Abstract
Objective
To evaluate the long-term efficacy and safety of ultrasound-guided radiofrequency ablation (RFA) for treating locally recurrent papillary thyroid cancer (PTC).
Methods
This retrospective study involved 32 patients with pathologically confirmed locally recurrent PTC. The ablation zone was assessed by contrast-enhanced ultrasound (CEUS) after RFA. At baseline, 6 and 12 months and every 6 months or 12 months thereafter, the following results were recorded: recurrence rate, largest diameter, volume, volume reduction rate (VRR) of recurrent lesions, serum thyroglobulin (Tg) level and complications.
Results
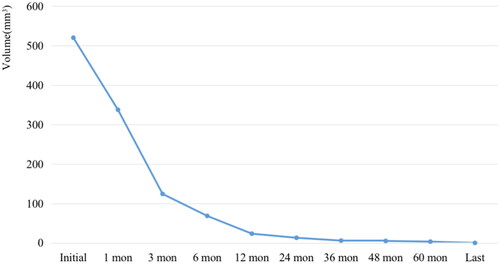
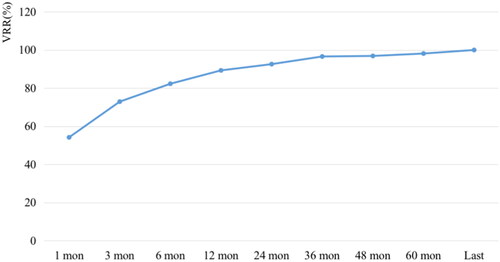
58 recurrent lesions in 32 patients were successfully ablated with RFA. The mean follow-up time was 73.19 ± 12.68 months (range, 60 to 98 months). At the last follow-up, almost all ablated lesions disappeared completely, and only one lesion showed scar-like changes. Nine (28.13%) patients developed new locally recurrent tumors; they were successfully treated with repeat RFA sessions. No new recurrent lesions were found during the follow-up. The largest diameter and volume of recurrent lesions decreased from 13.71 ± 6.48 mm and 520.43 ± 627.85 mm3 to 0 each at the end of observation period (p < .001). The average VRRs at 6, 12, 24, 36, 48, 60 months and last follow-up after RFA were 54.17%, 72.90%, 82.28%, 89.30%, 92.57%, 96.60%, 96.88%, 98.14% and 100% respectively. The median of serum Tg level was decreased from 1.48 ng/mL to 0.00 ng/mL (p < .05). No complications were reported during the follow-up.
Conclusions
US-guided RFA is an effective and safe option for treating locally recurrent PTC in selected patients, with favorable long-term outcomes.
Introduction
Thyroid carcinoma is the most common endocrine malignancy. With the incidence rates rising to 10.1 per 100,000 women [Citation1], thyroid cancer has become the seventh most common tumor in females [Citation2]. Papillary thyroid cancer (PTC) is the main contributor to total number of thyroid cancer, accounting for approximately 90% of overall histologic types [Citation3]. Owing to the indolent nature of PTC, the mortality rate is very low. However, in almost 20–35% of the patients, locally recurrent PTC is detected in the neck after initial thyroidectomy [Citation4], including thyroid surgery bed nodules and metastatic lymph nodes. Surgery followed by radioactive iodine therapy and thyroid hormone therapy is the standard treatment for recurrent PTC, but for both surgeons and patients, repeat surgery can be more challenging [Citation5]. Repeat surgeries are associated with temporary or permanent recurrent laryngeal nerve injury, postoperative hemorrhage and hypoparathyroidism, which can affect the quality of life of thyroid cancer survivors to varying degrees [Citation6].
In the last few decades, ultrasound-guided thermal ablation has shed some light on the future of precision medicine. According to some guidelines, radiofrequency ablation (RFA) can be performed in patients with recurrent thyroid cancer who refuse secondary surgery or in cases of high surgical risk [Citation7–9]. Several studies have demonstrated the efficacy and safety of RFA for treating locoregional recurrent PTC, with the mean volume reduction rate (VRR) ranging from 50.9% to 100% and the complication rate ranging from 0% to 10% [Citation10–13]. Very few long-term studies have presented data on the treatment efficacy of RFA. The study with the largest samples of 119 patients had a mean follow-up period of 47.9 ± 35.4 months [Citation14], Chung et al. [Citation15] reported the results of 29 patients who received long-term follow-up care for 80.0 ± 17.3 months. Some patients with new recurrent lesions after RFA were successfully treated with repeat operations. In this study, we aimed to determine the long-term efficacy and safety of RFA for locally recurrent PTC.
