Abstract
Objective
To evaluate the effect of HIFU (High-Intensity Focused Ultrasound) therapy on the survival and prognosis of patients with inoperable pancreatic cancer, and the clinical application of serological prognostic indicators.
Methods
We retrospectively analyzed the clinicopathological features, laboratory tests and follow-ups of 192 patients. Among the patients, 57 were treated with HIFU prior to chemotherapy (HIFU-priority), and 135 patients received chemotherapy followed by HIFU (HIFU-second). Univariate and multivariate Cox regression analysis was used to determine the prognostic value of tumor inflammation-related serological markers. A nomogram model was established based on the identified prognostic factors.
Results
Univariate analysis showed that receiving the treatment regimen in HIFU-priority was a significant protective factor for overall survival (OS, p < 0.001). Tumor stage, high C-reactive protein (CRP), high gamma-glutamyl transferase(γGT) high carbohydrate antigen 125 (CA125), high neutrophil-to-lymphocyte ratio (NLR), high lymphocyte-to-monocyte ratio (LMR) and liver metastasis were significant risk factors for poor prognosis (p < 0.05). CRP combined with normal tumor marker CA125 (CRP + CA125) was associated with longer OS (p = 0.005). Multivariate analysis shows that HIFU-priority is a protective factor for OS (Hazard Ratio, HR: 0.38; 95% confidence interval(CI): 0.25-0.57), tumor stage (HR: 1.61; 95% CI: 1.12-2.31), CRP + CA125 (HR: 1.46; 95% CI: 1.02-2.08) and γGT (HR: 1.44; 95% CI: 1.04-1.98) are risk factors for OS and serve as independent prognostic factors in the nomogram.
Conclusion
Early application of HIFU treatment improves the OS of patients with inoperable pancreatic cancer. CRP + CA125 and γGT are independent prognostic factors.
Introduction
Pancreatic cancer (PC) stands as a fatal malignancy within the gastrointestinal domain, characterized by a meager 5-year survival rate of 8% [Citation1].The lack of initial clinical indications, combined with swift spread to adjacent organs, contributes to prevalent diagnoses at advanced stages, accounting for 80-85% of cases, and aligns with a median survival duration of only 3-6 months [Citation2]. Surgical excision stands as the exclusive curative recourse, and progressions in supplementary chemotherapeutic modalities, namely FOLFIRINOX (comprising 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin), as well as the combination of gemcitabine and nab-paclitaxel, have yielded enhanced long-term outcomes and stands as the first-line treatment for patients with advanced PC [Citation3, Citation4]. Nevertheless, despite persistent endeavors to devise novel therapeutic approaches, overall survival for patients grappling with advanced disease and distant metastases has displayed limited enhancement throughout the preceding decade [Citation5]. Consequently, the identification of molecular markers with the capacity to predict prognosis and the tailoring optimal treatment strategies become of utmost significance in addressing the clinical requirements of individuals affected by advanced pancreatic cancer.
High-Intensity Focused Ultrasound (HIFU), an innovative noninvasive technique, has emerged as a promising modality for the treatment of inoperable PC [Citation6, Citation7]. HIFU harnesses focused ultrasonic energy to induce precise thermal ablation within tumor tissues, offering a potential avenue to alleviate symptoms, control tumor growth, and improve patient outcomes. Notably, HIFU serves as an innovative treatment option for local management of PC, demonstrating significant reductions in tumor-related pain and minimal notable side effects in various cases [Citation8–10]. Furthermore, emerging evidence suggests that HIFU ablation in combination with chemotherapy may yield enhanced clinical outcomes, further fueling interest in its potential synergy [Citation11].
Prognostic assessment holds the potential to optimize treatment strategies for patients afflicted by inoperable pancreatic cancer, particularly those in advanced stages, aiming to enhance their overall quality of life. Currently, several prognostic indicators encompassing histological grading, lymph node involvement, distant metastasis, inflammatory infiltration, and Carbohydrate antigen 19-9 (CA19-9) levels have been employed to prognosticate patient outcomes [Citation12–14]. However, the diverse nature of the disease and the limitations inherent to serum biomarkers has constrained the clinical utility of these predictive measures. Consequently, the need persists for a more precise and comprehensive evaluation framework that augments both sensitivity and specificity in prognostic assessment, thereby endowing it with heightened clinical relevance.
Within this context, the present retrospective investigation delves into the clinical efficacy of a sequential regimen comprising High-Intensity Focused Ultrasound (HIFU) in conjunction with standard chemotherapy for patients confronting inoperable pancreatic cancer. The findings underscore the significance of pretreatment monitoring encompassing C-reactive protein (CRP), carbohydrate antigen 125 (CA125), and gamma-glutamyl transferase (γGT), revealing their potential in assessing the survival prognosis of patients grappling with inoperable pancreatic cancer. Furthermore, through the establishment of a nomogram model, a refined and multifactorial clinical prognosis assessment system is proffered, furnishing a precise prognostic tool for this patient subset. This pivotal discovery not only furnishes a fresh foundation but also serves as a pivotal reference point for the advancement of clinical interventions in the realm of pancreatic cancer treatment.
Materials and methods
Patient enrollment and criteria
A total of 192 patients diagnosed with pancreatic adenocarcinoma received primary treatment at Fudan University Shanghai Cancer Center between January 2018 and October 2020 were included as the experimental cohort. For the validation cohort, 81 patients were collected from November 2020-Ferurary 2022 and were followed-up until August 2023. Patient inclusion criteria were as follows: (1) histopathologically confirmed pancreatic adenocarcinoma; (2) diagnosis of inoperable disease, encompassing stage IIb (determined as inoperable through surgical assessment), stage III, or stage IV tumor as per the 8th edition of the American Joint Committee on Cancer guidelines (Chicago, IL, USA), substantiated by radiological imaging modalities including contrast-enhanced abdominal CT scans, MRI, and/or magnetic resonance cholangiopancreatography (MRCP); (3) received systemic chemotherapy involving gemcitabine-based or 5-fluorouracil regimens; (4) absence of prior primary malignancies; and (5) a minimum follow-up duration exceeding 3 months. Exclusion criteria encompassed: (1) incomplete clinicopathological data; (2) follow-up duration of less than 3 months; and (3) acute inflammatory conditions, inclusive of disorders with potential to induce secondary diabetes such as hepatogenic diabetes, Cushing’s syndrome, glucagonoma, pheochromocytoma, hyperthyroidism, somatostatin, and other forms of diabetes, as well as hyperglycemia attributed to pharmaceutical agents.
Categorization was established based on the timing of thermal ablation in relation to standard chemotherapy. Specifically, thermal ablation performed prior to standard chemotherapy was designated as HIFU-priority group, while thermal ablation conducted subsequent to standard chemotherapy was designated as HIFU-second group. This categorization aimed to evaluate the influence of local thermal ablation on patient prognosis.
Collection of clinical data
Data for this study were extracted from electronic medical records at the Minimally Invasive Therapy Center and Integrative Oncology of Fudan University Shanghai Cancer Center, encompassing individuals who had previously undergone HIFU interventions. Prior to treatment, every patient provided written informed consent not only for the prescribed therapeutic procedure but also for potential research utilization of their medical records. Records pertaining to this investigation were meticulously preserved with utmost anonymity and confidentiality. The execution of all procedures adhered rigorously to the principles outlined in the Declaration of Helsinki and institutional protocols.
Relevant data were obtained from the institution’s electronic medical record database, encompassing patients’ fundamental particulars, tumor location, TNM staging, occurrences of jaundice and ascites, postoperative metastases and recurrences, and the presence or absence of liver impairment. Laboratory markers, including neutrophil count, lymphocyte count, monocyte count, platelet count, C-reactive protein (CRP), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 50 (CA50), carbohydrate antigen 242 (CA242), cancer antigen 125 (CA125), carcinoembryonic antigen (CEA), alanine transaminase (ALT), aspartate aminotransferase (AST), non-esterified fatty acid (NEFA), fibrinogen (FIB), and γ-glutamyl transferase (γGT), were systematically gathered. All laboratory indicators were routinely assessed preceding tumor diagnosis and any interventions.
Additionally, systemic inflammatory response parameters were calculated, comprising the systemic inflammatory response index (SIRI), calculated as the absolute product of neutrophil count and monocyte count divided by lymphocyte count [Citation12]. The ratios of neutrophils to lymphocytes (NLR), lymphocytes to monocytes (LMR), and platelets to lymphocytes (PLR) were also determined. Extraction of patient data encompassed a meticulous analysis of the clinical case system, wherein baseline clinical attributes, tumor particulars, treatment specifics, and medical records were collated via retrospective evaluation, subsequently undergoing comprehensive statistical analysis.
Principal instruments and apparatus
The HIFU treatment system utilized in this study was provided by Chongqing Haifu Technology Co., Ltd., China. A fasting duration of 12 h was observed by all patients prior to undergoing HIFU treatment. The HIFU treatment employed the subsequent parameters: 1) frequency: 1.04 MHz; 2) focal length: 151.0 mm; 3) diameter: 20 cm; 4) scanning technique: point-by-point method; 5) depth: the span from the tumor’s center to the skin on the ultrasound channel (ranging from 30 to 120 cm); 6) Target energy: 200-400 J per spot. Throughout the treatment procedure, real-time ultrasound-guided motion-integrated probe monitoring was employed to localize the pancreatic tumor, enabling segmentation of the tumor into successive slices spaced 5 mm apart. Vigilance over the patient’s blood pressure, pulse rate, respiratory rate, and peripheral oxygenation was upheld throughout the course of HIFU therapy.
Therapeutic protocols
The antecedent chemotherapy regimen involved the primary chemotherapy regimen featuring gemcitabine or fluorouracil. Irrespective of the combination treatment regimen for patients categorized under HIFU-priority or HIFU-second, the time gap between HIFU treatment and intravenous chemotherapy was maintained within a 7-day interval. Furthermore, a solitary HIFU treatment was conducted during the entirety of the follow-up period.
Follow-up procedures
The overall survival (OS) was delineated as the span between the date of pathologically confirmed diagnosis and either the date of decease or the date of the last follow-up. A consistent and systematic follow-up regimen was maintained, overseen by an independent investigator who conducted telephone interviews or reviewed medical records. During the initial year post-diagnosis, follow-up evaluations were performed at three-month intervals, subsequently extending to every six months. In cases where patients remained alive at the culmination of the last follow-up or had succumbed to causes unrelated to the condition, censoring was employed. The follow-up period was concluded on December 30, 2020.
Statistical analyses
All statistical assessments were carried out utilizing the Statistical Package for the Social Sciences Version 19.0 (SPSS, Inc.). Normally distributed continuous variables were depicted as mean ± standard deviation (SD) and underwent scrutiny using Student’s t-test. Categorical variables were represented as frequencies (%) and interrelations were appraised employing Pearson’s chi-square test. The overall survival (OS) timeframe spanned from the date of pathological diagnosis to either the date of mortality or the final follow-up. The Kaplan-Meier method was invoked to contrast OS across distinct patient groups, while the log-rank test was utilized to scrutinize the associations between prognostic factors and survival outcomes. Univariate and multivariate analyses were carried out through the Cox proportional hazards regression model to discern autonomous prognostic determinants for survival. Hazard ratios (HRs) calculated by the Cox regression model were disclosed as relative risks, accompanied by a corresponding 95% confidence interval (CI). Significance was considered when p < 0.05. The plotting of the nomogram plot analysis derived from the Cox regression model was executed using the statistical software package R4.1.2 and the nomogram of calibrated and validated using the validation cohort.
Results
Patients demographic profile
Among the cohort of 192 enrolled patients, the mean age was 63 years, comprising 105 males (54.7%) and 87 females (45.3%). As per the TNM staging system, 4 patients (2%) were categorized as stage IIb (unsuitable for surgical intervention), 52 patients (27.1%) were categorized as stage III, and 136 patients (70.8%) were classified as stage IV. Tumor localization exhibited 67 cases (34.9%) with masses situated in the pancreatic head and neck, 80 patients (41.7%) with masses in the pancreatic body, and 45 patients (23.4%) with masses in the pancreatic tail. Radical surgery was performed on only 10 patients (5.2%) out of the total, while the remaining 182 patients (94.8%) underwent palliative surgery, with 27 (14.1%) in the latter subgroup. Recurrence and metastasis were observed in 13 patients (6.8%) who had undergone radical or palliative surgery, with 49 patients (25.5%) experiencing liver metastasis. The summarization of patient characteristics in groups HIFU-priority and HIFU-second can be found in . No statistically significant discrepancies were detected in baseline clinical attributes between the two groups (p > 0.05).
Table 1. Clinical characteristics of the patients.
Independent prognostic factors for survival in patients with PC
In univariate analysis, the receipt of treatment under HIFU-priority demonstrated significant protective influence on overall survival (OS) (p < 0.001). Notable risk factors for OS encompassed tumor stage, elevated CRP, elevated γGT, elevated CA125, elevated NLR, elevated LMR, and the presence of liver metastasis (p < 0.05). The combination of normal CRP levels with the tumor marker CA125 exhibited an association with extended OS (p = 0.005). Parameters such as age, gender, jaundice, ALP, ALT, AST, LDH, and other blood biochemical indicators pertaining to liver function, along with CA50, CA242, and CEA, displayed no statistically significant disparities in patient OS. Multivariate analysis identified HIFU-priority treatment (HR: 0.38, 95% CI: 0.25-0.57, p < 0.001), TNM stage (HR: 1.61, 95% CI: 1.12-2.31, p < 0.05), CRP combined with CA125 (HR: 1.46, 95% CI: 1.02-2.08, p < 0.05), and γGT (HR: 1.44, 95% CI: 1.04-1.98, p < 0.05) as independent prognostic factors. Comprehensive findings of both univariate and multivariate analyses are presented in .
Table 2. Univariate and multivariate analyzes of overall survival in patients with pancreatic cancer.
Assessment of therapeutic efficacy through sequential HIFU and impact of serum markers on overall survival
The outcomes outlined in reveal that patients in HIFU-priority group exhibited a median survival period of 13.82 ± 13.50 months, while patients in HIFU-second group demonstrated a median survival period of 8.58 ± 3.01 months. Kaplan-Meier survival analysis exhibited a significantly prolonged median overall survival in HIFU-priority compared to HIFU-second group, with a statistically significant difference (p < 0.001, ). These findings suggest that treatment in HIFU-priority group may extend the overall survival of pancreatic cancer patients to a certain extent. Subsequently, a comprehensive analysis of the patients’ clinical characteristics and serological markers was conducted to identify potential prognostic predictors. Among these markers, blood biochemical indicators and serum tumor markers were evaluated based on clinically relevant ranges. For indices like SIRI, PLR, NLR, and LMR, ROC analysis was executed using X-Tile software to determine optimal cutoff values, followed by calculation of sensitivity and specificity. Subsequent Kaplan-Meier survival analysis was conducted utilizing these optimal cutoff values.
Figure 1. Kaplan-Meier survival analysis comparing the impact of HIFU treatment sequence, clinical stages, and serological markers on overall survival (OS).
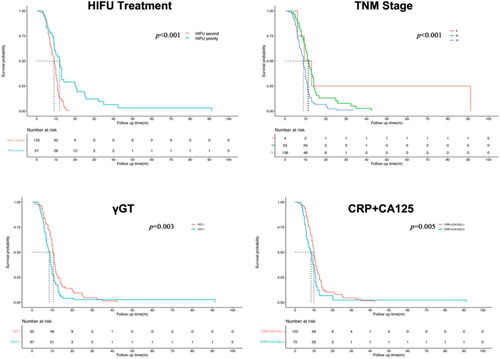
Results from ROC analysis indicated that the cutoff value for SIRI was 1.26 (AUC 0.569; sensitivity 58.7%; specificity 58.3%), PLR's cutoff value was 154.83 (AUC 0.594; sensitivity 49.1%; specificity 75.0%), LMR's cutoff value was 3.31 (AUC 0.582; sensitivity 49.1%; specificity 75.0%), and NLR's cutoff value was 0.28 (AUC 0.591; sensitivity 70.1%; specificity 50.0%). Kaplan-Meier survival analysis indicated statistically significant distinctions between elevated NLR, LMR, CRP combined with CA125, and γGT markers and poorer patient prognosis, with corresponding p-values of 0.023, 0.008, 0.005, and 0.004, respectively. The impact of CRP combined with CA125 and γGT changes on patient survival curves is visually depicted in .
Nomogram prognostic model for predicting OS in patients undergoing sequential HIFU treatment
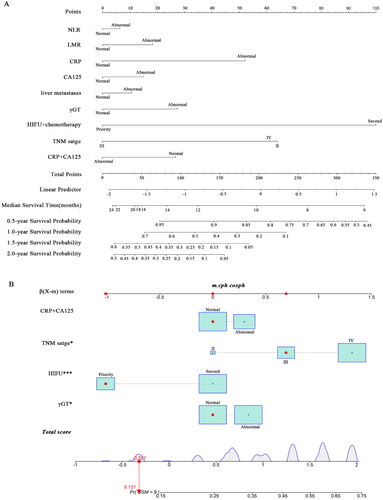
Drawing on the outcomes of multivariate analysis, a comprehensive nomogram model encompassing all the aforementioned independent prognostic factors was meticulously crafted to prognosticate the overall survival (OS) of patients. Within this nomogram framework, each autonomous prognostic determinant is assigned an appropriate scale and corresponding score. The cumulative summation of these scores is positioned along the lower total score scale bar and aligned with the median survival bar. This positioning designates the median OS and its associated survival probability. Elevated total scores correspond to reduced overall survival duration. Notably, the model discerns that HIFU-priority treatment exerts the most substantial impact on pancreatic cancer patient prognosis, followed by TNM stage, CRP, and γGT levels ().
Furthermore, a dynamic nomogram was devised to assess the likelihood of cumulative mortality at 9 months, effectively accounting for competing risks. This dynamic nomogram also traces the trajectory of pancreatic cancer patients displaying the longest survival span (). