?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
The temperature control of magnetic hyperthermia therapy mainly relies on circulating water cooling and regulating magnetic field intensity, which increases complexity in clinical applications. Using magnetic materials with appropriate Curie temperature has become an effective means to solve temperature monitoring and potentially achieve self-regulating temperature.
Methods
A self-temperature-regulating Fe83Zr10B7 magnetic material was prepared. Based on this material, a simplified model of magnetic hyperthermia for arm tumors was established and verified using the finite- element method. The influence of magnetic field intensity and frequency on the heating power and temperature rise rate of different-sized and shaped magnetic media was studied. Additionally, factors such as the size, quantity, and spatial arrangement of the magnetic media were analyzed for their impact on the damage to tumors with different volumes and shapes.
Results
Spherical shape is the most suitable for magnetic hyperthermia media, and the radius of the spherical magnetic media can be chosen according to the size of the tumor. For tumors with a radius below 10 mm, using magnetic media with a particle size of 3.5 mm is recommended. The optimal magnetic field conditions are H0 (10–12 kA/m) and f (110–120 kHz).
Conclusion
Based on the good magnetic properties and heating performance of the Fe83Zr10B7 magnetic material, it is feasible to use it as a magnetic medium for magnetic hyperthermia. The results of this study provide references for the selection of thermal seed size and magnetic field parameters in magnetic hyperthermia.
1. Introduction
Magnetic induction hyperthermia (MIH) will generate a higher temperature in a tumor by implanting a magnetic medium into the tumor with an applied magnetic field [Citation1–6]. Many works have revealed the biological effects of hyperthermia on macroscopic and microscopic levels [Citation7–10], suggesting that the magnetic medium absorbs energy and the surrounding temperature increases, leading to apoptosis and necrosis of tumor cells in the range of 41 °C and 46 °C [Citation11–14].
In clinical aspects, many researchers verified the excellent efficacy of MIH [Citation15–17]. Stea et al. [Citation18,Citation19] conducted MIH on glioma patients with thermoseeds, and the treatment temperature reached over 42 °C, proving the feasibility of thermoseeds implantation for interstitial hyperthermia in brain tumors. Deger et al. [Citation20,Citation21] conducted a phase II clinical trial of thermoseeds MIH in 57 prostate cancer patients. Subsequently, after two years of clinical observation, the content of the average PSA (prostate-specific antigen) of 12 patients decreased to normal levels. Johannsen et al. [Citation22] used the experimental results to prove the feasibility of magnetic fluid in the treatment of the prostate. Maier-Hauff et al. [Citation23] injected magnetic fluid into the intracranial of 14 patients with glioma of the brain, and the tumor volume of the patients decreased by 75% after AMF hyperthermia, indicating that MFH had a better inhibition on the growth of tumor cells and a stronger killing effect on them.
As a vital part of MIH, the character of the magnetic medium is an influential factor affecting the treatment results, which needs in-depth research [Citation24–26].Over the past decades, research on the magnetic media of MIH has focused on magnetic thermoseeds, ferromagnetic glass ceramics, and magnetic fluids. Masakazu et al. [Citation27] synthesized ferromagnetic hematite microspherical particles with SiO2 as the core and coated withγ-Fe2O3 magnetic particles, which met the requirements of hyperthermia temperature. Ji et al. [Citation28] studied the PFCS glass-ceramics magnetic properties, and induction heating capacity, the results showed that PFCS could induce apparent cell death of LoVo cancer. The theoretical formula for power absorption was derived by Wu et al. [Citation29] using the electromagnetic field constraint equation and compared the power absorbed per unit volume of several millimeter-level ferromagnetic thermoseedss. With using the thermal fluid model of Comsol Multiphysics, Astefanoaei, et al. [Citation30] studied the temperature field of tumor tissue with Fe67.7Cr13Nb0.3B20 magnetic nanoparticles used in hyperthermia, the results show that the nanoparticles can maintain a stable temperature range under suitable conditions.
Previous studies have discussed the role of materials with good magnetothermal properties in MIH, but there has been no in-depth study on how to achieve accurate temperature control during MIH implementation, which is what this paper wishes to discuss.
This paper uses Fe83Zr10B7 as the magnetic media, which is Fe-based bulk metallic glass with great properties such as high permeability, low coercivity, small anisotropy, and lower Curie temperature [Citation31]. This low Curie temperature point performance ensures that it does not warm up after reaching the Curie temperature point and thus has absolute temperature control performance for MIH. However, it has yet to be studied the application in MIH, thus enabling self-regulating temperature mechanism. In this paper, we provide some references for the application of self-regulating temperature nanomaterials in MIH by comparing the experimental results of self-regulating temperature magnetic materials with the results of finite element analysis.
