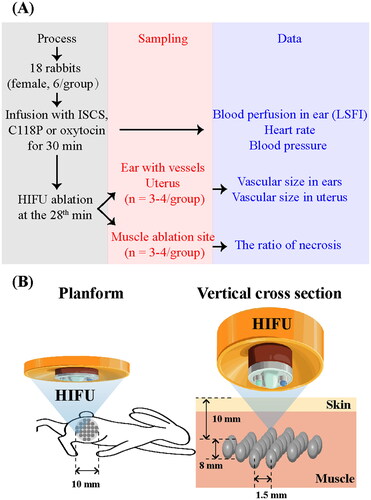
This in vivo study was approved by the Institutional Animal Affairs Committee of Shanghai University of Medicine and Health Sciences. As shown in , 18 large-eared, white female rabbits weighing 2.5 kg were divided into three groups. Each group was administered isotonic sodium chloride solution (ISCS), C118P (10 mg/kg, 5 mg/mL) (Nanjing Sanhome Pharmaceutical Co. Ltd., Nanjing, China) or oxytocin (2.25 U/kg, 1.33 U/mL) (Hybio Pharmaceutical Co., Ltd., Shenzhen, China) diluted with ISCS by slow infusion via the left marginal auricular vein for 30 min under isoflurane anesthesia. HIFU ablation was performed with an HY2900 HIFU therapy system (Wuxi Haiying Technology, Wuxi, China) on the left leg muscle 2 min before the end of the perfusion.
The HIFU device consists of a real-time diagnostic US device; integrated therapeutic transducers; a movement system allowing movement of the integrated transducer along the X, Y and Z planes; computer units for automated control; an US generator that produces the high-intensity US; and a degassed water circulation unit. The 1.5 MHz therapeutic transducer (25 cm diameter, 14 cm focal length) consisted of six spherically focused elements. It was fixed on top of a capsule that was filled with degassed water. The transducer was fixed to a computer-controlled, three-dimensional positioning device. Field mapping performed at Wuxi Haiying Technology yielded an ellipsoidal focal volume of 1.15 mm × 1.21 mm × 8.0 mm (−6 dB beam profile) based on the measurement of the field using a Tun001 hydrophone (NTR System, Inc., Onda Corp., Sunnyvale, CA). The waveforms were recorded using a TDS5052 oscilloscope (Tektronix, Inc., Beaverton, OR). The maximum electrical power from the amplifier to the therapy transducers was 1.02 kW. A corresponding total acoustic power of 479.2 W was measured using a Sartorius BS300 Balance (Beijing Sartorius Balance Co., Ltd., Beijing, China). The spatially averaged intensity level at −6 dB was 11,340 W/cm2. All intensities were obtained in water. The main HIFU parameters of the treatment in this study were the following: acoustic power, 63.5 W; irradiation depth (focal point), 1 cm below the skin (); irradiation time (Ton) of a single point, 1000 ms; irradiation interval time (Toff), 1000 ms. The ablation target region was set at a range of 1.0 cm diameter and 0.8 cm thickness with 35 ablation points in total. In this region, one irradiation ablation was performed for one focal point, with the distance between two points set at 1.5 mm.
Changes in blood perfusion in the right ear were recorded using laser speckle flow imaging (LSFI, Moor-FLPI-2, Moor Instruments, Axminster, UK), and heart rate and blood pressure were recorded throughout the perfusion process using an animal electrocardiogram monitor (ZS-2000, ZS Tech Co., Ltd., Shanghai, China). Animals (n = 3–4 per group) were sacrificed by anesthetic overdose following ablation, and the right ear with vessels and uterus and leg ablation sites were harvested for hematoxylin–eosin (HE) staining or nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) staining. For those ablation sites, one was cut perpendicular to the surface of the body into two equal pieces, and then one piece was sectioned and sliced parallel to the surface of the body at the site with the largest necrotic area, and the other sliced perpendicular to the surface of the body at thickest part of the necrosis.
The regions of interest in the marginal auricular vein and central auricular artery on images from LSFI were marked, and the perfusion index (PI) was extracted using moorFLPI-2 Review V5.0 (Moor Instruments, Axminster, UK). The PI, blood pressure and heart rate during the pre-perfusion period were considered to be 100%, and the relative values at 5, 10, 15, 20, 25 and 30 min after perfusion were calculated and used for statistical analysis. The circumferences or areas of blood vessels in the HE-stained images were measured. The necrotic area in the ablated tissues in the NADH-TR staining images was extracted and the necrosis rate was calculated. As most of them were conical, the necrosis rate was calculated using the formula:
Results were analyzed statistically and expressed as mean ± SEM. Statistical significance was set at p < .05. All statistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY). The data were analyzed by one-way analysis of variance (ANOVA) and assessed using the least significant difference. Correlations within the circumferences of the marginal auricular vein, central auricular artery and area ratio of the uterine blood vessels were evaluated using Pearson’s correlation coefficients with correlation and bivariate analysis.
