?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To investigate the pattern of change over time and predictors for metastasis in indeterminate lymph nodes (LNs) among patients with thyroid cancer post-ablation.
Methods
We enrolled patients who developed new cervical LNs after papillary thyroid carcinoma (PTC) ablation. Changes in the ultrasound characteristics of the indeterminate LN were recorded at months 1, 3, 6 and 12 after ablation. LN puncture pathology and long-term follow-up were standard of diagnosis. The indeterminate LNs were divided into benign and malignant groups, the differences between the two groups were compared, and the risk characteristics of malignant LNs were screened using generalized estimating equations (GEE).
Results
In total, we included 138 LNs from 99 patients, of which 48 were indeterminate LNs. When following up indeterminate LNs, non-cervical lymph node metastasis (non-CLNM) lesions demonstrated a statistically significant gradual decrease in volume (p = 0.012), though there was no significant change in the volume of CLNM lesions (p = 0.779). Compared to non-CLNM lesions, the diagnostic efficiency was the highest for CLNM lesions at 1–3 months after ablation, when the LN volume changed by −0.08 to 0.12 mL (p = 0.048). The third month after ablation became an important time point for review. Moreover, GEE analysis showed that microcalcifications, cystic changes, and vascularity were strongly associated with CLNMs (p = 0.004, p = 0.002, and p = 0.010, respectively).
Conclusions
There is a pattern of volume change of indeterminate LNs after PTC ablation, which, together with microcalcifications, cystic changes, and vascularity, can be used as criteria for differentiating the benignity and malignancy of indeterminate LNs.
Introduction
Papillary thyroid carcinoma (PTC) is the most common histological type of thyroid carcinoma, accounting for 80%–90% of differentiated thyroid carcinomas [Citation1]. The 2015 American Thyroid Association Management (ATA) Guidelines recommend surgery as the preferred treatment for thyroid carcinoma; this may, however, lead to complications such as recurrent laryngeal nerve injury and hypoparathyroidism [Citation2]. Thermal ablation has, thus, been gaining popularity in the treatment of PTC due to its advantages of safety, effectiveness, and minimal invasiveness; it has become the current research hotspot of interventional therapy [Citation3].
Although PTC is an inert cancer, 30%–80% of patients develop cervical lymph node metastases (CLNM) [Citation4,Citation5], a high risk factor for poor prognosis [Citation6]. The Korean Society of Thyroid Radiology (KSThR) and European Thyroid Association (ETA) define lymph nodes (LNs) without suspicious imaging features (microcalcifications, cystic changes, hyperechogenicity, and abnormal vascularity) as indeterminate LNs [Citation7,Citation8]. Indeterminate LNs occur frequently in the neck of patients with thyroid cancer post-ablation, and their risk of malignancy is about 19.5%, much higher than that of benign LNs (2.8%) [Citation9].
Ultrasound is the preferred method for examining LNs in thyroid carcinoma as it has high sensitivity and specificity for the diagnosis of suspicious LNs; however, the diagnosis of indeterminate LNs remains ambiguous [Citation6]. One study combined microvascular imaging with power Doppler ultrasound to correctly identify 80.8%–90.1% of indeterminate LNs as benign or malignant based on LN vascularity [Citation6]. Another study using contrast-enhanced ultrasound concluded that long-/short-axis ratio ≤1.5, peripheral or mixed blood flow, absence of hilar structure, centripetal or mass enhancement, and heterogeneous enhancement are high predictors for metastasis [Citation10]. In addition, the combination of computed tomography and ultrasound can increase the diagnostic accuracy for indeterminate LNs by 16.4% [Citation11]. Previous studies have involved a variety of examinations, but no uniform conclusions have been drawn. These studies simply focus on the ultrasound characteristics of the LN at a certain time point, and their patterns of change over time have rarely been reported. Moreover, previous studies explored indeterminate LNs before and after surgery; however, post-ablation changes have been rarely reported. Unlike conventional surgery, thermal ablation uses extremely high or low temperatures locally to induce irreversible cellular damage and, eventually, lead to apoptosis and coagulative necrosis of tumor cells, which can stimulate inflammatory and immune responses in the body and lead to reactive hyperplasia of LNs [Citation12], which appear as indeterminate LNs on ultrasonogram. Therefore, the identification of indeterminate LNs after ablation was more complicated. Simultaneously, this highly sensitive population, which can be actively monitored for PTC yet opt for ablation, is under greater psychological stress from cervical indeterminate LNs than is the general population. In summary, the differential diagnosis and clinical management of benign and malignant indeterminate LNs are worthy of further study.
