Abstract
Background
Benign breast lesions are often associated with hard nodule formation after microwave ablation (MWA), which persists for a long time and causes problems in patients. The aim of this study was to evaluate the efficacy of decorin in the treatment of hard nodule formation and its potential mechanism of action.
Methods
Using a Bama miniature pig model of mammary gland hyperplasia, immunohistochemistry, Masson’s trichrome and western blotting were firstly applied to compare the extent of fibrosis and activation of key members of the TGF-β1/SMAD and MAPK signaling pathways of hard nodule in the control and MWA groups, and then the extent of fibrosis and expression of signaling pathways in hard nodule were examined after application of decorin.
Results
The results showed that the MWA group had increased levels of TGF-β1, p-SMAD2/3, p-ERK1/2, and collagen I proteins and increased fibrosis at 2 weeks, 4 weeks, and 3 months after MWA. After decorin treatment, the expression levels of each protein were significantly downregulated, and the degree of fibrosis was reduced at 2 weeks, 4 weeks, and 3 months after MWA compared with the MWA group.
Conclusion
In conclusion, these results suggest that activation of TGF-β1 may play an important role in hard nodule formation and that decorin may reduce hard nodule formation after MWA in a model of mammary gland hyperplasia by inhibiting the TGF-β1/SMAD and MAPK signaling pathways.
Introduction
Benign breast lesions (e.g. fibroadenoma and mammary gland hyperplasia) are the most common diseases in adult women, and approximately 80% of breast nodules with biopsy indications are confirmed to be benign after pathological diagnosis [Citation1,Citation2]. A minority of nodules disappear spontaneously without treatment, but some of them are superficial, readily palpable, or accompanied by symptoms such as pain, swelling, or nipple discharge. In addition, some patients are worried about the enlargement of the nodules and the risk of malignancy in some pathological types and also suffer from a degree of psychological burden. There is, therefore, still a need for treatment in a large number of patients [Citation3–5]. Although surgery is considered one of the most commonly used methods, it often results in problems such as bleeding, hematoma, difficulty in completely removing all lesions, and scar formation. How to treat benign breast lesions in a minimally invasive, effective, and safe manner is still a matter of debate, and ablation therapy is generally considered to be a better alternative to surgical procedures.
Thermal ablation treatment includes microwave, radiofrequency, HIFU, laser, and other technologies, among which microwave ablation (MWA) has the advantages of a rapid increase in temperature, a large ablation range, complete necrosis in the coagulation zone, and repeatable treatment [Citation6]. Its treatment principle is the application of electromagnetic waves to induce charged ions to vibrate, causing them to collide with the surrounding molecules or ions and generate heat so that the local tissue temperature rises instantaneously. This then results in coagulation necrosis, and microwave ablation has achieved more satisfactory results in the treatment of thermal ablation of breast nodules [Citation7–9]. However, the use of microwave ablation often leads to the formation of local hard nodules that persist for a certain period of time, causing distress to patients.
After tissue injury, the tissue enters a state of self-repair, and fibrosis and scar formation occur. Transforming growth factor-β1 (TGF-β1) plays a crucial role in fibrosis and scar formation, mainly through TGF-β1-mediated SMAD and MAPK signaling pathways to complete a series of cellular transformations and repair [Citation10–12]. Decorin is a natural inhibitor of TGF-β1 and has been shown to exert beneficial antifibrotic effects in hypertrophic scars formed after burns [Citation13]. Therefore, this study aimed to investigate whether decorin could play an inhibitory role through the TGF-β1 signaling pathway in the formation of hard nodule after MWA in a model of mammary gland hyperplasia.
Materials and Methods
Animals
Two adult female Bama miniature pigs (age, 12 months; weight, 30–35 kg) determined to be healthy on the basis of physical examination were studied. The study protocol was approved by the ethics committee of Beijing Tiantan Hospital Affiliated with Capital Medical University (protocol number: 202102013). This study was performed in accordance with the Policy on Human Care and Use of Laboratory Animals of the United States Public Health Service. Food and water were withheld the evening before the study.