2. Numerical method
2.1. Establishment of the MIH model
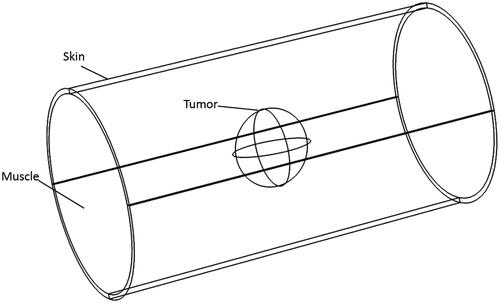
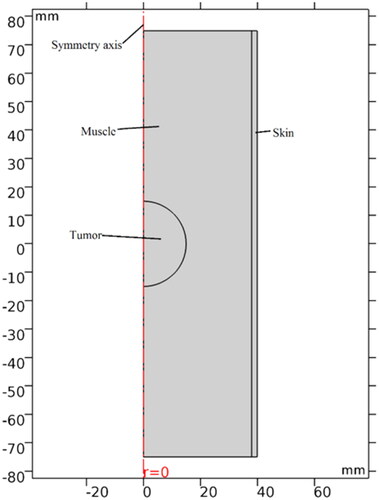
This paper adopts the human upper arm as the study subject and assumes the vascular tissues and skeletal tissues surrounding the tumor regions are muscle tissue; shows the simplified upper arm three-dimensional MIH model, the cylinder with a radius of 4 cm and height of 15 cm, the skin thickness is 2 mm. Additionally, set the tumor diameter to 1–4cm, which determines the size and position of the magnetic medium.
2.2. Physical parameters
shows the physical parameters of biological tissues [Citation32,Citation33]. shows the parameters of Fe83Zr10B7. As shown in , the diffuse peak state near an angle of 45° indicates that the sample strip phase structure is essentially amorphous, while diffuse peaks at 15–30° are peaks of amorphous glass substrate. The narrow magnetic hysteresis loop () means the heat generation of Fe83Zr10B7 is mainly caused by eddy current loss [Citation34]. The Curie temperature of the material was tested by TGA [Citation35,Citation36]and PPMS, as shown in , and the calculated Curie temperature was 75.13 °C and 75.98 °C respectively. In order to facilitate calculation, the Curie temperature set in this paper is 75 °C.
Figure 2. (a) The XRD pattern of Fe83Zr10B7. (b) The magnetic hysteresis loop of Fe83Zr10B7. (c) The thermogravimetric curve obtained by thermogravimetric analyzer under a static magnetic field, the inset is the first derivative of the thermogravimetric curve. (d) The M-T curve obtained by PPMS, the inset is the first derivative of the M-T curve.
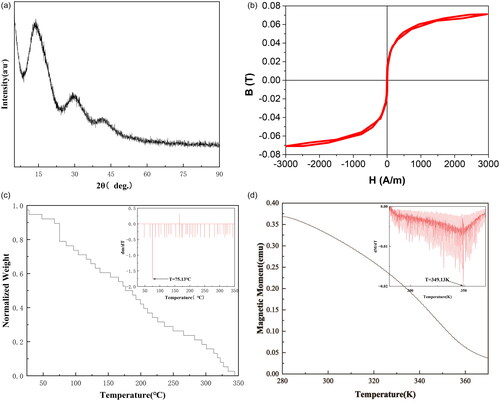
Table 1. Physical parameters.
Table 2. Fe83Zr10B7 parameters.
The detailed derivations are in Equationequations (1)-(6).
2.3. Equations and boundary conditions
Considering the metabolic heat production and blood perfusion of biological tissues, we use Pennes bio-heat transfer equation [Citation37] to calculate the temperature distribution of the tumor and surrounding tissues:
(1)
(1)
where ρ is the tissue density [kg/m3], Cp is the tissue heat capacity [J/kg/°C], t is the treatment time [s], T is the tissue temperature [°C], k is the tissue thermal conductivity [W/m/°C], ρb is the blood density [kg/m3], wb is the tissue blood perfusion rate [1/s], Cb is the specific heat of the blood [J/kg/°C], Tb is the blood temperature [°C], Qmet is the tissue metabolic heat production rate [W/m3]. Qml is the magnetic loss [W/m3], which can be considered as eddy current loss power in this paper [Citation34].
Deduce the eddy current loss as follows: set the cylinder with conductivity σ, radius R, and height h in an alternating magnetic field in which magnetic induction intensity B varies with time. Divide the cylinder into thin-walled cylinders with width dr, circumference 2πr and thickness h, and the induced electromotive force of any thin-walled cylinder is:
(2)
(2)
The instantaneous power of the wall is:
(3)
(3)
integrate the Equationequation (3)
(3)
(3) over 0∼R:
(4)
(4)
take the direction of the magnetic field parallel to the Z axis, and set the conditions outside the boundaries as magnetic insulation:
(5)
(5)
(6)
(6)
Where H0 is the magnetic field intensity [A/m], and the product of H0 [A/m] and frequency f[Hz] cannot exceed 4.85 × 108[Am−1s1] [Citation38]. In particular, the range of the product can be expanded to 5 × 109[Am−1S1] [Citation39,Citation40].