Therefore, this study investigated the predictors for metastasis and the variation pattern of the sonogram characteristics in cervical indeterminate LNs after PTC ablation by long-term ultrasound follow-up to provide meaningful guidance for the differential diagnosis of benign and malignant indeterminate LNs.
Materials and methods
Participants
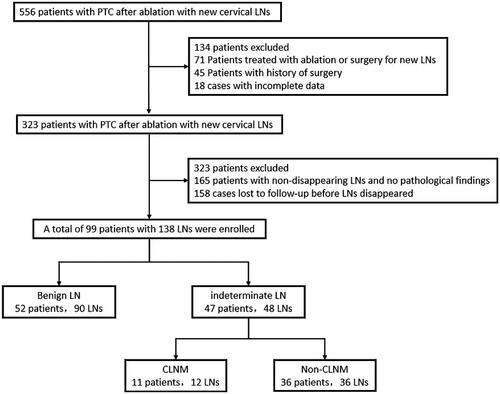
This retrospective study was approved by the ethics committee of the General Hospital of the Chinese People’s Liberation Army, and written informed consent was obtained from the patients. Patients who underwent PTC ablation and had new cervical LNs after ablation at our hospital between January 2019 and January 2022 were selected. Some patients with tumors larger than 1 cm in diameter but who voluntarily opted for ablation were also included in this study (). The exclusion criteria were: (1) preoperative presence of suspicious or indeterminate LNs; (2) incomplete clinical data; (3) history of other malignancies; (4) poor ultrasound image quality; and (5) history of neck surgery, radiotherapy, and chemotherapy (Protocol number: ChiCTR2200056848).
Evaluation of LNs
All patients underwent ultrasound examination of the neck using Resona R9 (Mindray), iU Elite (Philips Healthcare), SEQUOIA 512 (SIEMENS), or ACUSON S2000 (SIEMENS) scanners with a 7–12-MHz linear phased-array transducer. Patients were followed up with ultrasonography of the LNs at post-ablation months 1, 3, 6, and 12. Patients were placed in the supine position, and a full examination of the LNs in levels I to VII of the neck was performed, while representative gray-scale and color Doppler images of indeterminate LNs were taken. All examinations were performed by ultrasound physicians with more than 10 years of experience in thyroid ultrasound diagnosis.
Imaging analysis
The location, echogenicity, vascularity, and size of the LNs were evaluated during the follow-up. The vascularity was classified into four levels according to the distribution of blood flow within the LN: level 0, no flow signal; level 1, distributed in the center; level 2, distributed in the periphery; and level 3, both central and peripheral distribution. The diameters of the three dimensions of LN were recorded, and the volume was calculated using the equation [Citation13]: (V is the volume, a is the largest diameter, and b and c are the other two perpendicular diameters). The KSThR and ETA guidelines classify LN as benign, indeterminate, and suspicious based on ultrasound features and risk of metastasis. Imaging features of suspicious lymph nodes include microcalcifications, cystic changes, hyperechogenicity, and abnormal vascularity. Benign LNs were defined as LNs without suspicious imaging features but with the typical imaging feature of benign LNs, including a central echogenic hilum or a central radiating hilar vascularity. Indeterminate LNs were defined as LNs without suspicious or benign LN imaging features but with eccentric, malformed, or absent hilum, independent of nodule shape [Citation7,Citation8]. In this study, the changes in LN volume and sonographic features at follow-up were compared with those at the initial review.
Statistical analysis
SPSS 21.0 software (IBM) was used for statistical analysis. Continuous variables are expressed as mean ± SD, and t-test or Mann-Whitney U test was used for comparison between groups. Classification variables were compared using the χ2 test or Fisher’s exact test. For follow-up data, generalized estimating equation (GEE) logistic regression models were used to assess the relationship between the malignancy status of lymph nodes and ultrasound characteristics of the nodes. The trend of non-CLNM and CLNM volume changes was tested by one-way analysis of variance. A p-value <0.05 was considered significant.