Methods
This was a retrospective study performed at the Chinese PLA General Hospital, Beijing, China; the study was approved by our institutional review board (S2019-211-01), and informed consents was waived.
Patients
The inclusion criteria were as follows: (1) patient received thyroidectomy for PTC, (2) recurrent lesions were pathologically proven to be malignant by US-guided core-needle biopsy (CNB) or fine needle aspiration (FNA) before RFA, (3) presence of with well-documented clinical and imaging reports and (4) no evidence of distant metastases.
The following patients were excluded: (1) patients with lesions confirmed as other types of malignant thyroid carcinomas, (2) those who received implantation of iodine seeds, (3) those who failed to conduct the repeat follow-up examinations after RFA, (4) patients with severe organ failures unable to tolerate RFA and (5) patients with short follow-up duration (less than 60 months).
Pre-ablation evaluation
Ultrasonography (US) and contrast-enhanced ultrasound (CEUS) before and after RFA, including at the repeat follow-up evaluations, were performed using a Siemens Acuson Sequoia 512 Ultrasound System (Siemens, Mountain View, CA, USA) with a 15L8W linear array transducer, a Philips iU22 Ultrasound System (Philips Healthcare, Bothell, WA) with a L12-5 linear array transducer or a Mindray M9 Ultrasound System (Mindray, Shenzhen, China) with a L12-4 linear array transducer. US-guided RFA and CNB were all performed using a Siemens Acuson Sequoia 512 Ultrasound System with a 6L3 linear array transducer.
Each recurrent lesion was assessed by US and the following characteristics were recorded: location and US signs (margin, shape, cystic components, echogenicity, calcification, vascularization and three orthogonal diameters (the largest diameter and two perpendicular diameters)). The volume of each lesion was calculated using the equations V = πabc/6 (V is volume; a is the largest diameter; b and c are the other wo perpendicular diameters). The long-to-short-axis (L/S) ratio was calculated from the maximal transverse and longitudinal diameters [Citation16].
All the patients were evaluated by CEUS before RFA. CEUS was used to determine the blood perfusion patterns of lesions. The intensity (hyperenhancement, isoenhancement and hypoenhancement), enhancement diffusion (homogenous or heterogenous) and enhancement direction (centripetal fill-in, centrifugal fill-in and mixed fill-in) of the lesions were extracted from the contrast patterns. Sulfur hexafluoride (SonoVueR, Bracco. International, Milan, Italy) was used as the contrast agent. CEUS was performed after bolus injection of SonoVue (2.0–3.0 ml) followed by flushing with 5 ml of normal saline. Simultaneously, the timer on the US machine was started, and the imaging plane was kept as stable as possible. Each contrast imaging acquisition lasted at least 2 min after bolus injection. Before conducting the RFA procedure, the blood samples of all enrolled patients were assessed for coagulation, thyroid function and serum Tg level.
Ablation procedure
All RFA procedures were performed under real-time US guidance by an experienced a radiologist with over 20 years of experience in thyroid US and interventional US. A bipolar RFA generator (CelonLabPOWER; Olympus Surgical Technologies Europe, Hamburg, Germany) and a bipolar RF applicator with 9/15-mm active tip was used (CelonProSurge micro100-T09; Olympus Surgical Technologies Europe, Hamburg, Germany) during the ablation procedures.
