Abstract
Objective
To study the safety of improved hydrodissection based on the periparathyroidal fascial space during microwave ablation (MWA) for secondary hyperparathyroidism (SHPT).
Materials and Methods
Data from 337 patients (162 males and 175 females; mean age, 50.8 ± 12.8 [range, 16-84] years) who underwent MWA for SHPT were retrospectively reviewed. Among them, 177 patients underwent traditional hydrodissection (traditional group) and 160 patients underwent improved hydrodissection based on periparathyroidal fascial spaces (improved group). Safety enhancement was analyzed by comparing the complications between the two groups. The characteristics of the hydrodissected fascial spaces, complications, and the follow-up results were recorded. The baseline data, clinical parameters, laboratory indices and characteristics of SHPT lesions were analyzed to assess the risk factors associated with hoarseness.
Results
Hydrodissection was successfully performed in all the enrolled patients according to the protocol. Six periparathyroid fascial spaces were hydrodissected, depending on the location of the SHPT lesions. The incidence of hoarseness due to recurrent laryngeal nerve injury, the most common complication of thermal ablation for SHPT lesions, was lower in the improved group than in the traditional group (6.9% vs. 13.0%, p = 0.044). The median hoarseness recovery time in the improved group was shorter than that in the traditional group (2 vs. 6 months, p < 0.001). There was no difference in technical efficiency between the two groups (improved group vs. traditional group: 75.0% vs. 70.6%; p > 0.05).
Conclusions
Compared with traditional hydrodissection, improved hydrodissection based on periparathyroidal fascial spaces could enhance safety during MWA for SHPT.
Introduction
Secondary hyperparathyroidism (SHPT) is a common complication triggered and promoted by persistent calcium-phosphorus-vitamin D metabolism disorders in patients with end-stage renal disease [Citation1]. SHPT contributes to disturbances in mineral and bone metabolism and results in cardiovascular disease or even death. Parathyroidectomy (PTX) is the standard treatment for patients with SHPT who are refractory to medical therapy [Citation2,Citation3]. Ultrasound (US)-guided thermal ablation, including microwave ablation (MWA) and radiofrequency ablation, has recently been applied in clinical practice as a minimally invasive treatment for SHPT and has demonstrated its superiority [Citation4–6]. Previous studies showed that MWA or radiofrequency ablation could inactivate SHPT lesions and reduce parathyroid hormone (PTH) levels and were not inferior to PTX in terms of efficiency [Citation6,Citation7].
The parathyroid glands are located on the dorsal side of the thyroid gland and are much closer to the tracheoesophageal groove where the recurrent laryngeal nerve (RLN) is located. In most circumstances, the RLN is too tenuous to be visualized on US. Because the RLN is very sensitive to heat, RLN injury is the most common major complication of thermal ablation of SHPT lesions [Citation4,Citation5,Citation8]. Therefore, enhancing the safety of the thermal ablation of SHPT is a key factor in promoting its clinical application.
The hydrodissection technique is a protective measure used to reduce heat injury to adjacent structures during ablation and has been recommended in some guidelines for thyroid tumors [Citation9–11], which is simply copied in the thermal ablation of SHPT [Citation12]. However, to date, no study has adequately explored hydrodissection as a standard process for the thermal ablation of SHPT lesions.
Based on the clinical experience of > 500 patients undergoing MWA for > 1000 hyperparathyroidism lesions, an improved hydrodissection protocol based on periparathyroidal fascial spaces was established for the first time in our center, which included the operative procedure and extent requirement of hydrodissection, the US identifying and monitoring of the hydrodissected fascial spaces, and fascial spaces selected for hydrodissection. In the present study, a protocol for improved hydrodissection was described, and its safety enhancement was further investigated by comparing it with traditional hydrodissection in the MWA of SHPT.
Materials and methods
Study design and patients
This retrospective cohort study was approved by the Human Ethics Review Committee of the China-Japan Friendship Hospital. All patients provided written informed consent before ablation, and the requirement to obtain informed consent for study inclusion was waived because personal details were kept confidential.