This paper applies the biological heat transfer module in all domains. Set the initial temperature of the tumor and normal tissue at 37 °C, the initial temperature of the skin at 33.5 °C, and the initial temperature of magnetic media at 25 °C. The heat -transfer caused by the convection on the surface of the skin is considered in boundary condition:
(7)
(7)
where h is the convective heat transfer coefficient, valued as 6 W/m2/°C [Citation41], and Tair is the air temperature, taken as 25 °C. The other boundaries are assumed to maintain a constant temperature of 37 °C.
2.4. Selection of damage threshold
In order to express the damage degree of tissue in the treatment process more intuitively, the equivalent thermal dose theory of Sapareto and Dewey [Citation42] is adopted in this paper, which converts the heating time at arbitrary temperature to the equivalent heating time at 43 °C:
(8)
(8)
(9)
(9)
where t0 and tultimate represent the time the heating starts and ends, respectively. T (t,x,y,z) is the function of temperature over time at a certain point of tissue, and β is the constant function related to temperature. Rhoon and Yarmolenko [Citation43,Citation44] stated that the thermal dose threshold of reversible and irreversible damage to muscle tissue is 40 min and 80 min, respectively. Therefore, the equivalent thermal dose of 40 min and 80 min is adopted as the tissue reversible and irreversible damage threshold, respectively. It can be judged that the tumor is completely damaged if the integrated value of the lowest temperature of the tumor is greater than 80 min.
3. Results and discussion
3.1. Multi-physical field coupling method verification
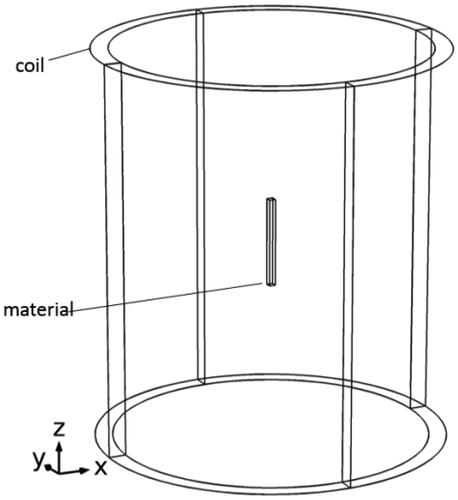
Use Fe83Zr10B7 to prepare a rectangular magnetic media with a thickness of 1.44 mm and a length of 20 mm. Put the magnetic media in an alternating magnetic field generated by LH-15 induction heating equipment provided by Dongguan Lihua mechanical equipment Co., Ltd. the temperature probe is fixed on the surface of the magnetic media, and the coil used in this paper with a radius of 78 mm and height 95 mm has nine turns. As shown in , the energizing solenoid coil with a height of 95 mm and radius of 78 mm is a 9-turn hollow copper tube. In order to avoid the experimental error caused by the joule heat of the copper pipe, circulating cooling water at 20 °C is used in the copper tube.
Figure 3. (a) The experimental scheme for measuring heating curves of Fe83Zr10B7 rectangular. (b) Cylindrical fresh pork tissue and Air-core copper coil used in MIH experiment. (c) Diagram of in vitro tissue experiment.
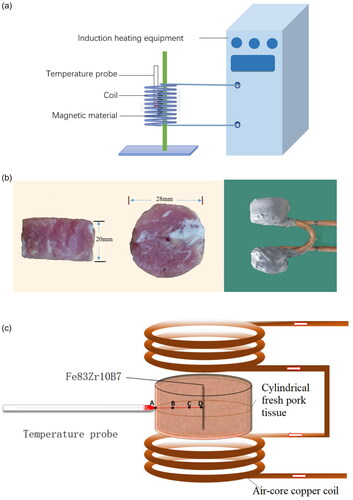
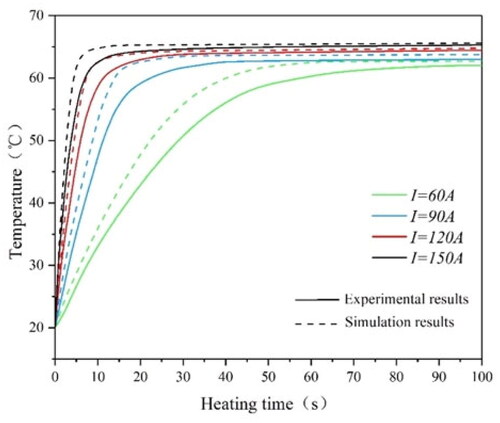
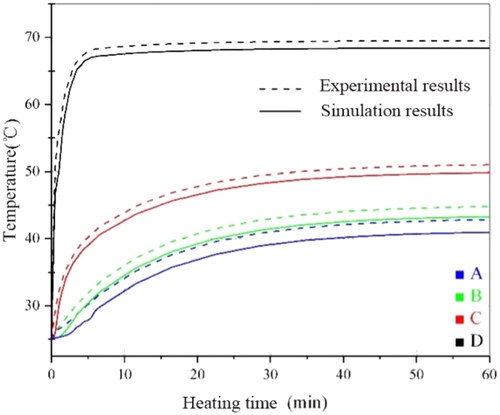
In order to verify the reliability of the experiment, a three-dimensional calculation model is designed by using Comsol Multiphysics in this paper. As shown in , the solenoid and the magnetic media are parallel. Moreover, the geometrical centers of both frames should coincide. The size of the coil and material is consistent with the experiment. As shown in , the experimental and simulated values of the surface temperature of Fe83Zr10B7 rectangular under different current conditions at 60 kHz are recorded. We also embed the magnetic media into a fresh pork tissue to measure the temperature rise; the experimental and simulated values are shown in , which is similar to . The simulated value is always slightly higher than the experimental value, and the reason may come from the deviations of environment between experiment and the simulation, but the final stable Curie temperature is basically unchanged; and the relative error is less than 2% which is within the allowable range.
