Abstract
Objective
To evaluate the therapeutic effect of high-intensity focused ultrasound (HIFU) treatment for adenomyotic patients with primary infertility and to explore the factors that affect the pregnancy outcomes.
Materials and methods
Twenty-seven adenomyotic patients with primary infertility who underwent HIFU at HUNAN Provincial Maternal and Child Health Care Hospital, China, between July 2018 and December 2022 were retrospectively reviewed. We evaluated the pregnancy outcomes and analyzed the factors that may affect pregnancy outcomes including time to conception, pregnancy approach, gestational age, delivery mode, neonatal outcomes, and complications during pregnancy and delivery.
Results
Among the 27 adenomyotic patients with primary infertility, 10 patients had a total of 11 pregnancies after HIFU treatment. Of these, eight (72%) cases were natural pregnancies and three (23%) were in vitro fertilization (IVF) pregnancies. The median time to conception was 10 (range 4–25) months. There were eight (72%) successful deliveries. The rate of full-term deliveries was 90%. Of the eight live births, four (50%) were born vaginally and four (50%) by cesarean section. No severe complications occurred. The mean birth weight of newborns was 3.1 (range: 2.3–3.9) kg; all newborns developed well without complications during postpartum and breastfeeding.
Conclusions
HIFU treatment for adenomyosis could improve fertility of patients with primary infertility. HIFU is a promising therapeutic approach for patients with adenomyosis and infertility who wish to achieve pregnancy and have live birth deliveries.
Introduction
Adenomyosis is a benign gynecological disease characterized by the presence of endometrial glands and stroma within the myometrium [Citation1]. With the widespread use of magnetic resonance imaging (MRI) and transvaginal ultrasonography in the diagnosis of gynecologic diseases, the rate of misdiagnosis of adenomyosis is significantly decreased [Citation2–8]. The common symptoms of adenomyosis include menstrual pain and heavy menstrual blood volume. In addition, the presence of adenomyosis appears to have a negative impact on fertility outcomes. Currently, it is generally believed that the prevalence of adenomyosis in infertile women ranges from 7% to 27% [Citation9]. Recently, several studies have demonstrated the potential role of adenomyosis in primary infertility [Citation10–12]. Various mechanisms have been proposed to explain the potential negative impact of adenomyosis on fertility and implantation. This includes the impairment of the uterine sperm transport system caused by the changes in the structure of myometrium and the presence of uterine dysperistalsis with a consequent reduction in the probability of embryo implantation [Citation13]. Furthermore, adenomyosis is associated with obstetric complications such as preterm delivery, malpresentation, placental abruption, placenta previa, and postpartum hemorrhage [Citation14].
The treatment of adenomyosis remains an intense challenge. Adenomyomectomy is considered a conventional method for women who wish to conceive [Citation15]. However, this treatment modality is associated with major surgical trauma and may cause pelvic adhesions, thereby increasing the risk of infertility [Citation16]. In addition, adenomyomectomy may also increase the risk of uterine rupture in the middle to late stages of pregnancy [Citation17]. Over the last decade, uterine artery embolization (UAE) has been used in the management of adenomyosis. Several studies have demonstrated its efficacy in the treatment of symptomatic adenomyosis, but UAE may impair ovarian function and thus decrease the chances of achieving pregnancy [Citation18]. As a noninvasive treatment, high-intensity focused ultrasound (HIFU) ablation has been utilized in the treatment of adenomyosis. Several studies have shown the safety and efficacy of HIFU treatment for adenomyosis and no damage to the surrounding myometrium as well as ovarian function [Citation19–24]. However, there remains no study on pregnancy outcomes of adenomyotic patients with primary infertility. Therefore, we aimed to evaluate the therapeutic effect of HIFU treatment for adenomyotic patients with primary infertility and to explore factors that affect the pregnancy outcomes.
Material and methods
The protocol of this retrospective study was approved by the ethics committee of Hunan Provincial Maternal and Child Health Care Hospital (No. 2023014), and the requirement for an informed consent to do the research was waived.