Results
Patient selection and grouping
We reviewed 556 patients with new cervical LNs after PTC ablation at our institution between January 2019 and January 2022. Following the inclusion and exclusion criteria, 138 LNs from 99 patients were included in our study. Among them, 90 were benign LNs and 48 were indeterminate LNs. Of the indeterminate LNs, there were 36 non-CLNM (75.00%) and 12 CLNM (25.00%) lesions (). The final diagnosis of LNs was based on fine-needle aspiration cytology (FNAC) and ultrasound follow-up within one year. If both FNAC and ultrasound follow-up yielded negative results, LNs were regarded as non-CLNM.
Predictors of indeterminate LN
The basic clinical information and tumor characteristics of the patients are shown in . Unclear boundary, irregular shape, LN level, and post-ablation Hashimoto’s thyroiditis (HT) were significantly associated with indeterminate LNs (p = 0.046, p = 0.004, p = 0.000, and p = 0.040, respectively). Benign LNs were prone to developing at level III (32.8%), while indeterminate LNs were at level VI (64.9%). The remaining features were not significantly associated with indeterminate LNs (all p > 0.05).
Table 1. Basic clinical information and ultrasound features of patients with papillary thyroid carcinoma after ablation.
Predictors of CLNM in indeterminate LN
The basic clinical information and tumor characteristics of the patients are shown in . Tumor volume and cystic change were associated with metastatic CLNM (p = 0.043 and p = 0.002, respectively). The remaining features were not significantly associated with CLNM (all p > 0.05).
Table 2. Basic clinical information and ultrasound features of patients with indeterminate lymph nodes for papillary thyroid carcinoma after ablation.
Indeterminate LN's predictors variation pattern with time and diagnostic value
GEE analysis of the internal sonographic features of indeterminate LNs revealed statistically significant differences in microcalcifications, cystic changes, and vascularity when comparing CLNM and non-CLNM lesions (p = 0.004, p = 0.002, and p = 0.010, respectively). In CLNM, nine (75.00%) showed microcalcifications and seven (58.33%) showed cystic changes, while in non-CLNM, eleven (30.56%) showed microcalcifications and four (11.11%) showed cystic changes. Regarding changes in vascularity, four (33.33%) showed increased vascularity and one (8.33%) showed decreased vascularity in CLNM. Eight (22.22%) showed decreased vascularity, and two (5.56%) showed increased vascularity in non-CLNM. The rest of the CLNM and non-CLNM showed no clear changes in vascularity. Thus, most indeterminate LNs showed no clear change in vascularity during follow-up, and the residual CLNM vascularity elevated and transformed into peripheral and mixed patterns, while non-CLNM vascularity decreased and gradually converged to the central pattern. In addition, there was no statistically significant difference in the change of unclear boundary, irregular shape, loss of fatty hilum and hyperechogenicity at follow-up (all p > 0.05) ().
Table 3. GEE analysis of the internal ultrasound characteristics of indeterminate lymph nodes.
Table 4. Volume changes of indeterminate lymph nodes with time.