Methods
Establishment of a model for mammary gland hyperplasia
Bama miniature pigs were fasted, dehydrated for 8 h, and anesthetized by intramuscular injection of 3% sodium pentobarbital in the posterior neck at a dose of 1–2 ml/kg. Generally, the presence of slow breathing, muscle relaxation, and a sluggish corneal response after administration of the drug indicated that appropriate anesthesia had been achieved, and tracheal intubation was performed via the mouth. Bama miniature pigs were placed on a thermostatic operating table in the supine position, their limbs were immobilized and the hair in the mammary region was removed with a razor.
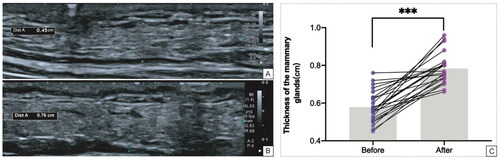
After being anesthetized, all mammary glands of the Bama miniature pigs were scanned using ultrasound. The ultrasound examinations were performed using an Aplio i800 ultrasound system (Canon Medical Systems, Japan) equipped with an i18LX5 transducer (5–18 MHz). The thickness of each mammary gland was recorded, with the thickness measurement site being 1 cm from the nipple in the outer upper quadrant. All glands were scanned for any occupying lesions. Ultrasound-guided puncture biopsy was performed on the mammary tissue, and the tissue samples were placed in a 10% formalin solution.
Estradiol benzoate was injected at 0.5 mg/(kg-d) into the muscle of the buttocks of the Bama miniature pigs for 25 days, followed by progesterone injection at 4 mg/(kg-d) continuously for 5 days.
After injection of the drug, all of the mammary glands of the Bama miniature pigs were scanned using ultrasound. The thickness of each mammary gland was recorded, and all glands were scanned for any occupying lesions. Ultrasound-guided puncture biopsy was performed on the mammary tissue, and the samples were placed in a 10% formalin solution.
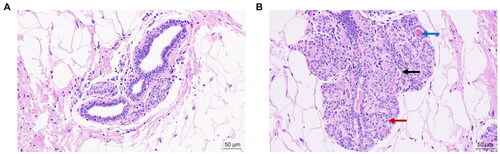
The fixed tissue samples were routinely dehydrated, paraffin-embedded, and serially sectioned into 5 mm samples. After staining with hematoxylin-eosin (H&E), the sections were mounted and the histology was observed with a light microscope.
Expression of TGF-β1 and activation of the TGF-β1/SMAD and MAPK signaling pathways in the control group and the MWA group
Ultrasound-guided microwave ablation and puncture sampling in a model of mammary gland hyperplasia
Eight hours before the experiment, the Bama miniature pigs were fasted without food or water. The experimental pigs were placed on a constant-temperature operating table after anesthesia, and their limbs were immobilized. The mammary glands of the animals were fully exposed. The ablation site was routinely sterilized and local anesthesia with 1% lidocaine hydrochloride was used.
Five glands were randomly selected for ultrasound-guided percutaneous MWA using the same ultrasound machine. Microwave ablation was performed using a microwave system (KY-2000, Canyon Medical, China) consisting of a microwave generator (2450 MHz) and a hollow water-cooled shaft antenna (with a 3 mm active tip of microwave transmission). The output power of the system was 30 W and the duration was 60 s. After the MWA, the wounds were disinfected and bandaged to prevent infection.
Five glands that did not undergo microwave ablation were randomly selected for the control group. Ultrasound-guided puncture biopsy of the hard nodules formed after microwave ablation and the normal glands of the control group were performed at 1, 2, and 4 weeks as well as 3 months after the MWA. Ultrasound images obtained at different times after the microwave ablation are shown in . A portion of the removed tissue was fixed in 10% formalin solution for immunohistochemical and Masson’s trichrome staining. The remainder of the tissue was stored in a −80 °C refrigerator for subsequent use in western blotting.
Immunohistochemical staining
Tissue samples were embedded in paraffin and sectioned into slices with a thickness of approximately 5 μm. After dewaxing with xylene and dehydration in alcohol, antigen repair and blocking of endogenous peroxidase activity were performed. The sections were then incubated in primary antibodies against anti-TGF-β1 (bs-0086R, Bioss, China, 1:100) at 4 °C overnight. After rinsing three times with PBS, the tissue sections were incubated with secondary antibodies corresponding to the species for 1 h at room temperature. Then, 3,3′-diaminobenzidine tetrahydrochloride (DAB) color development solution was added. After staining with hematoxylin, the sections were visualized with a microscope. Positively stained areas were measured using ImageJ software (National Institutes of Health, Bethesda, USA).
Masson’s trichrome staining
Masson’s trichrome staining was performed to examine the fibrosis in the hard nodules. The tissue samples fixed in 10% formalin were routinely dehydrated, paraffin-embedded, and serially sectioned into 5 µm samples. After staining with Masson’s trichrome, the sections were mounted and observed for histological examination with a light microscope. Collagen fibers were stained blue by Masson’s staining, and the image analysis software ImageJ (National Institutes of Health) was used to calculate the area of blue staining in the whole field of view to quantify the collagen volume fraction (CVF).
Western blotting
Protein expression levels of TGF-β1, phosphorylated-SMAD2/3 (p-SMAD2/3), SMAD2/3, phosphorylated-ERK1/2 (p-ERK1/2), ERK1/2, and type I collagen (collagen I) were determined by western blot analysis. The tissues were ground and solubilized in a non-denaturing lysis buffer (Applygen Technology Inc., Beijing, China). Homogenates were centrifuged at 12,000 rpm at 4 °C for 15 min, and the supernatants were collected. Protein concentrations were measured using a BCA Protein Assay Kit (No. 23227, Thermo Fisher Scientific, USA). The extracted protein samples were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed using the following antibodies: Anti-TGF-β1 (bs-0086R, Bioss, 1:1000), anti-p-SMAD2/3 (YP0362, ImmunoWay, USA, 1:1000), anti-SMAD2/3 (YT4332, ImmunoWay, 1:1000), anti-p-ERK1/2 (4370, Cell Signaling Technology, USA, 1:2000), anti-ERK1/2 (4695, Cell Signaling Technology, 1:1000), and anti-collagen I (bs-7158R, Bioss, 1:1000). After blocking in 5% skimmed milk for 2 h, the membranes were incubated with the primary antibodies overnight at 4 °C, and the corresponding secondary antibodies (BE0101, Easybio, China, 1:10,000) were incubated at 37 °C for 2 h. Finally, immunoreactive proteins were detected using enhanced chemiluminescence (ECL) reagent (P10200, New Cell & Molecular Biotech Co., Ltd, China). The strips were analyzed using ImageJ software (National Institutes of Health).
Expression of TGF-β1 and activation of the TGF-β1/SMAD and MAPK signaling pathways in the MWA group and the decorin-treated group
Ultrasound-guided microwave ablation and puncture sampling in a model of mammary gland hyperplasia
The preparation before microwave ablation was performed as before. Ten glands were selected for ultrasound-guided percutaneous MWA using the same ultrasound machine. Five glands were selected as the MWA group, and the other five glands were selected as the decorin-treated group. Glands in the decorin-treated group were injected with a dose of 50 µg of recombinant decorin (143-DE, R&D Systems Inc., USA) on days 1, 3, 5, and 7 after MWA [Citation14,Citation15], and the MWA group was injected with the same dose of saline at the same time.
Ultrasound-guided puncture biopsy of the hard nodules formed after microwave ablation in the MWA and decorin-treated groups was performed at 2 and 4 weeks as well as 3 months after MWA. A portion of the removed tissue was fixed in 10% formalin solution for immunohistochemical and Masson’s trichrome staining. The remainder of the tissue was stored in a −80 °C refrigerator for subsequent use in western blotting.
Staining experiments of tissues and detection of proteins
Immunohistochemical staining, Masson’s trichrome staining, and western blotting were performed on the obtained tissues using the same specific steps as before.
Statistical analysis
All data were tested for normality of distribution using the Shapiro–Wilk test. All data were normally distributed, and parametric statistical analyses were performed. The results are presented as means ± the standard deviation. The thickness of each mammary gland before and after modeling was compared using a paired sample t-test. Two-way repeated measures analysis of variance (ANOVA) was applied to determine statistically significant effects of different tissues and time points on the CVF and protein expression levels of hard nodules after MWA. All analyses were performed with IBM SPSS version 26.0 software (SPSS Inc., Chicago, IL, USA) with an α level set at 0.05.
Results
Establishment of a model for mammary gland hyperplasia
Ultrasound observation of changes in the thickness of the breast before and after modeling
The thickness of the breast before injection of the drug was 0.578 ± 0.082 cm, while after injection of the drug it was 0.783 ± 0.080 cm. Thus, the thickness increased by 0.205 cm (95% CI: 0.159–0.251 cm) after injection of the drug (), with a statistically significant difference (t = 9.216, p < 0.001) (). Ultrasound scanning of all mammary glands of Bama miniature pigs before and after injection of the drug did not reveal any significant lesions.
H&E Staining of breast tissues before and after the establishment of the model
Before the model was established, the number of follicles in the breast was small, the volume was small, and the follicles were not dilated (). After the model was established, the volume of the breast lobules increased, the number of follicles increased, the lumen of the gland was mildly dilated, and a small amount of secretion was visible in the lumen, which was consistent with the pathological manifestations of mammary gland hyperplasia ().
Expression of TGF-β1 and activation of the TGF-β1/SMAD and MAPK signaling pathways in the control group and the MWA group
Immunohistochemical staining
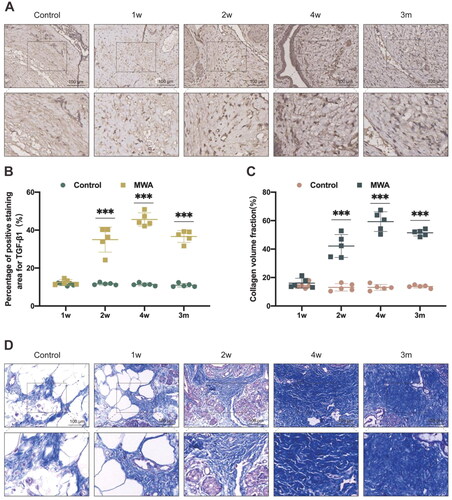
Both the control and MWA groups exhibited positive expression of TGF-β1 within the hard nodules, and it was mainly expressed in the cytoplasm. In the control group, the cells were intact and well arranged at 1, 2, and 4 weeks as well as 3 months after the MWA, and there was less TGF-β1 expression (yellow-brown staining). In the MWA group, the intracellular expression of TGF-β1 was also low at 1 week after the MWA, and at 2 weeks after the MWA, the intracellular expression of TGF-β1 was enhanced compared with that at 1 week. At 4 weeks as well as 3 months after the MWA, there was abundant intracellular expression of TGF-β1 (). The difference in the percentage of area stained positive for TGF-β1 between the control group and the MWA group at 2 and 4 weeks as well as 3 months after the MWA was statistically significant (all p < 0.001), and the difference in the percentage of area stained positive for TGF-β1 between the two groups at 1 week after the MWA was not statistically significant (p = 0.195) ().
Figure 4. (A) Immunohistochemical staining of TGF-β1 in the control and MWA groups at 1, 2, and 4 weeks as well as 3 months after the MWA. (B) Comparison of the positive staining area of TGF-β1 in the control and MWA groups. (C) Comparison of collagen volume fraction in the control and MWA groups. (D) Masson’s trichrome staining in the control and MWA groups at 1, 2, and 4 weeks as well as 3 months after the MWA. *p < 0.05, **p < 0.01 and ***p < 0.001.
Masson’s trichrome staining
Masson’s trichrome staining resulted in the collagen fibers appearing in light blue, cell nuclei as dark blue, while the remainder was stained red. In the control group, a very small amount of light blue tissue was seen in the hard nodules at 1, 2, and 4 weeks as well as at 3 months after the MWA. In the MWA group, a very small amount of light blue tissue was seen at 1 week after the MWA, and at 2 weeks after the MWA, a small amount of light blue-stained tissue was observed, which increased compared with that at 1 week. At 4 weeks and at 3 months after the MWA, a large area of disorganized light blue-stained tissue was seen, and collagen deposition could readily be discerned (). The difference in the collagen volume fraction between the control group and the MWA group at 2 and 4 weeks as well as at 3 months after the MWA was statistically significant (all p < 0.001), and the difference in the collagen volume fraction between the two groups at 1 week after the MWA was not statistically significant (p = 0.396) ().