The patients were in the supine position with the neck fully exposed. After the complete evaluation of the relationship between lesions and critical vessels or structures, 1% lidocaine was injected at the puncture site for local anesthesia. Moving-shot and hydrodissection techniques were used to achieve complete ablation and prevent thermal injury to the surrounding tissues and organs [Citation7]. During the ablation, 5% dextrose solution was injected continuously using another needle (23 gauge) to form at least 3 mm distance to protect the surrounding normal structures from thermal injury. The RFA extent exceeded the edge of targeted lesions to ensure complete ablation of the parenchyma and capsule under real-time ultrasound guidance. The RFA power was started at 3 W and was increased to 5 or 7 W depending on the situation. The ablation was terminated if the targeted ablation zones had changed into hyperechoic areas. Vital signs were monitored during the entire RFA procedure. After ablation, each patient was observed for 1–2 h in the hospital and side effects, minor or major complications were carefully evaluated according to the clinical signs and symptoms [Citation17].
Post-ablation assessment
US, CEUS, thyroid function test and serum Tg level measurement were performed at 1, 3, 6 and 12 months and every 6 or 12 months thereafter (). The percentage reduction in volume was calculated as VRR ([initial volume – final volume] × 100)/initial volume. If new suspicious lesions were found, US-guided CNB or FNA was performed. Complete disappearance was defined as complete invisible on plain ultrasound and absence of enhancement on CEUS.
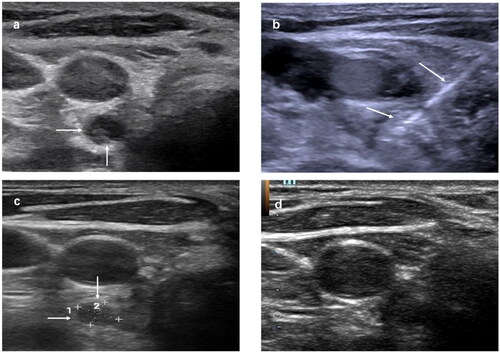
Figure 1. Recurrent papillary thyroid cancer at left level 3 after thyroidectomy and RI therapy in a 35-year-old female. (a) Transverse ultrasound image showing a 0.7-cm-sized hypoechoic mass at right level 6 (arrows). (b) An internally cooled electrode with a 1.5-cm-sized active tip was inserted into the recurrent tumor (arrows). (c) One month after RFA, the US scans showed the treated tumor had decreased with 79% VRR (d). Six months after RFA, the treated tumor was not found on US. RI: radioactive iodine; RFA: radiofrequency ablation; US: ultrasound.
Statistical analysis
SPSS statistical software (Version 26.0) was used for statistical analysis. Continuous data were expressed as mean ± SD (range) or median (range). The Wilcoxon signed rank tests were used to compare the largest diameter, tumor volume and serum Tg levels before RFA and at the last follow-up. Statistically significance was set at p < .05.
Results
We finally included 32 patients with 58 recurrent lesions from 107 patients who were treated with US-guided RFA in our department between November 2014 and January 2018.
The baseline patient characteristics are summarized in . We included 32 patients (13 men and 19 women) with 58 recurrent lesions. The mean age was 44.63 ± 11.22(range, 21–67 years) years. Of the 32 patients, 22 (68.75%) patients were treated with total thyroidectomy and lymph node dissection operation, 6 (18.75%) were underwent subtotal thyroidectomy and lymph node dissection, 1 (3.16%) were treated with thyroid lobectomy and lymph node dissection, 3 (9.38%) patients were treated without lymph node dissection and 21 (65.63%) patients received postoperative radioiodine therapy. Four (12.5%) patients had family history of cancer. The mean follow-up duration was 73.19 ± 12.68 months (range, 60–98 months).
Table 1. Patient baseline characteristics.
A total of 58 recurrent lesions were included in this study. Three (5.17%) tumors were on the thyroid bed, six (10.34%), on the cervical lymph node and 49 (84.49%), on the lateral aspect of the neck. The detailed characteristics of the recurrent lesions are shown in . The changes in the largest diameter and lesion volume at each follow-up are shown in and .
Figure 2. Changes in the largest diameter before RFA and at each follow-up. RFA: radiofrequency ablation.
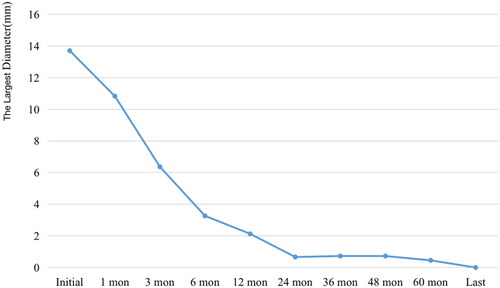
Table 2. Lesion characteristics.