3.2. Selection of material’s shape
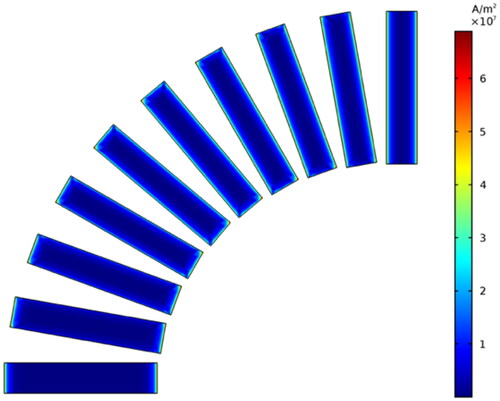
However, the uneven distribution of induced current generated by magnetic media in the magnetic field will lead to uneven heating power. Therefore, this paper studied the initial average heating power density of spherical, rectangular, and cylindrical media at different angles (0–90°) between the central axis and the magnetic field direction by taking the volume average of power density, as shown in . The curves of the initial average heating power density of three shapes with various angles under the conditions of Tmaterials=37 °C, f = 90kHz, and H0= 5 kA/m are demonstrated in . The sizes of these shapes are shown in brackets.
Figure 7. The angle between the direction of the applied magnetic field and the central axis of the cylindrical and the rectangular varies from 0° to 90°.
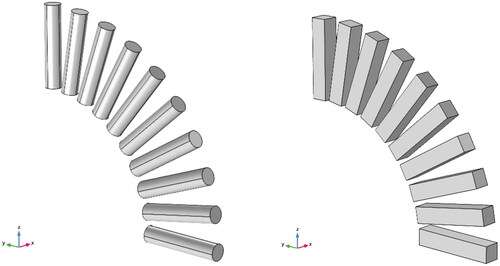
Figure 8. The initial average heating power density of three shapes of Fe83Zr10B7 material with various angles at f = 90kHz, H0=5kA/m and T = 37 °C.
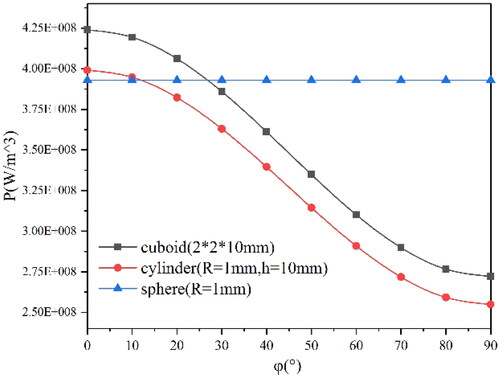
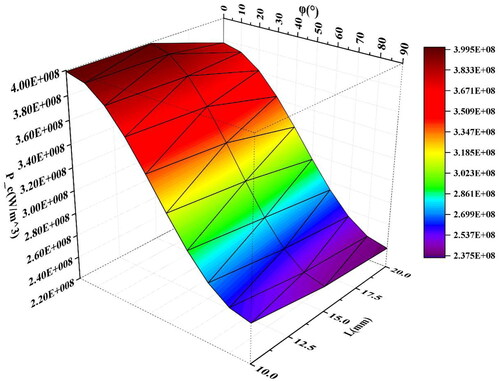
As shown in , for rectangular and cylindrical materials, the average heating power density decreases with the angle increase, due to the change of the distribution and magnitude of the induced current caused by the change of the angle. Take the simulation results of cuboid material as an example, as shown in ; with the increased angle, the induced current moves to the two bottom surfaces from the side surface, which is larger than the bottom surfaces, and causes a reduction of overall heating power. However, for the spherical media, the average heating power is independent of the direction of the applied magnetic field because of the symmetry. This paper also explores the influence of rectangular length on the average heating power density under the magnetic field condition of f = 90kHz and H0= 5 kA/m. As shown in and , the average heating power density tends to decrease slightly with the increase of L while R is constant.
In the actual course of hyperthermia, the shape of the tumor tissue may change after heating, which will cause a change in the position of the implanted medium and thus affect the heating power. Moreover, tumors of different sizes also require different lengths of material, which are difficult to monitor and control in treatment. Therefore, spherical material is preferred for multi-physical coupling analysis.