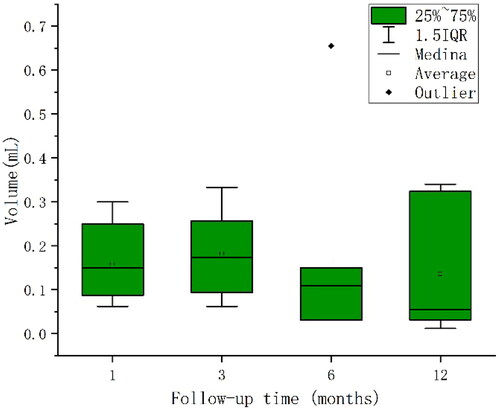
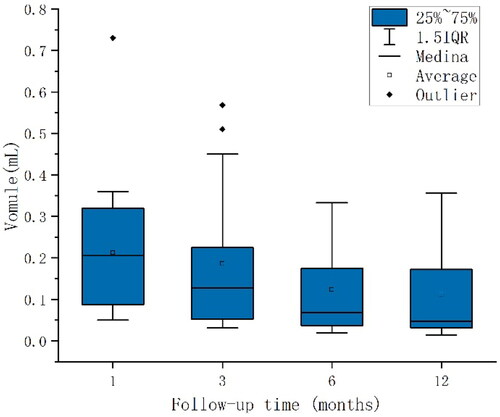
Follow-up of cervical indeterminate LNs at 1, 3, 6 and 12 months after PTC ablation, the mean volumes of CLNM were 0.16, 0.18, 0.17, and 0.14 ml, respectively, which were not statistically significant (p = 0.779) (). The mean volumes of non-CLNM were 0.21, 0.19, 0.12, and 0.11 ml, respectively, showing a gradual decrease with a statistically significant difference (P trend = 0.012)(). Of note, the non-CLNM volume gradually decreased after ablation, while CLNM volume did not change. The volume changes in LNs at post-ablation months 1–3, 3–6, and 6–12 were grouped equidistantly. Among them, there was a statistically significant change in volume between CLNM and non-CLNM lesions at post-ablation months 1–3 (p = 0.048), with the highest diagnostic efficiency (52.2%) when the LN volume change was between −0.08 and 0.12 ml, while no statistically significant change in LN volume was seen in postoperative months 3–6 and 6–12 (p = 0.387 and p = 0.744, respectively) (). In LN follow-up, the sensitivity of microcalcifications and cystic changes was relatively high (75.0% and 88.9%, respectively), and the sensitivity and specificity of elevated vascularity was 80.0%. The sensitivity of LN volume changes between −0.08 and 0.12 ml was 100.0%, the negative predictive values were all at a high level, 89.3%, 86.5%, 88.9%, and 100.0%, respectively, and the areas under the curve of the first four were 0.708, 0.813, 0.800 and 0.621, respectively ().
Figure 4. Ultrasonogram of left cervical non-metastatic lymph node at VI level in a patient after PTC thermal ablation. A. Lymph node volume was 0.23 ml at the 1st month after ablation; B. Lymph node volume was 0.14 ml at the 3rd month after ablation; C. Lymph node volume was 0.06 ml at the 6th month after ablation; D. Lymph node volume was 0.05 ml at the 12th month after ablation. A gradual decrease is observed.
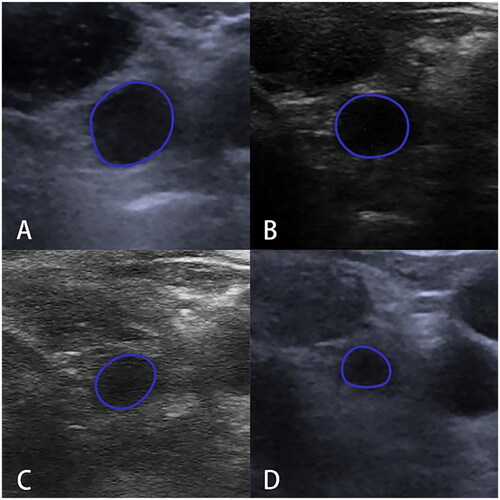
Table 5. Diagnostic value of ultrasonographic findings for predicting metastasis in indeterminate lymph nodes.
Discussion
In this study, unclear boundaries and irregular shapes were included as ultrasound features of tumors, and these were closely associated with indeterminate LNs. This is consistent with previous results and suggests these ultrasound features as important factors in predicting indeterminate LNs [Citation14,Citation15]. Furthermore, we concluded that indeterminate LNs were strongly associated with post-ablation HT. HT, the most common autoimmune disease of the thyroid, often causes LN enlargement due to a chronic inflammatory immune response [Citation15]. The enlarged LNs caused by it are frequent in level VI, often show loss of fatty hilum and have a rounded morphology [Citation16]; such LNs can also be called indeterminate LNs [Citation7,Citation8]. Furthermore, we concluded that the majority of indeterminate LNs occur in level VI. Therefore, after ablation, indeterminate LNs caused by HT should also undergo ultrasound for the diagnosis of potential CLNMs. Thus, the differential diagnosis of benign and malignant indeterminate LNs becomes more complicated.
We further analyzed the predictors of metastasis in indeterminate LNs and found that the larger the tumor volume, the higher the risk of malignancy in indeterminate LNs, while the maximum tumor diameter was not relevant. This is consistent with the conclusion of a related study by Park et al. [Citation17] showing that tumor volume, rather than tumor size, is a significant predictor of malignant propensity in indeterminate LNs. It has been previously reported that tumor cystic change is an independent predictor of CLNM [Citation18], and we demonstrated that it is also an important guide for the differential diagnosis of benign and malignant indeterminate LNs.