Western blotting
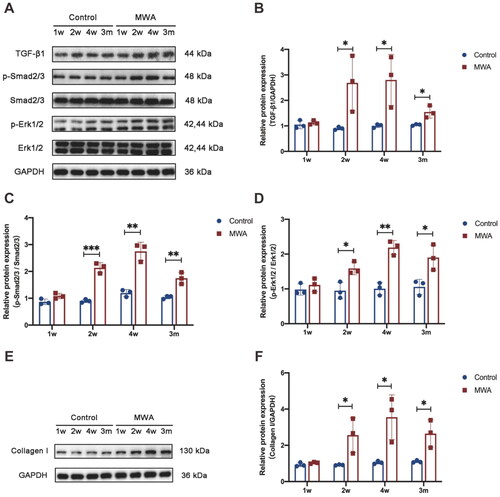
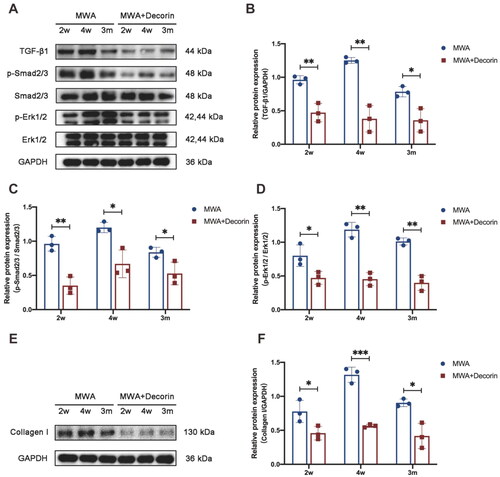
To determine whether activation of TGF-β1 plays a role in the formation of hard nodules after MWA, the expression of TGF-β1 and activation of the TGF-β1/SMAD and MAPK signaling pathways in the mammary gland hyperplasia model were investigated. As shown in , TGF-β1, p-SMAD2/3, p-ERK1/2, and collagen I proteins in the control group were expressed at similar levels at different time points (1, 2, and 4 weeks as well as 3 months after the MWA). In the MWA group, the expression levels of TGF-β1, p-SMAD2/3, p-ERK1/2, and collagen I were significantly increased at 2 and 4 weeks as well as at 3 months after the MWA compared with the control group for each protein (p < 0.05). This suggests that the TGF-β1/SMAD and MAPK signaling pathways play important roles in the formation of hard nodules in the MWA group.
Effect of decorin in blocking the formation of hard nodules after MWA
Immunohistochemical staining
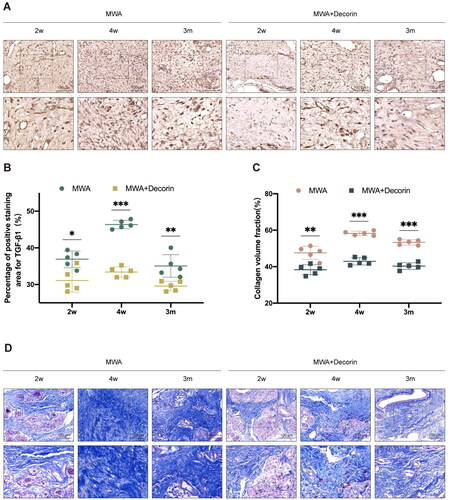
The intracellular TGF-β1 immunohistochemical staining was significantly reduced after decorin treatment (). The difference in the percentage of area positive for TGF-β1 staining area between the MWA group and the decorin-treated group at 2 and 4 weeks as well as 3 months after the MWA was statistically significant (p = 0.010, p < 0.001, and p = 0.006, respectively) ().