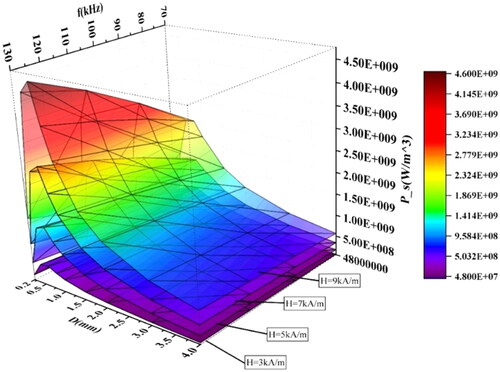
The conditions of are as follows; particle size D ranges from 0.2 mm to 4 mm, the f ranges from 70 to 130 kHz, and the H0and are 3 kA/m, 5 kA/m, 7 kA/m, and 9 kA/m respectively. It can be seen from the figure that under the condition of constant particle size, the average heating power increases with the increase of f and intensity, and the higher the H0, the greater the average heating power increases with frequency. Under the condition of constant H0 and f, the average heating power density of the material reaches the largest value when the size is 0.7mmr. The influence of f on average heating power gradually decreases as the size decreases or increases from 0.7 mm when the size is below 0.7 mm.
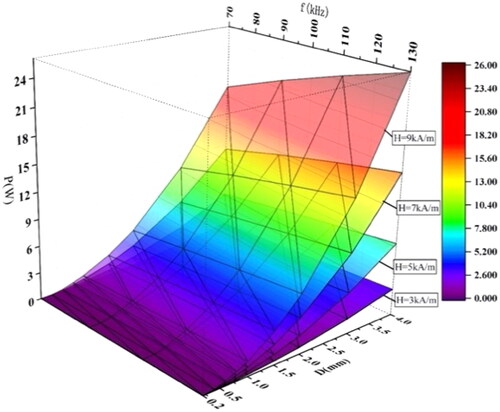
Although the magnetic media with a diameter of 0.7 mm has the largest heating power density, the overall heating power may be less than the larger magnetic media with a small heating power density. The product of the power density and the volume of the heating medium is the overall heating power. depicts the influence of particle size and f on heating power under different magnetic field strengths. As expected above, the overall heating power maybe not be maximum when the heating power density is maximum, and the heating power of spherical materials is positively correlated with particle size, f, and H0. The larger the H0 is, the more obvious the increase in heating power is.
Assuming radius R, heat capacity at constant pressure Cp, density ρ of the tumor are constant, the initial temperature is T0, the blood temperature is Tb=37 °C, and only blood perfusion can take away the heat, the average heat power loss caused by blood perfusion can be described as:
(10)
(10)
When t = 0, T(0)= T0; When t = tu, T(tu)= T.
In order to simplify the calculation, assume the temperature curve of the tumor is a quadratic function:
(11)
(11)
Then Equationequation (10)(10)
(10) can be described as:
(12)
(12)
Therefore, the average heating power of a tumor arising from temperature T0 to T in time t required is:
(13)
(13)
Take a tumor with R = 10mm as an example. If the temperature rises from 37 °C to 43 °C within 1 min, get p = 1.5 W through Equationequation (13)(13)
(13) . Considering the heating power will decrease with the increase in temperature, take P = 3W as the heating power. According to the results shown in , the heating power can reach 3 W when the diameter D is larger than 1.5 mm. Considering that magnetic media with a large size will increase the wound area and aggravate the patient’s pain, we use the spherical magnetic media with a diameter of 1.5 ∼ 4.0 mm as the magnetic media in the MIH simulation study.
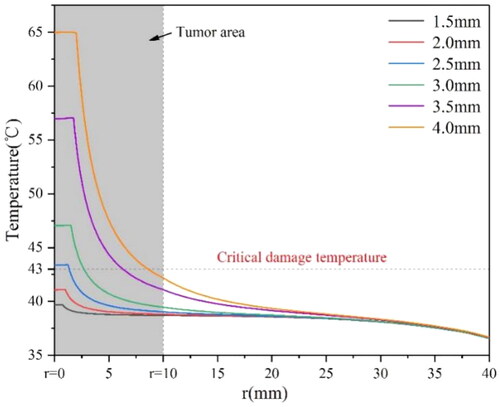
shows that when r reaches 3 mm, the temperature of some tumor tissues reaches the thermal damage temperature of 43 °C; when the r is 3.5 mm, the distance range at 43 °C is about 6 mm. When r reaches 4 mm, the tumor with a radius less than 7.5 mm can rise to over 43 °C. Therefore, for the tumor with a radius of 5 mm, the magnetic media with a diameter of 3.5 mm should be considered for treatment. F [Citation34] or the tumor with a radius of 10 mm, the magnetic media with a diameter of 4 mm should be considered. For larger tumors, multiple implantations would be suggested.