Long-term, multiple ultrasound follow-ups of indeterminate LNs after PTC ablation revealed the importance of microcalcifications, cystic changes, vascularity, and volume changes in differentiating CLNM from non-CLNM. In the progression of CLNM, liquefied necrosis, coagulative necrosis, hemorrhage, and calcium deposition due to the rapid proliferation of vascular and fibrous tissues occur within the LN due to infiltration of tumor cells [Citation19], manifesting as microcalcifications and cystic changes on ultrasonogram. One study concluded that ≥5 microcalcifications and cystic changes are independent predictors for PTC CLNM [Citation20,Citation21]. Moreover, LN-responsive hyperplasia occurs owing to the stimulation of ablation, followed by the gradual self-healing of the organism, leading to calcium salt deposits and liquid dark areas in non-CLNM, which may be the cause of false positives [Citation22,Citation23]. Most previous studies were statistically analyzed at a single time point, yielding a sensitivity, specificity, and accuracy of 75.4%, 47.9%, and 0.610, respectively, for microcalcifications and of 25.8%, 99.6%, and 0.478, respectively, for cystic lesions when diagnosing CLNM [Citation20,Citation24]. In this study, the long-term follow-up of indeterminate LNs yielded a specificity of 69.4% for microcalcifications and a sensitivity of 58.3% for cystic changes, both with an accuracy of 0.708 and 0.813, respectively, for the diagnosis of CLNM. All were significantly higher compared to previous studies observed at a single time point.
In the early stage of micro-infiltration of tumors, there is less structural destruction within the LN, leading to a central radiating hilar vascularity. With increased infiltration of tumor cells, on the one hand, tumor blood vessels are formed in the intercellular space induced by VEGF-C. On the other hand, tumor infiltration blocks the blood supply to the hilum, resulting in the lymphoid tissue having to obtain blood supply from the tumor margin, causing the formation of marginal blood vessels. Increased marginal vascularity in CLNM is a well-known imaging feature and is widely used in clinical practice. But when the tumor infiltrates further and gradually replace the lymphatic tissue, the original blood vessels of the LN proliferate and form central vessels within the LN that are not clearly connected to the hilum [Citation14,Citation23,Citation25,Citation26]. Therefore, judging LN benignity and malignancy from vascularity at a single time point alone may lead to false negatives. The 2015 ATA guidelines suggest that abnormal vascularity as a single imaging feature is not sensitive enough [Citation2]. In our study, the long-term observation of the vascularity of indeterminate LNs revealed that elevated vascularity is of great value in the diagnosis of CLNM, with a sensitivity, specificity and accuracy of 80.0% [Citation24,Citation27]. The diagnostic value of CLNM was improved compared to the previous diagnosis of CLNM by observing vascularity at a single time point, providing a novel direction for diagnosing cervical indeterminate LNs after PTC ablation by observing the vascularity of LNs.
LNs may show abnormal size and morphology in response to persistent inflammatory stimulation and appear as indeterminate on sonography [Citation6], which may lead to false positives and affect the diagnosis and differentiation of CLNM. Chu et al. demonstrated that the presence of immune cell infiltration in the transition zone, untreated tumor tissue, and peripheral blood indicates that ablation activates overall immunity. Moreover, the mechanical cell damage caused by thermal ablation releases various immunogenic intracellular substrates such as DNA, RNA, heat shock proteins, and so on that activate innate immunity and stimulate acquired immune responses [Citation12]. In addition, the destruction of ablated tissue, tumor cells, local extracellular matrix, and tissue components releases pro-inflammatory cytokines, causing aseptic inflammation [Citation28,Citation29]. In non-CLNM, lymphocyte proliferation within lymphoid follicles, expansion of lymphatic sinuses, and cortical thickening of lymph nodes are stimulated by a combination of the body’s immune and inflammatory responses, resulting in an increase in LN volume. Previous studies suggested that the kinetics of tissue response after ablation peaks between 72 h and 21 days and then gradually decreases [Citation30,Citation31]. In two studies of inflammatory markers after ablation with a 6-month follow-up, it was concluded that inflammatory markers began to increase from 18–24 h after the surgery and then gradually decreased [Citation32,Citation33]. Therefore, the ablation-induced immune response and inflammatory response stimulated the LN volume to increase; however, after 1 month, as the inflammatory response gradually diminished, the LN volume gradually decreased. Moreover, CLNM volume did not change definitively at the 1-year follow-up after ablation. Studies of post-surgical indeterminate LNs with regular monitoring have reached similar conclusions, with the majority (16/21) of CLNMs not changing definitively during 2–3 years of follow-up [Citation34].