Patients
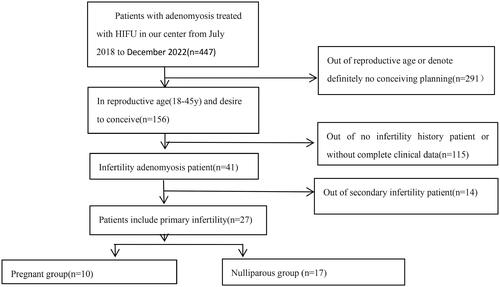
From July 2018 to December 2022, a total of 447 patients with adenomyosis were treated with HIFU at Hunan Provincial Maternal and Child Health Care Hospital. Based on the inclusion and exclusion criteria, 27 adenomyotic patients with primary infertility who wish to conceive were enrolled in this study ().
The inclusion criteria were as follows: (1) age range from 20 to 45 years; (2) the diagnosis of adenomyosis made by medical history, transvaginal ultrasound, and MRI; (3) patients with primary infertility.
The exclusion criteria were as follows: (1) patients with or suspected uterine malignancy; (2) patients with secondary infertility; (3) infertility due to male factors; (4) infertility due to ovarian disorders, including ovarian insufficiency; (5) no fertility requirements.
HIFU ablation
The procedure of HIFU treatment was described previously [Citation24]. Briefly, HIFU ablation was performed under conscious sedation (fentanyl at 0.8–1 lg/kg administered at 30–40 min intervals; midazolam hydrochloride, at 0.02–0.03 mg/kg, administered at 30–40 min intervals) using the Focused Ultrasound Tumor Therapeutic System (Model-JC200, Chongqing Haifu Medical Technology Co. Ltd., China) equipped with an ultrasound imaging device (MyLab 70, Esaote, Genova, Italy) for real-time guidance during the procedure. In this study, therapeutic ultrasound beams were generated by a transducer with a frequency of 0.8–1.0 MHz, a focal length of 15 cm, and a diameter of 20 cm.
The patients were placed in a prone position carefully, with the abdominal wall in contact with degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to compress and push the bowel away from the acoustic pathway. The power used was 400 W. The treatment began from the posterior to anterior, from inferior to superior part of the adenomyotic lesion. The focus was kept at least 1.5 cm away from both endometrium and boundary of the adenomyotic lesion. During the procedure, the sonication power was adjusted based on the feedback from the patients and the changed grayscale on the ultrasound imaging. The treatment was terminated when the hyperechoic area covered the entire adenomyosis or the contrast-enhancement ultrasound showed no blood supply in the lesion. The patients were required to report any discomfort during the procedure, and the vital signs including heart rate, blood pressure, respiration, and oxygen saturation were monitored. After HIFU treatment, cold saline was used to wash the bladder. All patients were discharged from hospital 1 day after HIFU.
Imaging evaluation
All patients underwent MRI examination within 1 week before and 1 day after HIFU treatment. Meanwhile, for better assessment of technical success, we performed contrast-enhanced ultrasound (SonoVue) study at the end of the procedure. We calculated the volume of adenomyosis treated and their non-perfused volume (NPV) according to the formula of the ellipsoid (V = 0.5233 × D1 × D2 × D3) where D1 is the long diameter, D2 is the left–right diameter, and D3 is the anteroposterior diameter.
Post-HIFU follow-up
Patients were scheduled for follow-up at 3, 6, 12, 24, and 36 months after HIFU. The reduction in volume of ablated adenomyosis was evaluated by transvaginal sonography, and the symptom improvement was assessed using the uterine fibroid symptom and quality-of-life questionnaire. Afterward, we collected clinical data and obstetric outcomes in patients who achieved pregnancy after HIFU treatment including age, characteristics of the adenomyosis, treatment parameters (power, energy, sonication time, NPV), and complications. We further analyzed the time to conception, pregnancy approach, delivery mode, neonatal outcomes, and complications during pregnancy and delivery. Likewise, symptom relief and shrinkage of adenomyotic lesion after HIFU treatment were evaluated.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software program, version 23 (IBM, Armonk, NY, USA). The normally distributed data were reported as means ± standard deviation, whereas the skewed distribution data were reported as medians and interquartile range (IQR). The Mann–Whitney U test or Fisher’s exact test was performed to compare the clinical parameters. Qualitative variables were expressed as absolute values and percentages. Quantitative variables were expressed with at least one measurement of central tendency and one of dispersion. In bivariate analysis, quantitative variables were compared using Wilcoxon test. A p value of <0.05 was considered as statistical significance. Dysmenorrhea was evaluated by Numeric Rating Score (NRS), and menorrhagia was evaluated by pictogram.