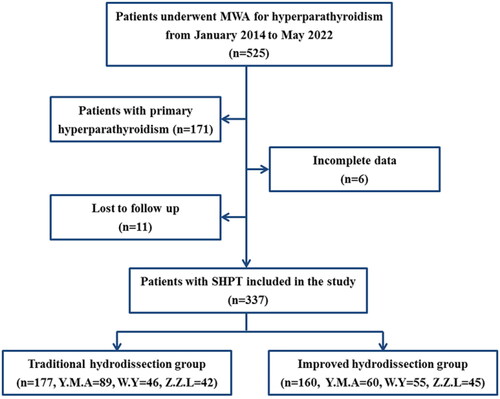
The medical records of all consecutive patients with SHPT who underwent MWA at our center between January 2014 and May 2022 were reviewed. The inclusion criteria were as follows: (1) end-stage renal disease patients with SHPT who were refractory to medication; (2) intact PTH (iPTH) ≥ 500 pg/mL or iPTH < 500 pg/mL with uncontrolled hypercalcemia/hyperphosphatemia and typical clinical symptoms [Citation13,Citation14]; 3) at least one enlarged parathyroid gland with a sharp margin, complete capsule clearly visible on US, and maximum diameter ≥ 0.6 cm [Citation15]; (4) increased 99mTc-sestamibi accumulation in both the early and delayed phases; (5) patients who refused or were ineligible for surgery; and (6) follow-up duration ≥ 3 months. The exclusion criteria were (1) primary hyperparathyroidism, (2) history of neck neoplasm resection, and (3) incomplete follow-up data. A flowchart of the patient selection process is shown in .
The patients were divided into two groups according to the hydrodissection technique: the traditional group and the improved group. Traditional hydrodissection was performed between January, 2014 and June, 2018. With the accumulation of experience, an improved hydrodissection technique based on the periparathyroidal fascial space was established and implemented in July 2018.
Equipment and operators
The B-mode US, contrast-enhanced US and MWA was undertaken with the Aplio 500 (Toshiba, Tokyo, Japan) with a 10.0-MHz linear-array transducer or the GE LOGIQ E9 (GE Healthcare, Pittsburgh, PA, USA) system equipped with a 9.0-MHz linear-array transducer. A 17-G internally cooled antenna with a 3-mm active tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System) was used for ablation. US contrast agent SonoVue (Bracco, Milan, Italy) or Sonazoid (Daiichi-Sankyo, Tokyo, Japan) was used in present study. Three radiologists (Y.M.A., W.Y., and Z.Z.L, all with 5 years of experience in parathyroid US) performed the hydrodissection and subsequent ablation.
Traditional and improved hydrodissection procedures
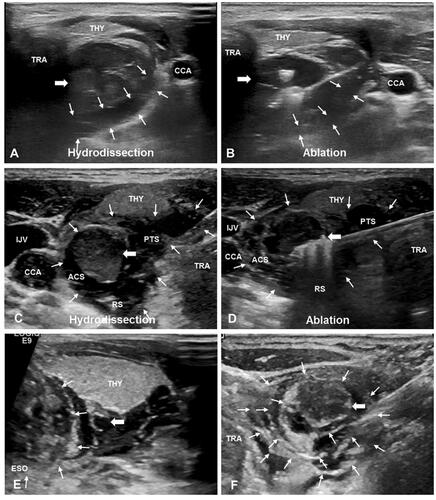
The patients were placed in the supine position with the neck extended. The ablation site was sterilized and draped using sterile towels. Lidocaine (1%) was injected subcutaneously at the puncture point. Then, an 18 G core needle connected to an extension tube and normal saline (NS) were inserted layer by layer under US guidance. When the needle tip reached the target position, NS was injected gently. For traditional hydrodissection, the needle tip was placed close to the parathyroid capsule, and NS was injected until the vital structures were separated by at least 5 mm from the SHPT lesion. The needle was then withdrawn. For improved hydrodissection, the needle tip was judged to be in the correct fascial space if the NS widened the fascial space and formed an anechoic area. If the soft tissue surrounding the needle tip became swollen, the needle tip was judged to be in an incorrect position and was further adjusted until it reached the proposed space. The needle tip was fixed tightly to the capsule of the SHPT lesion. During ablation, NS as the isolating fluid was continuously injected to maintain the width of the isolating band at ≥ 5 mm and keep the SHPT lesion displayed as an ‘island’ ().