3.3. Analysis of thermal damage
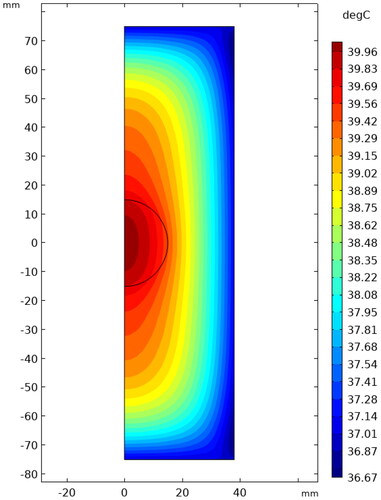
The model shown in has been established to investigate the effect of an alternating magnetic field on tissue damage. The blank area is air, and the magnetic field direction is parallel to the axis. shows the temperature distribution in the tissue under the condition of f = 240kHz, H0=15kA/m, t = 60 min. It can be seen that the highest temperature of the tissue reaches to 39.96°Cwithout the magnetic media, lower than the critical tissue damage temperature of 43 °C. The temperature gradually decreases along the symmetrical center due to the heat exchange between the skin and the surrounding air. In order to better study the effect of magnetic field conditions on tissue temperature, in this paper we calculate the average body temperature, excluding the skin, and use the equivalent heating time obtained by integrating it in time as the equivalent thermal dose.
Figure 15. The temperature distribution in normal tissue and tumor under the condition of f = 240kHz and H0=15kA/m for 60 min.
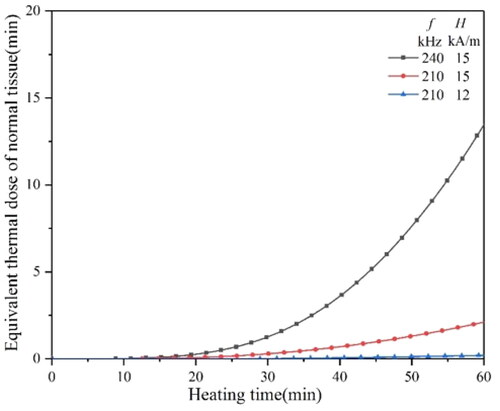
It can be seen from that under the condition of f = 240kHz and H0=15kA/m, the equivalent thermal dose of normal tissue at 60 min is less than 5 min, far less than the reversible tissue damage threshold of 40 min. Therefore, MIH under this magnetic field condition is feasible.
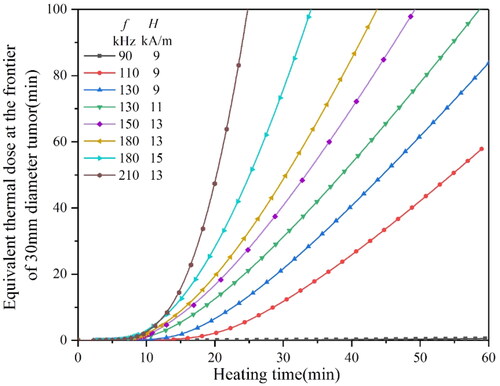
It can be seen from that when the H0 is 9 kA/m, the f should be more than 120 kHz to achieve an equivalent thermal dose of 80 min in a short time, while when the H0 is more than 10 kA/m, the f only needs 110 kHz or 120 kHz. indicates that, with the increase in tumor size, it needs higher H0 and f to reach the equivalent thermal dose of 80 min. When the H0 reaches 15 kA/m and H0 reaches above 200 kHz, the equivalent thermal dose of 80 min can be reached quickly.
Figure 17. The equivalent thermal dose curves of a 10 mm diameter tumor heated by a 3.5 mm diameter material under different magnetic conditions.
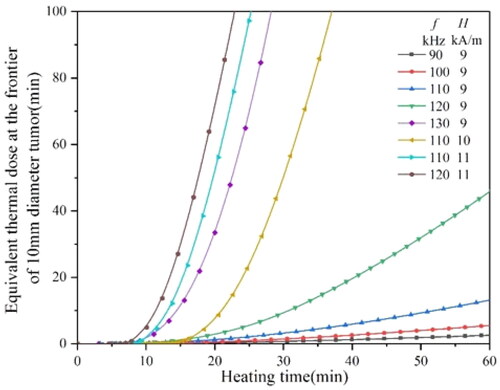
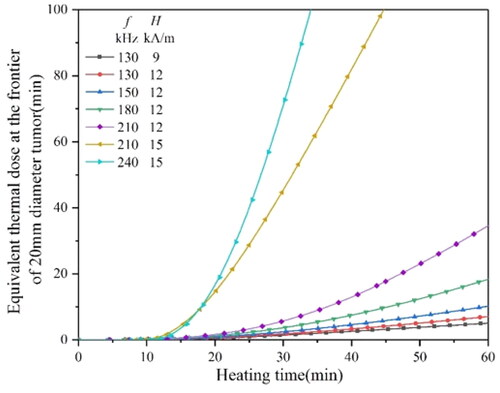
Figure 18. The equivalent thermal dose curves of a 20 mm diameter tumor heated by a 4 mm diameter material under different magnetic field conditions.