Results
Baseline characteristics of patients with primary infertility
The mean age of the 27 adenomyotic patients with primary infertility was 35.7 ± 4.8 (range: 26–45). The mean body mass index (BMI) was 24.6 ± 3.8 (range: 18–31.2) kg/m2. The mean uterine volume and lesion volume were 416.0 ± 169.7 (range: 122–912) mm3 and 149.5 ± 55.3 (range: 62–300) mm3, respectively. Among them, 10 of the 27 patients became pregnant. The mean age of the 10 women was 32.5 ± 3.8 (range: 26–37) years at the time of treatment. The mean BMI was 22.7 ± 3.1 (range: 19.8–30.2) kg/m2. The mean uterine volume and lesion volume of the 10 women were 392.6 ± 169.9 (range: 122–729) mm3 and 143.6 ± 59.8 (range: 62–267) mm3, respectively. The average time from HIFU to pregnancy was 10 (range: 4–25) months. Among these 10 patients, one had adenomyomectomy before HIFU treatment, and another patient experienced reduplicative failed assisted reproduction technology (). The type of the adenomyosis is described in .
Table 1. Baseline characteristics of the study Population of 10 adenomyotic patients with primary infertility who become pregnant after HIFU.
HIFU treatment and adverse effects
All patients completed HIFU treatment successfully in one session. The median treatment time was 72 (IQR 33–219) min. The median sonication power used was 375 (IQR 300–400) W, and the median ultrasound energy used was 220.8 (IQR 120–837.5) KJ. The median NPV after treatment was 78 (IQR 32–140) cm3 according to the contrast-enhanced MRI evaluation, which gave an NPV ratio of 46% (IQR 27%–80%) (). The patients tolerated HIFU treatment well. The most common intraprocedural side effects included mild lower abdominal pain, lower limb numbness or pain, inguinal pain, sacrococcygeal pain, with an incidence of 88.9% (24/27). Based on the Society of Interventional Radiology classification system, all of these adverse effects were classified as minor complications (class A) and subsided without any treatment. After HIFU treatment, no patients developed amenorrhea. Only three patients reported abdominal pain and sacrococcygeal pain after HIFU. The pain subsided within 24 h after taking oral indomethacin. One patient with intrinsic adenomyosis had vaginal discharge for 1 month after HIFU. She underwent a hysteroscopic 3 months later and hysteroscopic examination showed normal uterine cavity.
Table 2. Treatment parameters (n = 27).
Symptom improvement after HIFU
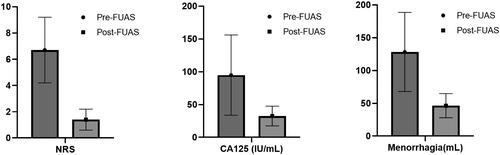
As shown in and , both dysmenorrhea and menorrhagia were relieved significantly (p < 0.05). The average pre-HIFU pain score was 6.67 ± 2.51, and it decreased to 1.44 ± 0.80 3 months after HIFU. The average menstruation volume was 128.15 ± 60.26 ml, and it decreased to 46.30 ± 18.38 ml. The median level of Ca125 was 78 IU/ml, and it decreased to 31 IU/ml.
Table 3. Symptom improvement after HIFU in primary infertile patients (n = 27).
Pregnancy outcomes
In the present study, 10 out of 27 patients had a total of 11 pregnancies (pregnancy rate: 37%). The median time to conception after HIFU was 10 (range: 4–25) months. Among these pregnancies, the natural conception rate was 72% (8/11). One patient reported a first-trimester spontaneous miscarriage, but subsequently achieved a full-term pregnancy at her second attempt. The remaining three patients (3/11, 23%) had IVF pregnancies, and one of them failed IVF attempts twice before HIFU treatment.
Among the 10 patients with 11 pregnancies, 8 (72%) patients had successful deliveries. The average gestational time was 37.8 ± 1.3 (range: 35–40) weeks. Seven patients (87%) had full-term deliveries and one patient (13%) had a preterm delivery at 35+5 weeks due to preterm premature rupture of membranes (PPROM). In these patients, four (50%) patients had vaginal deliveries and four (50%) patients had cesarean section deliveries. The indications for cesarean sections are shown in . The average birth weight of the eight newborns was 3.2 ± 0.5 (range: 2.3–3.9) kg, and one newborn was a low-birth-weight infant (<2.5 kg) ().