Figure 2. Ultrasound images of traditional hydrodissection and improved hydrodissection. (A) Traditional hydrodissection before ablation. A narrow anechoic isolating band (white thin arrows) was established outside the SHPT lesion (white thick arrow) by one-time injection. (B) During ablation, the isolating liquid (white thin arrows) was absorbed and diffused over time, there was no sufficient separating distance. (C) Improved hydrodissection before ablation. The pretracheal space (PTS), the retropharyngeal space (RS) and the anterior cervical space (ACS) were hydrodissected. The isolating fluid formed an anechoic isolating band (white thin arrows) and separated SHPT lesion (white thick arrow) as an ‘island’. (D) During ablation, the thickness of isolating band (white thin arrows) was maintained at least 5mm through continuous injection of NS. (E) Improved hydrodissection in the TGS (thin white arrows), which shows as a semicircular multilayer mixed-echoic area. (F) Improved hydrodissection in the CPS (thin white arrows). After injection of NS, the ‘onion skin’ sign emerged around SHPT lesion (white thick arrow), which showed a multiple layer and annular anechoic area. Note: THY, thyroid; ESO, esophagus; TRA, trachea; CCA, common carotid artery; IJV, internal jugular vein; CPS, circumferential periparathyroidal space; TGS, tracheoesophageal groove space = TGS.
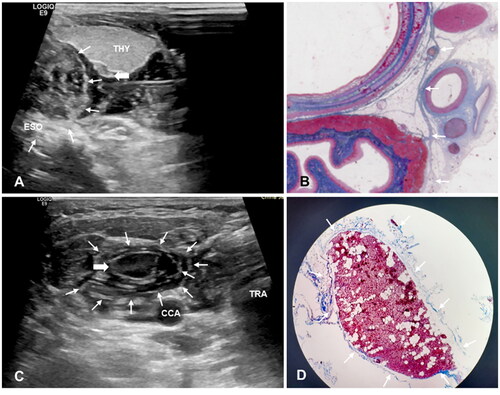
The fascial space under successfully improved hydrodissection could be identified on US by the following features: (i) possession of an obvious and smooth border; (ii) tension attributed to restriction of fluid flow; (iii) formation of an anechoic or mixed-echoic isolating band after hydrodissection and pushing the surrounding critical structures away from the SHPT lesion; and (iv) ‘onion skin’ sign in the TGS/CPS: a multilayer anechoic area with an obvious border and tension surrounding the tracheoesophageal groove or SHPT lesion ().
Figure 3. Ultrasound Image of hydrodissected TGS and CPS and the pathological images. (A) The TGS (white thin arrows) shows as a semicircular multilayer mixed-echoic area. (B) Pathological image shows corresponding fascia (white thin arrows) distribution in the tracheoesophageal groove area. (C) The CPS (white thin arrows) shows as an annular multilayer mixed-echoic area around SHPT lesion (white thick arrow). (D) The MASSON stains of resected SHPT lesion showed there were several layers of circular distributed collagen fibers (white thin arrows) around SHPT lesion (white thick arrow). Note: THY, thyroid; ESO, esophagus; TRA, trachea; CCA, common carotid artery; IJV, internal jugular vein; CPS, circumferential periparathyroidal space; TGS, tracheoesophageal groove space.
The evaluation standards for the successful establishment of improved hydrodissection are as follows: i) the width of the anechoic isolating band is at least 5 mm and is maintained by continuous injection of NS if necessary during ablation; ii) the hydrodissected fascial space should be close to the SHPT lesion; and 3) the PTC tip is fixed adjacent to the capsule of the target SHPT lesion.