3. Results
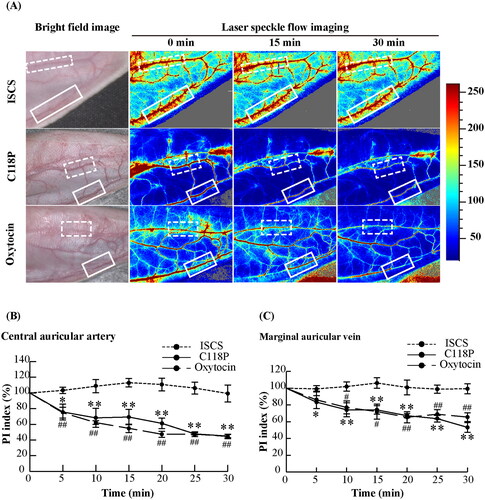
3.1. C118P steadily reduced blood perfusion in the ear as oxytocin
LSFI indicated that the blood perfusion of the central auricular artery and marginal auricular vein decreased gradually after perfusion of C118P and oxytocin, whereas ISCS perfusion did not result in any changes (). Image and statistical analyses () suggested no difference in the changes in blood perfusion between C118P and oxytocin when analyzed at the same time point. Blood perfusion of the central auricular artery significantly decreased starting 5 min after perfusion of either C118P or oxytocin; the PIs for C118P and oxytocin after 5 min were 75.80%±10.09% and 74.86%±6.83% of the pre-perfusion level (p < .05 and p < .01, respectively), and were 45.05%±1.99% and 44.63%±2.82% at the end of perfusion, respectively (both p < .01). The earliest effects on blood perfusion in the marginal auricular vein occurred 5 and 10 min after perfusion in the C118P and oxytocin groups, respectively. The PIs decreased significantly to 83.41%±7.56% and 86.08%±10.54% of the pre-perfusion level for C118P and oxytocin (both p < .05), and were 53.25%±5.84% and 65.56%±5.19% at the end of perfusion, respectively (both p < .01).
Figure 2. Changes of blood perfusion for rabbit auricular vessels in the ISCS, C118P and oxytocin groups. (A) LSFI for the three groups. Dotted and solid line frames indicate the central auricular artery and marginal auricular vein, respectively. The chromaticity bar indicates the PI value for pseudo colors, indicating the relative flow of blood perfusion. (B) Statistical analysis of the PI changes in the three groups. Mean value of PI was significantly different from that of the pre-perfusion level: *p< .05, **p< .01 in the C118P group, and #p< .05, ##p< .01 in the oxytocin group. N = 6 per group.
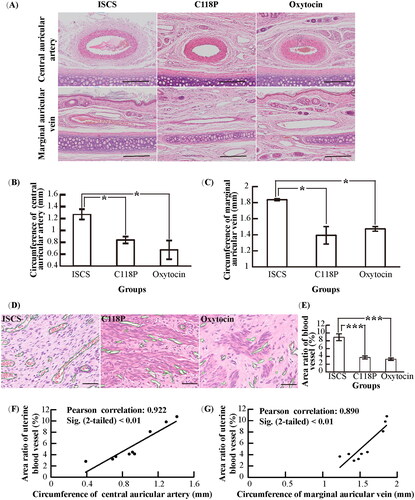
3.2. C118P and oxytocin contract blood vessels in the ear and uterus
Microscopic imaging with HE staining indicated that the central auricular artery, marginal auricular vein () and uterine blood vessels () in the C118P and oxytocin groups were smaller than those in the ISCS control group. Statistically, the circumferences of the central auricular artery and marginal auricular vein in the C118P and oxytocin groups were significantly lower than those in the ISCS group (both p < .05) (); the area ratios of the uterine blood vessels (the uterine blood vessel cross-sectional area per unit area of the uterine tissue) were 3.72%±0.39% and 3.27%±0.30% in the C118P and oxytocin groups, respectively, which were significantly lower than the 8.9%±0.87% observed in the ISCS group (both p < .001) (). Correlation analysis indicated that the circumferences of the central auricular artery and marginal auricular vein were positively correlated with the area ratio of the uterine blood vessels (r = 0.922, p < .01 and r = 0.890, p < .01, respectively) ().
Figure 3. Vascular changes in rabbit ears and uterus in the ISCS, C118P and oxytocin groups. (A) Microscopic imaging of HE staining for central auricular arteries and marginal auricular veins. Bar: 250 μm. (B, C) Statistical analysis of the circumferences for central auricular arteries (B) and marginal auricular veins (C). Data were significantly different: *p< .05 (n = 3 per group). (D) Microscopic imaging of HE staining for uterine blood vessels. The green line shows the blood vessel outline. Bar: 50 μm. (E) Statistical analysis of the area ratio for uterine blood vessels. Data were significantly different: ***p< .001 (n = 4 per group). (F) Correlation analysis between central auricular arteries and uterine blood vessels. (G) Correlation analysis between marginal auricular veins and uterine blood vessels.
3.3. C118P improves the ablative efficiency of HIFU in muscle more than oxytocin
Ultrasound revealed that the ablation zones in the C118P and oxytocin groups showed hyperechoic changes after HIFU ablation, which indicated that organic changes had occurred; in contrast, echogenic changes in the ISCS group were not noticeable (). Tissue dissection after animal sacrifice revealed that cone-shaped necrotic foci occurred in the muscle ablation zones, and their range in the C118P and oxytocin groups was wider than that in the ISCS group ().
Figure 4. The ablative efficiency of HIFU in the ISCS, C118P and oxytocin groups. (A) Ultrasound imaging and macroscopic view of postmortem samples for muscle tissues under HIFU ablation. Bar: 10 mm. (B) Microscopic imaging of HE and NADH-TR staining for ablated muscles. The contour in images for NADH-TR staining indicates a necrotic area. Bar: 2.5 mm. (C) Statistical analysis of the necrosis ratio. Data were significantly different: *p< .05 (n = 3 per group).
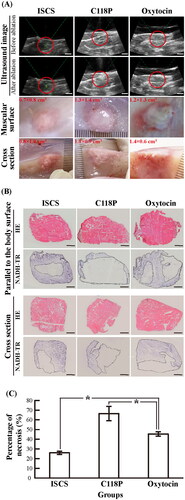
Histochemical staining revealed necrosis in the non-stained tissue with NADH-TR (). Based on microscopic imaging statistics, the necrosis rate after ablation in the C118P group was significantly higher than that in the oxytocin group (66.5%±7.3% vs. 45.5%±2.3%, p < .05). The necrosis rate in the oxytocin group was also significantly higher than that in the lowest ISCS group (45.5%±2.3% vs. 26.0%±1.7%, p < .05) ().
3.4. The effect of C118P on heart rate and blood pressure was greater than that of oxytocin
As shown in , perfusion of C118P and oxytocin significantly decreased the heart rate after 10 and 20 min, respectively (both p < .01), with this effect continuing until the end of the perfusion. The lowest heart rate occurred at 20–25 min, at 78.2%±3.7% of the pre-perfusion rates in the C118P group and 86.1%±4.3% in the oxytocin group. Although there was no significant difference between the two groups at 20–25 min, the heart rate was always lower in the C118P group, especially at 15 and 30 min (both p < .05).
Figure 5. Statistical analysis of the changes in heart rate (A) and blood pressure (B, C) in the ISCS, C118P and oxytocin groups. Mean value was significantly different from that of the pre-perfusion level: **p< .01 in the C118P group, and #p< .05, ##p< .01 in the oxytocin group. Data were significantly different between the C118P and oxytocin groups: &p< .05.
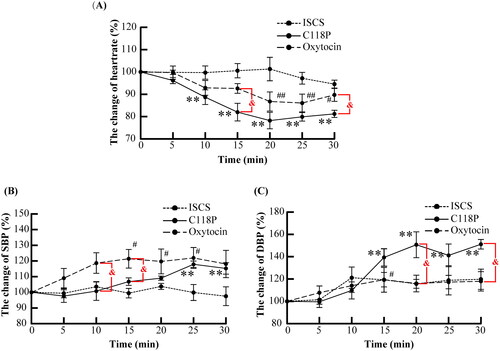
The changes in blood pressure were the opposite of that in heart rate. Both systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the C118P group increased and were significantly higher than the pre-perfusion levels at the end of the perfusion (both p < .01). Oxytocin did not change DBP, but increased SBP 15 min after perfusion (p < .01); however, SBP in the oxytocin group was not significantly higher than that in the C118P group in 20–30 min.