Table 4. Obstetric characteristics of the 10 pregnancies achieved after HIFU treatment.
In this study, first-trimester miscarriage was observed in two patients, and one had medical-induced abortion due to fetal chromosome abnormality. One patient had postpartum hemorrhage associated with uterine contraction. The patient lost 600 ml of blood after placenta delivery, but the bleeding was controlled using oxytocin, which did not cause more serious consequences.
Statistical analysis of factors influencing reproductive outcomes after HIFU treatment
To investigate the factors affecting reproductive outcomes, we compared the baseline characteristics and treatment results between successfully pregnant and non-pregnant patients. The results showed significant differences between the two groups in age (p = 0.006), BMI (p = 0.045), NPV ratio (p = 0.036), duration of infertility (p = 0.000), and types of adenomyosis (p = 0.030) (intrinsic vs. extrinsic vs. intramural vs. full thickness). The intrinsic adenomyotic patients (Type I) with primary infertility had better postoperative reproductive outcomes than patients with other types of adenomyosis. Furthermore, the higher NPV ratio, the younger patient, the shorter duration of infertility, and the lower BMI level were associated with greater improvement in reproductive outcomes ().
Table 5. Patient characteristics between pregnant and nulliparous groups.
The pregnancy outcome was used as the dependent variable. The other variables that may affect the pregnancy outcomes were used as independent variables. Seven independent variables related to the pregnancy outcomes were first identified by the univariate analysis: age and duration of infertility (p < 0.05) (). Then multivariate ordered logistic regression analysis was used to investigate the factors that independently affect pregnancy outcome. The analysis results showed that the risk factor affecting the pregnancy outcome was duration of infertility (p < 0.032, OR = 0.383, 95% confidence interval 0.159–0.920) ().
Table 6. Univariate analysis related to pregnancy outcome.
Table 7. Multivariate ordered logistic regression analysis for pregnancy outcome.
Discussion
Infertility is defined as a disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse. Primary female infertility refers to women who have never been diagnosed with clinical pregnancy and meet the infertility criteria [Citation25]. In this study, 27 patients with adenomyosis were diagnosed as having primary infertility. A previous study showed that the pregnancy rate was 30.3% (10/33) after adenomyomectomy in infertility adenomyosis patients [Citation15]. It was lower than that in our result (37%). The postoperative complications including uterine shrinkage, premature ovarian insufficiency, ureter fistula, and subfascial hematoma, occurred in four patients in that study [Citation15]. Recently, another study [Citation26] enrolled a total of 93 patients with adenomyosis and infertility, and 50 were treated with HIFU and 43 underwent laparoscopic excision (LE). Patients treated with HIFU showed a significantly higher pregnancy rate (52% vs. 30.2%) than those who underwent LE. In comparison with LE, HIFU achieved better postoperative reproductive outcomes.
Several studies have shown that HIFU treatment does not impair ovarian function, and it may increase pregnancy rate and live birth rate [Citation26–31]. In 2006, Ricabinovi et al. reported a 36-year-old patient with an 84-cm3 focal adenomyotic lesion who had difficulty in conceiving [Citation29]. After one session of ultrasound guided focused ultrasound ablation treatment, an NPV of 33 cm3 was achieved. The patient conceived spontaneously and delivered a healthy term infant via vaginal delivery. Zhou et al. [Citation27] treated 68 patients with adenomyosis who wished to conceive with HIFU and completed follow-up. Of these 68 patients, 54 conceived at a median of 10 months (range: 1–31 months) post-HIFU, and 21 of them delivered healthy babies. Our results showed that 37% of patients with primary infertility became pregnant with 29% live birth rate.
A previous study has shown that adenomyosis is strongly associated with lifelong primary infertility in this primate [Citation10]. Exacoustos et al. confirmed the potential role of adenomyosis in primary infertility later [Citation11]. Recently, Huang et al. reported the pregnancy outcomes in adenomyotic patients with infertility after HIFU and analyzed factors that may affect fertility outcomes [Citation26]. Their results showed that 52% (26/50) of the patients with adenomyosis who had a history of infertility became pregnant following HIFU treatment with a 36% live birth rate. The patients with diffuse adenomyosis were associated with less improvement in reproductive outcomes than those with focal adenomyosis [Citation26]. Our results also showed that age, BMI, NPV ratio, and duration of infertility were significantly correlated with reproductive outcomes (). The patients with younger age were associated with greater improvement in reproductive outcomes after HIFU than patients with older age.
Currently, a consensus has been reached that adenomyosis is an independent risk factor for primary infertility. Bourdon et al. showed that focal adenomyosis is associated with primary infertility in reproductive-age women, and most of these patients with infertility exhibited focal adenomyosis of the outer myometrium (Kishi type II) [Citation12]. In the present study, we found that 13.4% (7/52) of patients with internal adenomyosis (Kishi type I), 18.6% (8/43) of patients with external adenomyosis (Kishi type II), 66.6% (2/3) of patients with intramural adenomyosis (Kishi type III), and 17.2% (10/58) of patients with indetermined type of adenomyosis [Kishi type IV] had primary infertility. Therefore, our results showed that every type of adenomyosis was associated with primary infertility, but type III had a higher percentage of primary infertility than other types.
Our results also showed a significantly higher pregnancy rate in patients with intrinsic adenomyosis (Kishi type I: 71.4%) and intramural adenomyosis (type III: 100%) than in patients with extrinsic adenomyosis (type II: 25%) and indetermined type of adenomyosis (10%) after HIFU treatment. This phenomenon may be explained by that most of the external adenomyotic lesions and some of full-thickness adenomyosis were accompanied by pelvic endometriosis and had uterorectal adhesion and that those non-uterine infertility factors will aggravate the difficulty of pregnancy [Citation30].
Many factors are related to primary infertility. Our study showed that age, BMI, types of adenomyosis, NPV ratio, and duration of infertility are significantly correlated with reproductive outcomes after HIFU (). This can help screen the therapeutic indications and provide individual treatment programs for patients. For example, encouraging young women to intervene early, choose an appropriate treatment, stop and delay disease progression, or control weight, and all the above measures can further promote fertility and provide patients with effective treatment counseling and advice. Previous studies have shown that the NPV ratio of adenomyosis is related to the long-term efficacy of the treatment [Citation26]. Liu et al. [Citation31] investigated that the average NPV ratio calculated immediately after HIFU was 57.4% ± 24.4%, and the re-intervention rate was lower in patients with a higher NPV ratio. In this study, the NPV ratio of 55% achieved in the pregnant group compared with the 43% in the non-pregnant group seemed to have a better clinical effect, which was similar to the results of some previous studies [Citation26–29]. Our results also revealed that HIFU treatment had a better therapeutic effect in intrinsic adenomyosis than extrinsic and full-thickness adenomyosis. Compared with the extrinsic type and indetermined adenomyosis, intrinsic adenomyosis was easier to treat with HIFU to achieve a higher NPV ratio. This phenomenon can be explained by that more intrinsic adenomyotic lesions are located in the anterior wall of the uterus, and there is no energy absorption in the acoustic pathway, which makes the energy efficiency easier to deposit in the lesion, and patients have better intraoperative tolerance. In contrast, more extrinsic adenomyotic lesions are invaded in the posterior wall of the uterus and close to the sacrum, and thus intraoperative tolerance is worse, treatment intensity is lower, and the NPV ratio is often lower than that of intrinsic adenomyosis.
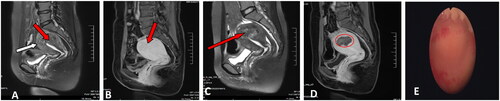
This study also has a meaningful phenomenon that needs further elaboration. A patient with intrinsic adenomyosis had a minor damage in the endometrium at the uterine fundus of the uterus (). She experienced vaginal fluid for nearly a month after HIFU. We were constantly worried about whether the patient had endometrium damage and uterine adhesions, and thus we gave her 3-month full-cycle treatment of estrogen and progesterone, together with hysteroscopy 6 months after HIFU. During the procedure of hysteroscopy, the uterine cavity was found to be roughly normal, without obvious endometrial defect and adhesion (). This also indicated that the endometria has rich blood flow and better repair function, and minor endometria damage will not lead to intrauterine adhesion. However, we still needed to be more careful and minimize the risk of endometrium and serosa damage in treatment. Previous studies [Citation8,Citation32] have shown that external adenomyosis frequently coexists with endometriosis, especially with deep infiltrating endometriosis, compared to internal adenomyosis. Therefore, we have suggested that patients with internal adenomyosis can try to conceive naturally after HIFU, but patients with external adenomyosis should be evaluated accurately to figure out if assisted reproductive technology is needed for a successful pregnancy.
Figure 3. MRI Features of internal adenomyosis. (A) Internal adenomyosis: a lesion with ill-defined margin located in the anterior wall of the uterus had developed in the thickened junctional zone (black arrow) and the myometrium outside the adenomyotic lesion was preserved. The thickness of the intact myometrium was 4 mm (white arrow). (B) Pre-HIFU enhanced MRI showed an internal adenomyotic lesion located at the posterior wall of the uterus (arrow). (C) T2-weighted image 1 day after HIFU. (D) A contrast-enhanced MRI obtained 1 day after HIFU showed the internal adenomyotic lesion was ablated (circled). (E) A hysteroscopic showing a normal uterine cavity after 6 months. This patient achieved successful IVF whom underwent previous twice failed IVF that ended in uncomplicated cesarean deliveries at 40 weeks because of the advice of obstetrician.
This study is limited because it is a retrospective study, and some bias may have occurred because the patients were not randomized for enrolment. This study is also limited because the sample size is small, and thus it is difficult to compare the factors that affect pregnancy outcomes between the pregnant group and the non-pregnant group. In addition, these patients were from a single center, and the results may have bias because of the skill and experience of physicians. In the future, multicenter or prospective randomized controlled trials in different populations are needed to validate the findings of this study and enhance the study’s external validity.
Conclusions
Based on the results of our study with small sample size, HIFU could improve the fertility outcomes of adenomyotic patients with primary infertility. Patients with intrinsic adenomyosis (Kishi type I) or patients with intramural adenomyosis had a significantly higher pregnancy rate than that of patients with extrinsic adenomyosis (Kishi type II) and patients with indetermined adenomyosis (Kishi type IV) after HIFU treatment.
In the future, multicenter or prospective randomized controlled trials with a large number of subjects are needed to validate the findings of this study. Also, it is important to explore other treatment options for adenomyotic patients with primary infertility and investigate the impact of HIFU on different types of adenomyosis in more details.
Disclosure statement
Zhibiao Wang is a senior consultant to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the article.
Data availability statement
The full access to the data will be available to researchers for purposes of research or regulatory decision making with a signed data access agreement after approval of a proposal. All data requests will be reviewed by the research committee at Chongqing Medical University and by the corresponding authors to verify whether the request is subject to any intellectual property or confidentiality obligations.
Additional information
Funding
References
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus—revisited. Am J Obstet Gynecol. 1972;112(5):583–593. doi: 10.1016/0002-9378(72)90781-8.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16(11):2427–2433. doi: 10.1093/humrep/16.11.2427.
- Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207(2):114.e1–114.e7. doi: 10.1016/j.ajog.2012.06.027.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16–24. doi: 10.1016/j.bpobgyn.2018.01.013.
- Tellum T, Nygaard S, Lieng M. Noninvasive diagnosis of adenomyosis: a structured review and meta-analysis of diagnostic accuracy in imaging. J Minim Invasive Gynecol. 2020;27(2):408–418.e3. doi: 10.1016/j.jmig.2019.11.001.
- Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27(12):3432–3439. doi: 10.1093/humrep/des332.
- Pinzauti S, Lazzeri L, Tosti C, et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol. 2015;46(6):730–736. doi: 10.1002/uog.14834.
- Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(7):1393–1401. doi: 10.1093/humrep/dex088.
- Dueholm M, Aagaard J. Adenomyosis and IVF/ICSI treatment: clinical considerations and recommendations. Expert Rev Endocrinol Metab. 2018;13(4):177–179. doi: 10.1080/17446651.2018.1493923.
- Barrier BF, Malinowski MJ, Dick EJ, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82 Suppl 3:1091–1094. doi: 10.1016/j.fertnstert.2003.11.065.