This study also concluded that LN volume changes between −0.08 and 0.12 ml at post-ablation months 1–3 had the highest diagnostic efficiency for CLNM, with an accuracy of 62.1%. Therefore, the 3rd month after ablation becomes an important time point for review. If the LN volume change did not fluctuate within the aforementioned range at post-ablation month 3, it was more inclined to be benign, and its continued volume reduction at follow-up further corroborated the benign tendency of LN. Conversely, malignancy was considered. Because there are no clear suspicious imaging features of indeterminate LNs, the change in LN volume becomes an important and non-negligible index to determine their benignity and malignancy at the follow-up after ablation, providing meaningful guidance for the diagnosis of indeterminate LNs after PTC ablation.
There are some limitations to this study: On the one hand, the sample size of CLNM included is small. Since PTC is more inert, most patients who choose ablation have papillary thyroid microcarcinomas <1 cm in diameter, and their chance of developing CLNM is only 1%–2%[Citation2]; thus, the sample size of CLNM included in this study is more in line with the incidence and metastasis rate of this disease. On the other hand. The immunological and pathological processes mentioned in the discussion were not derived from our own study. We will expand the sample size for further in-depth study.
In conclusion, for indeterminate LNs after PTC ablation, the month 3 is an important time point for review. By observing the changes in microcalcifications, cystic changes, vascularity, and LN volume, a reasonable judgment can be made on whether it is benign or malignant, providing meaningful guidance for clinical decisions.
Geolocation information
No. 28 Fuxing Road, Haidian District, Beijing, China.
Acknowledgment
None.
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Funding
References
- Lin P, He R-Q, Huang Z-G, et al. Role of global aberrant alternative splicing events in papillary thyroid cancer prognosis. Aging. 2019;11(7):2082–2097.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98(10):3949–3957.
- Wu X, Li B, Zheng C, et al. Risk factors for Central lymph node metastases in patients with papillary thyroid microcarcinoma. Endocr Pract. 2018;24(12):1057–1062.
- Xu Y, Xu L, Wang J. Clinical predictors of lymph node metastasis and survival rate in papillary thyroid microcarcinoma: analysis of 3607 patients at a single institution. J Surg Res. 2018;221:128–134.
- Lee S, Lee JY, Yoon RG, et al. The value of microvascular imaging for triaging indeterminate cervical lymph nodes in patients with papillary thyroid carcinoma. Cancers. 2020;12(10):2839.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–395.
- Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2(3):147–159.
- Yoo RE, Kim JH, Bae JM, et al. Ultrasonographic indeterminate lymph nodes in preoperative thyroid cancer patients: malignancy risk and ultrasonographic findings predictive of malignancy. Korean J Radiol. 2020;21(5):598–604.
- Guo Q, Sun C, Chang Q, et al. Contrast-enhanced ultrasound-based nomogram for predicting malignant involvements among sonographically indeterminate/suspicious cervical lymph nodes in patients with differentiated thyroid carcinoma. Ultrasound Med Biol. 2022;48(8):1579–1589.
- Yoo R-E, Kim J-H, Hwang I, et al. Added value of computed tomography to ultrasonography for assessing LN metastasis in preoperative patients with thyroid cancer: node-by-node correlation. Cancers. 2020;12(5):1190.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Yan L, Zhang Y, Jiang B, et al. Radiofrequency ablation for cervical metastatic lymph nodes in children and adolescents with papillary thyroid carcinoma: a preliminary study. Front Endocrinol. 2021;12:624054.
- Li F, Pan D, He Y, et al. Using ultrasound features and radiomics analysis to predict lymph node metastasis in patients with thyroid cancer. BMC Surg. 2020;20(1):315.
- Min Y, Huang Y, Wei M, et al. Preoperatively predicting the central lymph node metastasis for papillary thyroid cancer patients with hashimoto’s thyroiditis. Front Endocrinol (Lausanne). 2021;12:713475.