Figure 6. (A) Immunohistochemical staining of TGF-β1 in the MWA and decorin-treated groups at 2 and 4 weeks as well as 3 months after the MWA. (B) Comparison of the positive staining area of TGF-β1 in the MWA and decorin-treated groups. (C) Comparison of collagen volume fraction in the MWA and decorin-treated groups. (D) Masson’s trichrome staining in the MWA and decorin-treated groups at 2 and 4 weeks as well as 3 months after the MWA. *p < 0.05, **p < 0.01 and ***p < 0.001.
Masson’s trichrome staining
After treatment with decorin, the area of light blue staining in the hard nodules was significantly reduced compared with that in the MWA group (). The difference in the collagen volume fraction between the MWA group and the decorin-treated group at 2 and 4 weeks as well as 3 months after the MWA was statistically significant (p = 0.002, p < 0.001, and p < 0.001, respectively) ().
Western blotting
To further determine whether decorin could reduce the formation of hard nodules after MWA, we examined the expression levels of TGF-β1, p-SMAD2/3, p-ERK1/2, and collagen I at 2 and 4 weeks as well as 3 months after the MWA. As shown in , the TGF-β1, p-SMAD2/3, p-ERK1/2, and collagen I expression levels in the decorin-treated group were lower than those in the MWA group at 2 and 4 weeks as well as 3 months after the MWA (p < 0.05). This suggests that decorin can reduce the formation of hard nodules through inhibition of the TGF-β1/SMAD and MAPK signaling pathways.
Discussion
Microwave ablation of benign breast lesions, which is considered to be a good alternative to surgery, also has certain problems. The prolonged presence of hard nodules after microwave ablation adversely impacts patient well-being. Benign breast lesions include various pathological types such as fibroadenoma and mammary gland hyperplasia. In this study, an animal model of mammary gland hyperplasia was used to determine the role of decorin in inhibition of the formation of hard nodules after MWA. This study initially examined changes in TGF-β1 expression levels to assess whether TGF-β1 activation may be associated with the formation of hard nodules after MWA, and the extent of tissue fibrosis was determined by Masson’s trichrome staining. The results revealed increased TGF-β1 expression in the MWA group and an increased degree of fibrosis after MWA. To better understand the possible mechanisms of hard nodule formation, the activities of p-SMAD2/3 and p-ERK1/2, which are downstream targets of TGF-β1 signaling, and the final effector molecule collagen I were examined. These results suggest that the TGF-β1-induced signaling pathways are involved in hard nodule formation after MWA. To further investigate the role and mechanism of decorin in reducing the formation of hard nodules, the extent of fibrosis in the hard nodule tissues was determined by Masson’s trichrome staining, and the activation of TGF-β1 signaling and downstream signaling molecules was also assayed. Treatment with decorin resulted in reduced fibrotic changes in the hard nodule tissues, significantly reduced activation of TGF-β1 signaling, and reduced expression of downstream signaling molecules. This suggests that one of the mechanisms by which decorin reduces hard nodule formation after MWA is through inhibition of the TGF-β1/SMAD and MAPK signaling pathways.
TGF-β1 is an important cytokine that causes fibrosis and scarring, and its downstream SMAD and MAPK signaling pathways play an extremely important role in tissue fibrosis and scar formation, with important functions in regulating cell growth and differentiation [Citation16,Citation17]. TGF-β1 is a membrane protein receptor-dependent factor that binds primarily to cell membrane receptors and activates SMAD2 and SMAD3 phosphorylation. Activated SMAD2/3 binds to SMAD4 to form SMAD2/3/4 complexes, which then translocate to the nucleus and trigger target gene transcription, resulting in collagen overexpression, which leads to scar formation [Citation18,Citation19]. The TGF-β1-MAPK signaling pathway, as a non-classical signaling pathway, has an important role in tissue fibrosis and scar formation, and especially this subclass of ERK has a close relationship with the inflammatory response and scar formation after tissue injury[Citation20]. Therefore, we hypothesized that the formation of hard nodules after MWA is associated with TGF-β1/SMAD and MAPK signaling pathways. Microwave ablation involves thermal injury, and a previous study [Citation21] has shown that the expression of TGF-β1 is increased after thermal injury, and inhibition of long-term TGF-β1-dependent fibrosis of wounds by targeting the signaling molecule CD47 can improve the rate of healing from thermal injury, which is consistent with our findings.