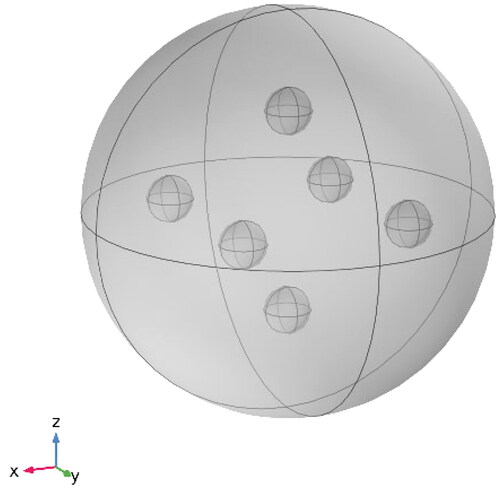
In this paper, we use a multi-point placement method to distribute the heat generated by the magnetic media more uniformly for tumors with a diameter larger than 30 mm. The heating model is shown in , 2 spherical magnetic mediums are implanted along the x-axis, Y-axis, and z-axis, respectively, and each two coaxial magnetic mediums are symmetrically distributed around the center of the tumor.
shows that when the H0 is above 9 kA/m, and the magnetic f is above 130 kHz, the equivalent thermal dose of 80 min could be reached within 60 min. Only if the H0 is above 13 kA/m and the f is above, 180 kHz can the equivalent thermal dose reach 80 min in a short time.
4. Conclusion
In this paper, the multiphysics coupling model of MIH is established by using Comsol Multiphysics. The spherical magnetic media is selected as the optimal research object after analyzing the influence of shapes in the magnetic field. The optimal treatment conditions are obtained by studying the thermal damage caused by different sizes and positions of magnetic media under different magnetic field conditions. For tumors with a radius of 10 mm, the optimal magnetic field conditions are H0 = (10–12kA/m) and f = (110–120kHz) when using the magnetic media with a diameter of 3.5 mm for treatment. For tumors with a radius of 20 mm, the optimal magnetic field conditions are H0 = (15–16kA/m) and f = (210–240kHz) when using the magnetic media with a diameter of 4 mm for treatment. For tumors with a radius of 20 mm, the optimal magnetic field conditions are H0 = (13–15kA/m) and f = (180–210kHz) when using six spherical magnetic mediums with a diameter of 3.5 mm for treatment. This paper can be used as a reference for applying Fe83Zr10B7 in MIH.
The simulation models in this study ignore the blood vessels, bones, and complex tissues surrounding tumors, which may affect the accuracy of the simulation results. In addition, the Fe83Zr10B7 is a strip material; the laboratory has not yet been able to make it into a spherical or cylindrical shape; it may affect the accuracy of simulation results due to the human error exist in the machining process, which is a problem to be considered in future research. In conclusion, considering the suitable Curie temperature and the magnetic properties of Fe83Zr10B7, it is feasible to be used as self-regulating temperature magnetic media in the future MIH.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Beik J, Abed Z, Ghoreishi FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221.
- Elming P, Sørensen B, Oei A, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. 2019;11(1):60.
- Peiravi M, Eslami H, Ansari M, et al. Magnetic hyperthermia: potentials and limitations. J Indian Chem Soc. 2022;99(1):100269.
- Sánchez OS, Castelo-Grande T, Augusto PA, et al. Cubic nanoparticles for magnetic hyperthermia: process optimization and potential industrial implementation. Nanomaterials. 2021;11(7):1652.
- Lebrun A, Zhu L. Magnetic nanoparticle hyperthermia in cancer treatment: history, mechanism, Imaging-Assisted protocol design, and challenges. John Wiley & Sons, Ltd; 2018. p. 631–667. DOI:10.1002/9781119127420.ch29
- Ganguly S, Margel S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers. 2021;13(23):4259.
- Oei AL, Kok HP, Oei SB, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020;163–164:84–97.
- Dobšíček Trefná H, Schmidt M, van Rhoon GC, et al. Quality assurance guidelines for interstitial hyperthermia. Int J Hyperthermia. 2019;36(1):277–294.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56.
- Mw D, Bl V, M L M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Inter J Hyperthermia. 2003;19(3):267–294.
- Sedighi O, Alaghmandfard A, Montazerian M, et al. A critical review of bioceramics for magnetic hyperthermia. J Am Ceram Soc. 2022;105(3):1723–1747.
- Kazantseva NE, Smolkova IS, Babayan V, et al. Magnetic nanomaterials for arterial embolization and hyperthermia of parenchymal organs tumors: a review. Nanomaterials. 2021;11(12):3402.
- Fatima H, Charinpanitkul T, Kim KS. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials. 2021;11(5):1203.