The relationship between the PTC needle and the ablation antenna is not fixed for differently positioned SHPT lesions. The principle is to isolate the SHPT lesion as an ‘island’ throughout the ablation procedure to protect the surrounding vital structures. In most cases, the SHPT lesion is located dorsal to the thyroid, close to the tracheo-esophageal groove, so the injection needle is often inserted through the isthmus of the thyroid to isolate the pretracheal space, while the ablation antenna is inserted through the lateral neck parallel to the long axis of the SHPT lesion.
Preablation assessment and MWA procedure
Pretreatment assessment and MWA were performed as previously described [Citation12,Citation16]. All SHPT lesions disclosed by US should be completely ablated in one session. If the SHPT lesion was too large or if unilateral RLN injury occurred and ablation of the contralateral SHPT lesion was suspended, a two-session ablation had to be performed.
Follow-up and outcomes
Laboratory variables collected included serum iPTH, calcium, phosphate, and alkaline phosphatase levels at 2 h, 1 d, 7 days, and 1 month after MWA and then at 3-month intervals. Post-ablation complications were also recorded. If the patient had hoarseness, movement of the vocal cord was evaluated by US and laryngoscopy at each follow-up. All patients were followed until death, kidney transplantation, loss, or at the end of the study.
Complete ablation was defined as a non-enhancement ablation zone completely covering the SHPT lesion on contrast-enhanced US. Technical success was defined as the achievement of complete ablation, according to the protocol. According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, technical efficiency was defined as the lowest iPTH level within 7 days after MWA of < 300 pg/mL [Citation13,Citation17].
The primary outcomes were successful hydrodissection and intraoperative complications. Secondary outcomes included technical efficiency and incidence of severe hypocalcemia in both groups. The baseline data, clinical parameters, laboratory indices and parathyroid gland characteristics were analyzed to assess the risk factors for hoarseness associated with thermal injury.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Data with a normal distribution are presented as the mean ± standard deviation (SD), and the median and 25%-75% interquartile range (IQR) were used for data do not fit a normal distribution. Comparisons between parameters were performed using the independent sample t-test, Mann–Whitney U test, or chi-squared test. Logistic stepwise regression with the forward selection of variables was adopted to explore the risk factors for hoarseness. All results were tested using bilateral tests, and the significance was set at p < 0.05.
Results
Demographic and SHPT lesion characteristics
A total of 337 SHPT patients, consisting of 162 males and 175 females) with 731 SHPT lesions (median lesion volume: 0.572 ml [25-75% IQR:0.073 ml-3.035 ml]) were enrolled in the present study. The mean age was 50.8 ± 12.8 (range, 16-84) years. Overall, 177 patients (335 lesions) were included in the traditional group and 160 patients (396 lesions) were included in the improved group. The baseline characteristics, which included sex, age, dialysis vintage, SHPT-related symptoms, laboratory examination results (serum iPTH, calcium, phosphate, and alkaline phosphatase), and SHPT lesion characteristics (location, number, and size), were not significantly different between the two groups (p > 0.05). The baseline characteristics of the patients are summarized in .
Table 1. Baseline characteristics.
Hydrodissection outcomes
General information on hydrodissection
In the present study, six periparathyroid fascial spaces were hydrodissected, depending on the location of the SHPT lesions. Four of these fascial spaces corresponded to the classic anatomical spaces, including the pretracheal space (PTS), retropharyngeal space (RS), anterior cervical space (ACS), and carotid space (CS). Two other delicate fascial spaces, the TGS and CPS, were first disclosed by the US.
Traditional and improved hydrodissection were successfully performed in all enrolled patients according to the protocol. The median time consuming of improved hydrodissection is 284s per one lesion, which was longer than that of traditional hydrodissection 199s (p < 0.001). For SHPT lesions in normal locations, isolating fluid was injected into the PTS in 365/378 (96.6%), RS in 306/378 (81.0%), TGS in 59/378 (15.6%), and CPS in 274/378 (72.5%) lesions. All four spaces were hydrodissected in 51 lesions, three spaces in 217 lesions, two spaces in 312 lesions, and one space in 378 lesions.
Of the 18 ectopic SHPT lesions, 10 were located at the suprasternal fossa, 2 were inside the thyroid gland, 2 were close to the submandibular gland, 2 were outside the lateral lobe of the thyroid gland, and 2 were laterally abutted to the carotid sheath. For ectopic SHPT lesions, isolating fluid was injected into the PTS in 12/18 (66.7%), RS in 6/18 (33.3%), ACS in 4/18 (22.2%), CS in 2/18 (11.1%), and CPS in 16/18 (88.9%) lesions.
Anatomy and ultrasonic characteristics of periparathyroidal fascial spaces
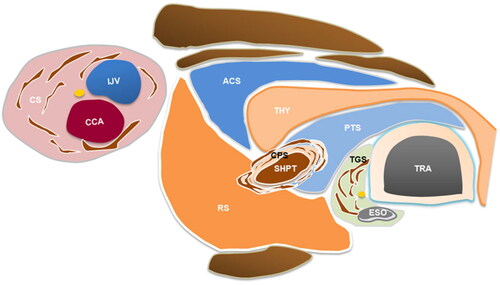
The location and ultrasonic characteristics of the six periparathyroid fascial spaces after hydrodissection were as follows: (1) The PTS, located between the thyroid and trachea, formed a stable semicircular arc or triangular anechoic isolating band on US after hydrodissection, pushing the trachea and esophagus away from the SHPT lesion and thyroid. The RLN and superior laryngeal nerves are located inside the PTS. (2) The RS, located posterior to the thyroid and carotid sheath, formed a stable anechoic isolating band on US after hydrodissection, and pushed the SHPT lesion and thyroid away from the longus colli, carotid sheath, and cervical sympathetic ganglion. (3) The ACS, located between the infrahyoid muscles and thyroid, formed an irregularly anechoic isolating band on US after hydrodissection and pushed the SHPT lesion and thyroid away from the infrahyoid muscles and the carotid sheath. (4) The CS, delimited by the three layers of the deep cervical fascia, formed a circular anechoic region on US after hydrodissection and pushed the SHPT lesion away from the common carotid artery, internal jugular vein, sympathetic plexus, supra- and infrahyoid cervical lymph nodes, and the vagus nerve. (5) The TGS enclosing the trachea and esophagus formed a mixed-echoic semicircular multilayer structure on US after hydrodissection and pushed the SHPT lesion away from the RLN, trachea, and esophagus. (6) The CPS, the space around the parathyroid gland, consists of multilayered continuous collagenous fibers and forms an onion-skin-like structure with an obvious border and tension on US after hydrodissection and pushes the SHPT lesion away from the surrounding critical structures. A schematic of the periparathyroidal space is shown in .
Figure 4. The schematic diagram of different fascial spaces around parathyroid. The schematic diagram of hydrodissected PTS, RS, ACS, CS, CPS and TGS. Note: THY, thyroid; ESO, esophagus; TRA, trachea; CCA, common carotid artery; IJV, internal jugular vein; SHPT, secondary hyperparathyroidism; PTS, pretracheal space; RS, retropharyngeal space; ACS, anterior cervical space; CS, carotid space; CPS, circumferential periparathyroidal space; TGS, tracheoesophageal groove space.
Strategy for hydrodissected fascial space selection
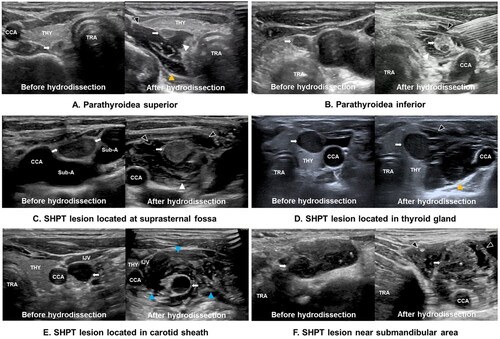
Hydrodissection strategies depend on the location of the SHPT lesions. In the present study, the location of the SHPT lesion and the corresponding fascial spaces that needed hydrodissection were divided into two categories: (1) SHPT lesions in a common position (parathyroidea superior/inferior); the fascial spaces including the PTS, RS, and ACS were sufficiently hydrodissected. TGS and CPS were hydrodissected to further increase the safe distance when the SHPT lesion was adjacent to the nerve (i.e. especially for upper glands those are close proximity to RLN). (2) Ectopic SHPT lesion: i) when the SHPT lesion was located at the suprasternal fossa where the large vessel and nerve were located, the PTS and CPS were hydrodissected; ii) when the SHPT lesion was located at the lateral neck where the thyroid, carotid sheath, and cervical sympathetic chain are located, the ACS, CS, and CPS were hydrodissected; iii) when the SHPT lesion was located in the submandibular area and near the infrahyoid muscles, the ACS and CPS were hydrodissected; iv) when the SHPT lesion was located in the thyroid gland, the ACS, PTS, and/or RS were hydrodissected according to the relationship between the SHPT lesion and the thyroid capsule ().
Figure 5. (A-F) Ultrasound images of SHPT lesions (White arrow) at different locations before and after improved hydrodissection. Pretracheal space hydrodissection (white arrowhead), retropharyngeal space hydrodissection (yellow arrowhead), anterior cervical space hydrodissection (black arrowhead), and carotid space hydrodissection (blue arrowhead) are shown as anechoic or mixed echoic bands on the images. Note: THY, thyroid; ESO, esophagus; TRA, trachea; CCA, common carotid artery; IJV, internal jugular vein; Sub-A, subclavian artery; SHPT, secondary hyperparathyroidism.
Ablation outcome
Complete ablation was achieved in all 337 cases according to the protocol (330 cases in one session, 7 cases in two sessions). The technical success rate was 100%. The overall median ablation time per lesion was 117 s (IQR, 37-414 s). The median ablation time in the improved group was shorter than that in the traditional group (243 s vs. 340 s, p < 0.001). While there was no difference regarding the duration including ablation plus hydrodissection between the two groups. According to the post-operative CEUS and laboratory results, the technical efficiency rate was 72.7% (245/337). In the subgroup analysis, the technical efficiency rate was 75.0% (120/160) in the improved group and 70.6% (125/177) in the traditional group. However, there was no difference in the technical efficiency rates between the two groups (p > 0.05).
No perioperative death occurred. Hoarseness due to RLN injury was the exclusive major complication encountered in 34 cases, including 11 cases (6.9%, 11/160) in the improved group and 23 cases (13.0%, 23/177) in the traditional group. Further univariate and ultivariate analyses showed that the rate of hoarseness in the improved group was lower than that in the traditional group (p = 0.044, p = 0.007). Permanent hoarseness occurred in three cases in the traditional group and in none in the improved group; however, there was no significant difference between the two groups (p = 0.50). The remaining 31 patients with hoarseness recovered completely during the follow-up. However, the median recovery time in the improved group was shorter than that in the traditional group (2 vs. 6 months, p < 0.001). Severe hypocalcemia episodes occurred in 83 cases (83/337, 24.6%), including 45 (45/177, 25.4%) cases in the traditional group and 38 (38/160, 23.8%) cases in the improved group. There was no significant difference in the severe hypocalcemia rates between the two groups (p > 0.05).
Discussion
In the last decade, US-guided MWA has been preliminarily used in the clinic to manage SHPT by a few teams and has achieved promising results [Citation4,Citation5,Citation7,Citation12,Citation18]. Because of the unique location of SHPT lesions, which are often tightly close to vital structures such as the trachea, esophagus, and nerves, ensuring safety is one of the key and challenging factors for MWA of SHPT. In addition, due to defective migration during the embryonic period or parathyroid cell contamination during PTX, the prevalence of ectopic hyperparathyroidism can reach 16% in patients with SHPT [Citation19]. Ectopic parathyroid glands may occur in different areas, such as the suprasternal fossa, retroesophageal region, intrathyroidal region, mandibular region, or outside/inside the carotid sheath [Citation20], which increases the risk of thermal ablation. High-frequency US can reveal the most adjacent vital structures [Citation21]. However, tenuous nerves, especially the superior laryngeal nerves and RLNs, are generally invisible on US in most situations and vulnerable to heat injury. Therefore, there is a relatively higher risk of thermal ablation of SHPT lesions regardless of whether they are located in normal or ectopic positions.
Hydrodissection is the most important technique for reducing heat injury during ablation by ensuring a safe distance between SHPT lesions and adjacent critical structures. However, thus far, the hydrodissection technique remains an empirical procedure, and there is still a lack of specific research on this key technique as a standard procedure during ablation. As a result, the incidence of hoarseness, the most common major complication directly associated with RLN heat injury, has been reported to be as high as 9.2% [Citation4,Citation5,Citation8,Citation18].
In the present study, by summarizing the clinical experience of hundreds of SHPT cases treated with MWA and comparing the anatomy of the periparathyroidal fascial space with US characteristics intraoperatively, a standard improved hydrodissection technique based on the periparathyroidal fascial space was established for the first time. In the present study, the key factors associated with improved hydrodissection have been described in detail, including the hydrodissection procedure, extent requirement of hydrodissection, US evaluation and monitoring of hydrodissection, and strategy of fascial space selection. The standardized hydrodissection procedure is similar to that of blunt separation during thyroidectomy.
The results of the present study showed that all 160 enrolled patients underwent successful improved hydrodissection, regardless of the normal or ectopic location. Six fascial spaces were described depending on the location of the SHPT lesions. Some corresponded to the classic anatomical spaces (i.e. PTS, RS, ACS, and CS), which are generally used to achieve blunt separation in surgery, and a few were first disclosed on US, which was verified by comparing the US imaging and microscopic pathology in the present study (i.e. TGS and CPS).
According to our experience, although a one-time injection of NS around the parathyroid gland in the traditional group could achieve a transient separation distance of more than 5 mm, it is difficult to maintain a sufficiently safe distance during the ablation process, mainly because of the absorption and diffusion of liquid, sometimes leading to inevitable thermal damage. Under US guidance and monitoring, persistent and sufficient hydrodissection based on fascial spaces in the improved group could greatly enhance the safety of ablation, even with the same technique success rate (improved vs. traditional, 75.0% vs. 70.6%), especially in long procedure times, multiple lesions, and special anatomical locations of lesions. According to the results, although hydrodissection was performed in both groups, the incidence of RLN injury in the improved group was significantly lower (6.9% vs. 13.0%) than that in the traditional group. Moreover, the recovery time was shorter (2 months vs. 6 months) and permanent hoarseness, a symptom indicating severe injury or complete inactivity of the RLN, did not occur in the improved group. These results fully indicate the safety enhancement of the improved hydrodissection technique. In addition, the incidence of RLN injury in the improved group was even lower than 11.8% after PTX [Citation18], which further confirmed the safety advantage of improved hydrodissection ().
Table 2. The factors Influencing the development of hoarseness.
In summary, the improved hydrodissection technique based on periparathyroidal fascial space has several advantages: (1) After hydrodissection, the fascial space is filled with anechoic NS, which makes the SHPT lesion appear as an ‘island’, and the island status can effectively inhibit heat spillover. (2) Through effective hydrodissection, it is difficult to cause heat injury to the surrounding vital structures, even to the fascia around the parathyroid gland. Therefore, postoperative adherence could be avoided. (3) Continuous injection of NS during ablation not only helps to maintain a sufficient separation distance, but also produces further thermal deposition effects through the convection of liquid, which is especially beneficial for protecting nerves and other critical structures within fascial spaces and reduces thermal ablation complications. Although the time to establish hydrodissection was slightly longer in the improved hydrodissection group than in the traditional hydrodissection group, there was no difference in the overall length of the procedure between the two groups.
The present study has several limitations. First, most of the fascial spaces visualized on US in the present study were not compared to gross specimens. Second, there are inherent problems related to the retrospective cohort study design.
Conclusion
The establishment of improved hydrodissection based on periparathyroidal fascial spaces could enhance the safety of the ablation procedure and help promote wider clinical application of thermal ablation in SHPT as a minimally invasive treatment.
Ethics approval
This retrospective study was approved by the Institutional Review Board of our hospital. Written informed consent was obtained from each patient prior to the ablation. The patients consented to publish their examination results and radiological images anonymously, and the requirement for written informed consent for publication of their data was waived by the ethics committee of China-Japan Friendship Hospital.
| Abbreviations | ||
| SHPT | = | secondary hyperparathyroidism |
| PTH | = | parathyroid hormone; |
| US | = | ultrasound |
| PTX | = | parathyroidectomy |
| MWA | = | microwave ablation |
| NS | = | normal saline |
| RLN | = | recurrent laryngeal nerve |
| PTS | = | pretracheal space |
| RS | = | retropharyngeal space |
| ACS | = | anterior cervical space |
| CS | = | carotid space |
| TGS | = | tracheoesophageal groove space |
| CPS | = | circumferential periparathyroidal space |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–921. doi:10.2215/CJN.06040710.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the dopps study. Clin J Am Soc Nephrol. 2015;10(1):98–109. doi:10.2215/CJN.12941213.
- Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30(12):2027–2033. doi:10.1093/ndt/gfv334.
- Cao XJ, Zhao ZL, Wei Y, et al. Efficacy and safety of microwave ablation treatment for secondary hyperparathyroidism: systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):316–323. doi:10.1080/02656736.2020.1744741.
- Zhou X, Shen Y, Zhu Y, et al. Ultrasound-guided microwave ablation for secondary hyperparathyroidism: a systematic review and meta-analysis. Int J Hyperthermia. 2021;38(1):1285–1294. doi:10.1080/02656736.2021.1965664.
- Ren M, Zheng D, Wu J, et al. Efficacy and safety of radiofrequency ablation versus parathyroidectomy for secondary hyperparathyroidism in dialysis patients: a single-center retrospective study. Sci Rep. 2022;12(1):10289. doi:10.1038/s41598-022-14623-x.
- Diao Z, Qian L, Teng C, et al. Microwave ablation versus parathyroidectomy for severe secondary hyperparathyroidism in patients on hemodialysis: a retrospective multicenter study. Int J Hyperthermia. 2021;38(1):213–219. doi:10.1080/02656736.2021.1885754.
- Wei Y, Peng LL, Zhao ZL, et al. Complications encountered in the treatment of primary and secondary hyperparathyroidism with microwave ablation - a retrospective study. Int J Hyperther. 2019;36:1264–1271.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655. doi:10.3348/kjr.2018.19.4.632.
- Xu D, Ge M, Yang A, et al. Expert consensus workshop report: guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther. 2020;16(5):960–966. doi:10.4103/jcrt.JCRT_558_19.
- Mauri G, Hegedus L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197. doi:10.1159/000516469.
- Zhuo L, Peng LL, Zhang YM, et al. Us-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282(2):576–584. doi:10.1148/radiol.2016151875.
- Isakova T, Nickolas TL, Denburg M, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751. doi:10.1053/j.ajkd.2017.07.019.
- Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–288. doi:10.1111/1744-9987.12058.
- Ying W, Zhen-Long Z, Xiao-Jing C, et al. A study on the causes of operative failures after microwave ablation for primary hyperparathyroidism. Eur Radiol. 2021;31(9):6522–6530. doi:10.1007/s00330-021-07761-9.
- Wei Y, Peng LL, Zhao ZL, et al. Risk factors of severe hypocalcemia after us-guided percutaneous microwave ablation of the parathyroid gland in patients with secondary hyperparathyroidism. J Bone Miner Res. 2020;35(4):691–697. doi:10.1002/jbmr.3934.
- Kdigo 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
- Gong L, Tang W, Lu J, et al. Thermal ablation versus parathyroidectomy for secondary hyperparathyroidism: a meta-analysis. Int J Surg. 2019;70:13–18. doi:10.1016/j.ijsu.2019.08.004.
- Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp Clin Endocrinol Diabetes. 2012;120(10):604–610. doi:10.1055/s-0032-1327628.
- Roy M, Mazeh H, Chen H, et al. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg. 2013;37(1):102–106. doi:10.1007/s00268-012-1773-z.
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16(4):749–766. doi:10.3348/kjr.2015.16.4.749.