Owing to the close association between TGF-β1 signaling and collagen production, blocking the TGF-β1 signaling pathway may prevent hard nodule formation. Decorin is a small proteoglycan that is a natural inhibitor of the TGF-β signaling pathway [Citation22,Citation23]. Decorin binds TGF-β via core proteins rather than side chains, resulting in an antifibrotic response [Citation24]. Previous studies have shown that decorin downregulates TGF-β production and collagen synthesis in proliferative scar fibroblasts in vitro [Citation25,Citation26]. Several in vivo experiments with decorin injection or synthesis by expression vectors have also shown that this proteoglycan has antifibrotic effects [Citation27]. We have previously demonstrated that hard nodule formation after MWA is associated with the TGF-β1/SMAD and MAPK signaling pathways, and we subsequently speculated that decorin may play an inhibitory role in this process. We confirmed that decorin can further inhibit hard nodule formation after MWA in a model of mammary gland hyperplasia by inhibiting the TGF-β1 signaling pathway.
Hypertrophic scarring following thermal injury is a dermal fibroproliferative disorder that can lead to considerable morbidity. The development of hypertrophic scars is often linked to TGF-β1 overexpression [Citation28]. Elevated levels of TGF-β1 have been found in the serum of recovering burn patients [Citation28,Citation29], and HTS staining is more intense for this cytokine than in normal skin samples or mature scars. Therefore, TGF-β1 is a key cytokine in wound healing and hypertrophic scarring. A previous study [Citation30] has shown that the sustained delivery of recombinant decorin from nanofiber dressings potentially obstructs scar formation during the process of wound healing. We suggest that the changes in ablation foci correspond to the local thermal damage repair process, and the change pattern may be related to tissue repair and cell proliferation. Therefore, this study is the first to link the formation of local hard nodules after thermal ablation to the TGF-β1signaling pathway and to verify the potential of decorin treatment.
The present study has some limitations. The antifibrotic effect of decorin in the tissue appeared to be dose- and time-dependent. In addition, our results should be further validated by in vitro experiments. Future studies should investigate the dose- and time-dependent responses of the antifibrotic effect of decorin, as well as the underlying molecular mechanism in vitro.
In conclusion, the present study demonstrated involvement of the TGF-β1 cascade in the formation of hard nodules after MWA in a mammary gland hyperplasia model. Decorin was shown to reduce the expression of collagen I and tissue fibrosis in hard nodules in vivo through inhibition of the TGF-β1 signaling pathway. Therefore, inhibition of TGF-β1 activity by decorin treatment may be a feasible approach to promote the disappearance of hard nodules after MWA.
Acknowledgements
The authors acknowledge all the co-authors for their hard work in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Wei Zhang, upon reasonable request.
Additional information
Funding
References
- Isozaki H, Yamamoto Y, Sakai K, et al. Mucinous carcinoma of the breast: clinicopathological features and long-term prognosis in comparison with invasive ductal cancer; a single hospital’s 30+-year experience. Acta Med Okayama. 2020;74(2):137–143.
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116(33-34):565–574.
- Han X, Li Q, Wang C, et al. The association of occupational stress and depressive symptoms among employed persons with benign breast disease: the mediating role of psychological capital. Psychopathology. 2019;52(3):205–211.
- Barrow TM, Peng C, Wilson A, et al. Psychosocial stress is associated with benign breast disease in young chinese women: results from project ELEFANT. Breast Cancer Res Treat. 2019;173(1):217–224.
- Figueroa JD, Gierach GL, Duggan MA, et al. Risk factors for breast cancer development by tumor characteristics among women with benign breast disease. Breast Cancer Res. 2021;23(1):34.
- Ahmed M, Brace CL, Lee FT, Jr., et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.
- Liu G, Zhang Y, Hu E, et al. Feasibility and efficacy of microwave ablation for treating breast fibroadenoma. Int J Hyperthermia. 2021;38(1):471–478.