4. Discussion
This study selected leg muscles similar in texture to uterine fibroids to simulate ablation therapy because HIFU treatment and the study for animal uterine fibroids are difficult. Although ablation experiments in this study have suggested that C118P improves HIFU ablative efficacy in muscles (), this point alone cannot be used to arbitrarily speculate that the same effect applies to all muscle tissues. There is no current evidence that C118P has the same vasoconstrictive or blood-flow-reducing effect on the muscle and uterus, owing to methodological limitations.
It is difficult to determine whether the blood vessels of the muscle tissues are constricted (as they vary in thickness and are thus not comparable); however, the blood perfusion in the muscle can be inferred. Muscle blood perfusion can be detected using specific techniques for deeper tissues, such as digital subtraction angiography (DSA), magnetic resonance imaging (MRI) [Citation18] or radionuclide tracers [Citation19], which are expensive. In this study, a simple, fast and inexpensive LSFI, which can only measure blood perfusion on the surface [Citation20], was used for auricular vessels to speculatively evaluate changes in blood perfusion in the muscles or other peripheral tissues. As C118P decreased blood perfusion in the auricular vessels (), it could be inferred that it might also decrease the blood supply to the muscle, supporting its mechanism of improving the efficacy of HIFU ablation by reducing blood perfusion.
The change in the blood supply to the uterus might be different from that in the peripheral tissues. Fortunately, the positive correlation between the circumferences of the auricular vessel and the area ratio of the uterine blood vessels () suggests that C118P also contracted the uterine blood vessels. As the working principle of VDAs is to reduce blood perfusion by constricting blood vessels, it is speculated that C118P, as a VDA, could also decrease uterine blood perfusion, thereby improving the efficacy of HIFU ablation for uterine fibroids.
Clinically, the synergistic effect of oxytocin and HIFU is not ideal, and there is a need for a better substitute. In this study, C118P did not yield a better vasoconstrictive or blood flow-reducing effect than oxytocin ( and ); nevertheless, its ablation efficacy with HIFU in the muscle was superior to that of oxytocin (). This might be because the vasoconstrictive effect of oxytocin is dependent on the number of receptors in vascular smooth muscles, which cannot be further enhanced by augmenting oxytocin when the receptor is fully saturated [Citation21]. As an analogue of CA4P, C118P constricts blood vessels by depolymerizing tubulin in the vascular endothelium [Citation12], and thus augmenting the dose of C118P can enhance the vasoconstrictive effect as most tubulin is polymerized. Regardless of the cause, C118P appears to be a promising replacement for oxytocin as it shows evidence of being a better modulator in improving the efficiency of HIFU ablation.
The risks associated with C118P administration still need to be determined. VDAs have been reported to induce transient increases in blood pressure [Citation22,Citation23]. This study also revealed that C118P increased DBP and decreased heart rate more drastically than oxytocin (). This indicates that C118P administration in elderly individuals and those with a high risk of cardiovascular disease should be avoided when it is used to aid HIFU ablation, or electrocardiography should be performed for real-time monitoring. Prolonged high blood pressure (>150/100 mmHg) should be avoided by temporarily suspending the perfusion of C118P or administering antihypertensive drugs. Other modified approaches include interventional administration of C118P at the lesion site via the aorta and injection of other drugs (e.g., lipiodol) to induce embolism, thereby inhibiting blood perfusion [Citation14].
5. Conclusion
This study confirmed that C118P, a novel VDA, could improve the efficacy of HIFU ablation of muscle tissues with a better effect than that of the positive control treatment (oxytocin). In addition, C118P reduced blood perfusion in rabbit ears, with positive correlations between the circumferences of the auricular vessels and the area ratio of uterine blood vessels. It is speculated that C118P can also reduce blood perfusion in the uterus or other peripheral tissues and assist in the HIFU ablation of uterine fibroids or other benign tumors.
Acknowledgements
The authors thank Nanjing Sanhome Pharmaceutical Co. Ltd. for the kind gift of C118P.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used and analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bachu VS, Kedda J, Suk I, et al. High-intensity focused ultrasound: a review of mechanisms and clinical applications. Ann Biomed Eng. 2021;49(9):1975–1991.
- Jeng CJ, Ou KY, Long CY, et al. 500 cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol. 2020;59(6):865–871.