Before RFA, the mean largest diameter was 13.71 ± 6.48 mm (range, 4–23 mm), and the mean volume was 520.43 ± 627.85 mm3 (range, 12.57–2879.79 mm3) (p < .001). Almost all the recurrent lesions had disappeared at the latest follow-up. The mean VRRs of the tumors were 54.17%, 72.90%, 82.28%, 89.30%, 92.57%, 96.60%, 96.88%, 98.14% and 100% at 1-month, 3-month, 6-month, 12-month, 24-month, 36-month, 48-month, 60-month and last follow-up visit, respectively ( and ). Furthermore, 57 tumors had completely disappeared, and only one lesion showed a scar-like appearance. The median of serum Tg level was decreased from 1.48 ng/mL (range, 0.04–21.3 ng/mL) to 0.00 ng/mL (range, 0.00–37.00 ng/mL) (p < .05).
Table 3. Long-term follow-up results of RFA.
During the follow-up, nine (28.12%) patients developed new recurrent tumor in the neck. They were successfully treated with a second RFA session, except for one patient who refused further RFA and underwent repeat surgery. No patient showed recurrence at the treatment site. All patients tolerated the RFA procedure well. No major and minor complications or side effects were observed during and after RFA.
Discussion
We found that US-guided RFA is an effective and safety option for treating locally recurrent PTC in selected patients. Almost all the recurrent lesions were completely disappeared, and only one lesion developed a scar-like appearance. The median serum Tg levels had significantly decreased after RFA. New recurrent lesions were found in nine (28.12%) patients, and they were successfully treated with repeat ablation. No other new recurrent lesions were found in the mean follow-up period of 73.19 months, and there were no major or minor complications recorded in our study.
Previous studies have highlighted the efficacy and feasibility of RFA. In several published systematic reviews and meta-analyses, the mean VRR ranged from 50.9 to 100% [Citation18–21], similar to our results. While a previous study with the largest samples of 119 patients had a mean follow-up of only 47.9 months [Citation14] and another study with the longest follow-up of 80.0 months included only 29 patients [Citation15], our study reported the results of a large sample size of 32 patients, 58 recurrent lesions with a long follow-up duration of 73.19 months.
These are also some studies reported the efficacy of ethanol ablation (EA) as treatment of metastatic lymph nodes from PTC [Citation22–24], Suh et al. [Citation25] reported a systematic review and meta-analysis result, which compared the efficacy and safety of RFA and EA for treating locally recurrent thyroid cancer, the efficacy of RFA was found to be similar to that of EA, the pooled proportion of complete disappearance after RFA (68.8%) was higher than that after EA (53.4%) (p = .3384), the recurrence after RFA was lower than that after EA (0.00% vs. 2.4; p = .9766). However, EA is more likely to develop an irreversible local injury with infraction, thrombosis, coagulative necrosis and fibrosis [Citation26]. Pain leakage of the injected ethanol into the surrounding tissue during EA, this complication can be overcome by hydrodissection technique in RFA. Compared with RFA, EA is supported as an adjunct to surgery for recurrent differentiated thyroid cancer by the Thyroid Cancer Care Collaborative [Citation27].
Repeat RFA is considered a safe option for high-risk patients with locally recurrent PTC. The recurrence of PTC is usually associated with the baseline characteristics of the patients and biological behavior of the lesion at the primary site [Citation28]. A previous study has showed that the incidence of cervical lymph node metastasis was significantly higher in patients aged below 45 years [Citation29]. In our study, the recurrence rate of PTC after RFA was 28.12% (n = 9). Patients with new recurrent PTC underwent a second RFA session, and only one patient developed another new recurrent tumor that was successfully treated with the third RFA session. One of the patients who developed new recurrent PTC was treated with repeat surgery–this indicates that the ablation procedures had no effect on subsequent repeat operations.