- Gupta R, Sharma D. (Carboxymethyl-stevioside)-coated magnetic dots for enhanced magnetic hyperthermia and improved glioblastoma treatment. Colloids Surf B Biointerfaces. 2021;205:111870.
- Kobayashi T, Kida Y. Interstitial hyperthermia of malignant brain tumors by an implant heating system using stereotactic techniques. Stereotact Funct Neurosurg. 1992;59(1-4):123–127.
- Kobayashi T, Kida Y, Tanaka T, et al. Interstitial hyperthermia of malignant brain tumors by implant heating system: clinical experience. J Neurooncol. 1991;10(2):153–163.
- Akiyama S, Kawasaki S, Kodera Y, et al. A new method of thermo-chemotherapy using a stent for patients with esophageal cancer. Surg Today. 2006;36(1):19–24.
- Stea B, Kittelson J, Cassady JR, et al. Treatment of malignant gliomas with interstitial irradiation and hyperthermia. Int J Radiat Oncol Biol Phys. 1992;24(4):657–667.
- Mack CF, Stea B, Kittelson JM, et al. Interstitial thermoradiotherapy with ferromagnetic implants for locally advanced and recurrent neoplasms. Int J Radiat Oncol Biol Phys. 1993;27(1):109–115.
- Deger S, Taymoorian K, Boehmer D, et al. Thermoradiotherapy using interstitial self-regulating thermoseeds: an intermediate analysis of a phase II trial. Eur Urol. 2004;45(5):574–579; discussion 580.
- Deger S, Boehmer D, Turk I, et al. Interstitial hyperthermia using self-regulating thermoseeds combined with conformal radiation therapy. Eur Urol. 2002;42(2):147–153.
- Johannsen M, Gneveckow U, Eckelt L, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia. 2005;21(7):637–647.
- Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81(1):53–60.
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541.
- Raouf I, Khalid S, Khan A, et al. A review on numerical modeling for magnetic nanoparticle hyperthermia: progress and challenges. J Therm Biol. 2020;91:102644.
- Tang Y D, Flesch R, Jin T, et al. Computational evaluation of malignant tissue apoptosis in magnetic hyperthermia considering intratumoral injection strategy. Int J Heat Mass Transf. 2021;178:121609.
- Kawashita M, Domi S, Saito Y, et al. In vitro heat generation by ferrimagnetic maghemite microspheres for hyperthermic treatment of cancer under an alternating magnetic field. J Mater Sci Mater Med. 2008;19(5):1897–1903.
- Ji P, Wang Y, Zhang M, et al. P2O5-Fe2O3-CaO-SiO2 ferromagnetic glass-ceramics for hyperthermia. Int J Appl Ceram Technol. 2018;15(5):1261–1267.
- Wu J, Wang H, Zhang X, et al. Power absorption of milliscaled implants in alternating magnetic field for magnetically induced hyperthermia: institute of electrical and electronics engineers inc[C]. Datong: institute of Electrical and Electronics Engineers Inc.; 2016.
- Astefanoaei I, Dumitru I, Stancu A, et al. A thermo-fluid analysis in magnetic hyperthermia. Chinese Phys B. 2014;23(4):044401.
- Schwarz RB, Johnson WL. Formation of an amorphous alloy by solid-state reaction of the pure polycrystalline metals. Phys Rev Lett. 1983;51(5):415–418.
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues.3. PARAMETRIC models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41(11):2271–2293.
- Hasgall PA, Neufeld E, Gosselin MC, et al. ITIS database for thermal and electromagnetic parameters of biological tissues, version 2.2. ITIS Database. 2011.
- Wu Y. Design and optimization of electromagnetic induction devices by finite element analysis [D]. South China University of Technology, 2021. DOI:10.27151/d.cnki.ghnlu.2021.002803
- Arulmurugan R, Vaidyanathan G, Sendhilnathan S, et al. Co-Zn ferrite nanoparticles for ferrofluid preparation: study on magnetic properties. Physica B Condensed Matter. 2005;363(1-4):225–231.
- Zhang W, Zuo XD, Niu Y, et al. Novel nanoparticles with Cr3+ substituted ferrite for self-regulating temperature hyperthermia. Nanoscale. 2017;9(37):13929–13937.
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm (reprinted from journal of applied physiology, vol 1, pg 93-122, 1948). J Appl Physiol (1985). 1998;85(1):5–34.
- Atkinson WJ, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng. 1984;31(1):70–75.
- Stauffer PR, Cetas TC, Fletcher AM, et al. Observations on the use of ferromagnetic implants for inducing hyperthermia. IEEE Trans Biomed Eng. 1984;BME-31(1):76–90.
- Mao N, Song MJ, Pan DM, et al. Computational fluid dynamics analysis of convective heat transfer coefficients for a sleeping human body. Appl Therm Eng. 2017;117:385–396.
- Hergt R, Dutz S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J Magn Magn Mater. 2007;311(1):187–192.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43 °C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels?. Eur Radiol. 2013;23(8):2215–2227.
- Yarmolenko PS, Moon E J, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343.