- Liu Y, Lv H, Zhang S, et al. The impact of coexistent hashimoto’s thyroiditis on central compartment lymph node metastasis in papillary thyroid carcinoma. Front Endocrinol . 2021;12:772071.
- Park KN, Kang KY, Hong HS, et al. Predictive value of estimated tumor volume measured by ultrasonography for occult central lymph node metastasis in papillary thyroid carcinoma. Ultrasound Med Biol. 2015;41(11):2849–2854.
- Kahramangil B, Kose E, Donmez M, et al. Efficacy of surgeon-performed, ultrasound-guided lymph node fine needle aspiration in patients with thyroid pathologic conditions. Surgery. 2018;164(4):657–664.
- Ghafoori M, Azizian A, Pourrajabi Z, et al. Sonographic evaluation of cervical lymphadenopathy; comparison of metastatic and reactive lymph nodes in patients with head and neck squamous cell carcinoma using gray scale and doppler techniques. Iran J Radiol. 2015;12(3):e11044.
- Guang Y, He W, Zhang W, et al. Clinical study of ultrasonographic risk factors for central lymph node metastasis of papillary thyroid carcinoma. Front Endocrinol. 2021;12:791970.
- Zou Q, Ma S, Zhou X. Association of sonographic features and clinicopathologic factors of papillary thyroid microcarcinoma for prevalence of lymph node metastasis: a retrospective analysis. Arch Endocrinol Metab. 2021;64:803–809.
- Liu S, Xie X, Tang X, et al. Non-tuberculosis extensive abdominal lymph node calcification leading to portal hypertension with esophageal and gastric variceal bleeding: a rare case report. BMC Gastroenterol. 2022;22(1):245.
- (a) Qs LI, Liu H, Yan SL, et al. 2017. Superficial organ ultrasound medicine. 2th edn. Science Press, Beijing. (b) Leong SP, Pissas A, Scarato M, et al. The lymphatic system and sentinel lymph nodes: conduit for cancer metastasis. Clin Exp Metastasis. 2022;39:139–157.
- Chung SR, Baek JH, Rho YH, et al. Sonographic diagnosis of cervical lymph node metastasis in patients with thyroid cancer and comparison of european and korean guidelines for stratifying the risk of malignant lymph node. Korean J Radiol. 2022;23(11):1102–1111.
- Yang WT, Metreweli C, Lam PK, et al. Benign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power doppler US. Radiology. 2001;220(3):795–802.
- Na DG, Lim HK, Byun HS, et al. Differential diagnosis of cervical lymphadenopathy: usefulness of color doppler sonography. AJR Am J Roentgenol. 1997;168(5):1311–1316.
- Zu D-M, Feng L-L, Zhang L, et al. Evaluation of mesenteric lymph nodes in a pediatric population with mesenteric lymphadenitis using superb microvascular imaging. Med Sci Monit. 2019;25:5336–5342.
- Nijkamp MW, Borren A, Govaert KM, et al. Radiofrequency ablation of colorectal liver metastases induces an inflammatory response in distant hepatic metastases but not in local accelerated outgrowth. J Surg Oncol. 2010;101(7):551–556.
- Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–1942.
- Gravante G, Ong SL, Metcalfe MS, et al. The effects of radiofrequency ablation on the hepatic parenchyma: histological bases for tumor recurrences. Surg Oncol. 2011;20(4):237–245.
- Goldberg SN, Gazelle GS, Compton CC, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452–2463.
- Bin Waleed K, Yin X, Yang X, et al. Short and long-term changes in platelet and inflammatory biomarkers after cryoballoon and radiofrequency ablation. Int J Cardiol. 2019;285:128–132.
- Tonguc T, Strunk H, Gonzalez-Carmona MA, et al. US-guided high-intensity focused ultrasound (HIFU) of abdominal tumors: outcome, early ablation-related laboratory changes and inflammatory reaction. A single-center experience from Germany. Int J Hyperthermia. 2021;38(2):65–74.
- Hu M, Xia C, Zhou Y, et al. Ultrasound surveillance of abnormal cervical lymph nodes in patients with papillary thyroid carcinoma after surgery. J Ultrasound Med. 2021;40(1):29–37.