In addition, RFA was tolerated by most patients with few complications. For recurrent thyroid cancers, the incidence rates of complication after repeat surgery range from 7.5–18.5% [Citation28,Citation30], and the overall complication rates of RFA is 10.98% (95% CI: 4.82–17.15), including major complications such as nerve injuries [Citation20]. However, no life-threatening complications have been reported [Citation31,Citation32]. Choi Y et al. [Citation5] compared the complications between RFA and repeat surgery in the treatment of locally recurrent thyroid cancer. Hypocalcemia had occurred only in the repeat surgery group (n = 18), and the number of complications was significantly higher in the repeat surgery group. Our results showed that RFA was well-tolerated by all the patients, and even among patients who underwent repeat RFA sessions, no complications or procedure-related deaths were reported. These results can be attributed to the following factors: (1) at our center, RFA was performed by experienced operators who used the moving-shot and the hydrodissection techniques skillfully and, thus, protected the adjacent structures from being injured during the procedure [Citation7]; (2) RFA was usually performed under local anesthesia; hence, there was no risk of possible complications caused by general anesthesia and (3) all RFA procedures were performed under real-time US guidance to monitor any changes in the ablation zone, to achieve complete ablation and to prevent damages to adjacent organs and tissues.
Limitations
Our long-term follow-up data suggested that RFA is a feasible option for the treatment of locally recurrent PTC; however, our study also had several limitations. Firstly, this was a retrospective study, and the RFA procedures were performed by one experienced operator at a single center, which may have resulted in a selection bias. Secondly, this study focused on PTC; therefore, further studies on other subtypes are needed. Furthermore, this study did not provide data on the comparison of RFA with repeat surgery or other treatments.
Conclusion
US-guided RFA is an effective and safe option for treating locally recurrent PTC in selected patients, with favorable long-term outcomes.
Acknowledgements
We are grateful to the patients who participated in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pizzato M, Li M, Vignat J, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):264–272.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
- Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022;32(5):560–570.
- Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19(12):1373–1380.
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36(1):359–367.
- Stavrakis AI, Ituarte PH, Ko CY, et al. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142(6):887–899.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Mauri G, Hegedüs L, Bandula S, et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Lee SJ, Jung SL, Kim BS, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol. 2014;15(6):817–826.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170.
- Chung SR, Baek JH, Choi YJ, et al. Efficacy of radiofrequency ablation for recurrent thyroid cancer invading the airways. Eur Radiol. 2021;31(4):2153–2160.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903.
- Steinkamp HJ, Cornehl M, Hosten N, et al. Cervical lymphadenopathy: ratio of long- to short-axis diameter as a predictor of malignancy. Br J Radiol. 1995;68(807):266–270.
- Mauri G, Pacella CM, Papini E, et al. Image-Guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13(2):117–125.
- Zhao Q, Tian G, Kong D, et al. Meta-analysis of radiofrequency ablation for treating the local recurrence of thyroid cancers. J Endocrinol Invest. 2016;39(8):909–916.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Qiu Y, Xing Z, He Y, et al. Ultrasound-guided thermal ablation for cervical lymph node metastasis from thyroid carcinoma: a meta-analysis of clinical efficacy and safety. Lasers Med Sci. 2022;37(3):1747–1754.
- Frich PS, Sigstad E, Berstad AE, et al. Long-Term efficacy of ethanol ablation as treatment of metastatic lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2022;107(5):e2141–e2147.
- Hay ID, Lee RA, Davidge-Pitts C, et al. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013;154(6):1448–1454;discussion 1454–1445.
- Kim BM, Kim MJ, Kim EK, et al. Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. Eur Radiol. 2008;18(4):835–842.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26(3):420–428.
- Pomorski L, Bartos M. Histologic changes in thyroid nodules after percutaneous ethanol injection in patients subsequently operated on due to new focal thyroid lesions. APMIS. 2002;110(2):172–176.
- Urken ML, Milas M, Randolph GW, et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the thyroid cancer care collaborative. Head Neck. 2015;37(4):605–614.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
- Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016;26(6):807–815.
- Conzo G, Calò PG, Sinisi AA, et al. Impact of prophylactic Central compartment neck dissection on locoregional recurrence of differentiated thyroid cancer in clinically node-negative patients: a retrospective study of a large clinical series. Surgery. 2014;155(6):998–1005.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.