- Dogra VS, Zhang M, Bhatt S. High-intensity focused ultrasound (HIFU) therapy applications. Ultrasound Clin. 2009;4(3):307–321.
- Lozinski T, Filipowska J, Krol P, et al. Oxytocin administration in high-intensity focused ultrasound treatment of myomata. Biomed Res Int. 2018;2018:7518026.
- Łoziński T, Ludwin A, Filipowska J, et al. Oxytocin and misoprostol with diclofenac in the preparation for magnetic resonance-guided high-intensity ultrasound treatment of symptomatic uterine fibroids: a prospective cohort study. Ultrasound Med Biol. 2021;47(6):1573–1585.
- Chen HM, Zhang XC, Cao HX, et al. Application value of pituitrin in the treatment of uterine fibroids with high intensity focused ultrasound. Chin J Fam Plann Gynecotokol. 2014;7:46–48.
- Lang BHH, Woo YC, Chiu KW. Evaluation of pain during high-intensity focused ultrasound ablation of benign thyroid nodules. Eur Radiol. 2018;28(6):2620–2627.
- Serrone J, Kocaeli H, Douglas Mast T, et al. The potential applications of high-intensity focused ultrasound (HIFU) in vascular neurosurgery. J Clin Neurosci. 2012;19(2):214–221.
- Smolarczyk R, Czapla J, Jarosz-Biej M, et al. Vascular disrupting agents in cancer therapy. Eur J Pharmacol. 2021;891(891):173692.
- Prise VE, Honess DJ, Stratford MR, et al. The vascular response of tumor and normal tissues in the rat to the vascular targeting agent, combretastatin A-4-phosphate, at clinically relevant doses. Int J Oncol. 2002;21(4):717–726.
- Gaya AM, Rustin GJ. Vascular disrupting agents: a new class of drug in cancer therapy. Clin Oncol (R Coll Radiol). 2005;17(4):277–290.
- Zhang C, Zhang X, Wang G, et al. Preclinical pharmacokinetics of C118P, a novel prodrug of microtubules inhibitor and its metabolite C118 in mice, rats, and dogs. Molecules. 2018;23(11):2883.
- Yang M, Su Y, Wang Z, et al. C118P, a novel microtubule inhibitor with anti-angiogenic and vascular disrupting activities, exerts anti-tumor effects against hepatocellular carcinoma. Biochem Pharmacol. 2021;190:114641.
- He J, Liu C, Li T, et al. Pictorial imaging-histopathology correlation in a rabbit with hepatic VX2 tumor treated by transarterial vascular disrupting agent administration. Int J Med Sci. 2020;17(15):2269–2275.
- Li M, Hung A, Yang AWH. Guizhi fuling wan for uterine fibroids: a systematic review of in vivo studies. J Ethnopharmacol. 2019;245:112177.
- Mozzachio K, Moore AB, Kissling GE, et al. Immunoexpression of steroid hormone receptors and proliferation markers in uterine leiomyoma and normal myometrial tissues from the miniature pig, Sus scrofa. Toxicol Pathol. 2016;44(3):450–457.
- Cook AL, Rogers TD, Sowers M. Spontaneous uterine leiomyosarcoma in a rhesus macaque. Contemp Top Lab Anim Sci. 2004;43(1):47–49.
- Galanakis N, Maris TG, Kontopodis N, et al. Perfusion imaging techniques in lower extremity peripheral arterial disease. Br J Radiol. 2022;95(1135):20211203.
- Anderson HL, Yap JT, Miller MP, et al. Assessment of pharmacodynamic vascular response in a phase I trial of combretastatin A4 phosphate. J Clin Oncol. 2003;21(15):2823–2830.
- Heeman W, Steenbergen W, van Dam G, et al. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt. 2019;24(8):1–11.
- Balki M, Erik-Soussi M, Ramachandran N, et al. The contractile effects of oxytocin, ergonovine, and carboprost and their combinations: an in vitro study on human myometrial strips. Anesth Analg. 2015;120(5):1074–1084.
- Cooney MM, Radivoyevitch T, Dowlati A, et al. Cardiovascular safety profile of combretastatin a4 phosphate in a single-dose phase I study in patients with advanced cancer. Clin Cancer Res. 2004;10(1 Pt 1):96–100.
- Gill JH, Rockley KL, De Santis C, et al. Vascular disrupting agents in cancer treatment: cardiovascular toxicity and implications for co-administration with other cancer chemotherapeutics. Pharmacol Ther. 2019;202:18–31.