- Cui R, Wu H, Xu J, et al. Volume reduction for >/=2 cm benign breast lesions after ultrasound-guided microwave ablation with a minimum 12-month follow-up. Int J Hyperthermia. 2021;38(1):341–348.
- Yang Q, Li H, Chen BH, et al. Ultrasound-guided percutaneous microwave ablation for 755 benign breast lesions: a prospective multicenter study. Eur Radiol. 2020;30(9):5029–5038.
- Wu ZY, Zhang HJ, Zhou ZH, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-beta1/SMAD pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10(3):1046–1056.
- Cui J, Jin S, Jin C, et al. Syndecan-1 regulates extracellular matrix expression in keloid fibroblasts via TGF-beta1/SMAD and MAPK signaling pathways. Life Sci. 2020;254:117326.
- Li Y, Yu Z, Zhao D, et al. Corilagin alleviates hypertrophic scars via inhibiting the transforming growth factor (TGF)-beta/SMAD signal pathway. Life Sci. 2021;277:119483.
- Zhang Z, Li XJ, Liu Y, et al. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns. 2007;33(5):634–641.
- Abdel MP, Morrey ME, Barlow JD, et al. Intra-articular decorin influences the fibrosis genetic expression profile in a rabbit model of joint contracture. Bone Joint Res. 2014;3(3):82–88.
- Zhu KQ, Engrav LH, Tamura RN, et al. Further similarities between cutaneous scarring in the female, red duroc pig and human hypertrophic scarring. Burns. 2004;30(6):518–530.
- Ren LL, Li XJ, Duan TT, et al. Transforming growth factor-beta signaling: from tissue fibrosis to therapeutic opportunities. Chem Biol Interact. 2023;369:110289.
- Beaven E, Kumar R, Bhatt HN, et al. Myofibroblast specific targeting approaches to improve fibrosis treatment. Chem Commun (Camb). 2022;58(98):13556–13571.
- Liu J, Jin J, Liang T, et al. To Ub or not to Ub: a regulatory question in TGF-beta signaling. Trends Biochem Sci. 2022;47(12):1059–1072.
- Lee JH, Massague J. TGF-beta in developmental and fibrogenic EMTs. Semin Cancer Biol. 2022;86(Pt 2):136–145.
- Tang M, Bian W, Cheng L, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF-beta/SMAD and ERK signaling pathways. Int J Mol Med. 2018;41(3):1487–1499.
- Soto-Pantoja DR, Shih HB, Maxhimer JB, et al. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014;37:25–34.
- Jarvinen TAH, Ruoslahti E. Generation of a multi-functional, target organ-specific, anti-fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin. Br J Pharmacol. 2019;176(1):16–25.
- Jarvinen TA, Ruoslahti E. Targeted antiscarring therapy for tissue injuries. Adv Wound Care (New Rochelle). 2013;2(2):50–54.
- Dong Y, Zhong J, Dong L. The role of decorin in autoimmune and inflammatory diseases. J Immunol Res. 2022;2022:1–11.
- Honardoust D, Varkey M, Hori K, et al. Small leucine-rich proteoglycans, decorin and fibromodulin, are reduced in postburn hypertrophic scar. Wound Repair Regen. 2011;19(3):368–378.
- Ahmed Z, Bansal D, Tizzard K, et al. Decorin blocks scarring and cystic cavitation in acute and induces scar dissolution in chronic spinal cord wounds. Neurobiol Dis. 2014;64:163–176.
- Krishna P, Regner M, Palko J, et al. The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring. Laryngoscope. 2010;120(11):2247–2257.
- Wang J, Jiao H, Stewart TL, et al. Increased TGF-beta-producing CD4+ T lymphocytes in postburn patients and their potential interaction with dermal fibroblasts in hypertrophic scarring. Wound Repair Regen. 2007;15(4):530–539.
- Yang L, Scott PG, Giuffre J, et al. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82(9):1183–1192.
- Vijayan AN, Solaimuthu A, Murali P, et al. Decorin mediated biomimetic PCL-gelatin nano-framework to impede scarring. Int J Biol Macromol. 2022;219:907–918.