- Exacoustos C, Morosetti G, Conway F, et al. New sonographic classification of adenomyosis: do type and degree of adenomyosis correlate to severity of symptoms?. J Minim Invasive Gynecol. 2020;27(6):1308–1315. doi: 10.1016/j.jmig.2019.09.788.
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114(6):1271–1277. doi: 10.1016/j.fertnstert.2020.06.018.
- Buggio L, Dridi D, Barbara G. Adenomyosis: impact on fertility and obstetric outcomes. Reprod Sci. 2021; 28(11):3081–3084. doi: 10.1007/s43032-021-00679-z.
- Wu X, Jiang W, Xu H, et al. Characteristics of uterine rupture after laparoscopic surgery of the uterus: clinical analysis of 10 cases and literature review. J Int Med Res. 2018;46(9):3630–3639. doi: 10.1177/0300060518776769.
- Yoon SH, Lee GJ, Cho HJ, et al. Clinical efficacy of a novel method of fertility preserving adenomyomectomy in infertile women with diffuse adenomyosis. Medicine (Baltimore). 2023;102(13):e33266. doi: 10.1097/MD.0000000000033266.
- Takeuchi H, Kitade M, Kikuchi I, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol. 2006;13(2):150–154. doi: 10.1016/j.jmig.2005.12.004.
- Zhou Y, Shen L, Wang Y, et al. Long-term pregnancy outcomes of patients with diffuse adenomyosis after double-flap adenomyomectomy. J Clin Med. 2022;11(12):3489. doi: 10.3390/jcm11123489.
- Jacob GP, Oraif A, Power S. When helping hurts: the effect of surgical interventions on ovarian reserve. Hum Fertil (Camb). 2016;19(1):3–8. doi: 10.3109/14647273.2016.1148826.
- Du C, Wang Y, Qu D, et al. Magnetic resonance imaging T2WI hyperintense foci number and the prognosis of adenomyosis after high-intensity focused ultrasound treatment. Int J Gynaecol Obstet. 2021;154(2):241–247. doi: 10.1002/ijgo.13587.
- Li W, Mao J, Liu Y, et al. Clinical effectiveness and potential long term benefits of high-intensity focused ultrasound therapy for patients with adenomyosis. J Int Med Res. 2020;48(12):300060520976492. doi: 10.1177/0300060520976492.
- Jeng C, Ou K, Long C, et al. 500 Cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol. 2020;59(6):865–871. doi: 10.1016/j.tjog.2020.09.013.
- Lee J, Hong G, Lee K, et al. Safety and efficacy of ultrasound guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221. doi: 10.1016/j.ultrasmedbio.2019.08.022.
- Keserci B, Duc NM. The role of T1 perfusion-based classification in predicting the outcome of magnetic resonance-guided high intensity focused ultrasound treatment of adenomyosis. Int J Hyperthermia. 2018;34(3):306–314. doi: 10.1080/02656736.2017.1326634.
- Gong C, Wang Y, Lv F, et al. Evaluation of high intensity focused ultrasound treatment for different types of adenomyosis based on magnetic resonance imaging classification. Int J Hyperthermia. 2022;39(1):530–538. doi: 10.1080/02656736.2022.2052366.
- Parazzini F, Mais V, Cipriani S, et al. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):103–106. doi: 10.1016/j.ejogrb.2008.12.010.
- Huang YF, Deng J, Wei XL, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with high-intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia. 2020;37(1):301–307. doi: 10.1080/02656736.2020.1742390.
- Zhou CY, Xu XJ, He J. [Pregnancy outcomes and symptom improvement of patients with adenomyosis treated with high intensity focused ultrasound ablation]. Zhonghua Fu Chan Ke Za Zhi. 2016;51(11):845–849.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care. Fertil Steril. 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005.
- Rabinovici J, Inbar Y, Eylon SC, et al. Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis: a case report. Hum Reprod. 2006;21(5):1255–1259. doi: 10.1093/humrep/dei458.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714. doi: 10.1111/aogs.13159.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis. Medicine (Baltimore). 2016;95(3):e2443. doi: 10.1097/MD.0000000000002443.
- Khan KN, Fujishita A, Koshiba A, et al. Biological differences between intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod Biomed Online. 2019;39(2):343–353. doi: 10.1016/j.rbmo.2019.03.210.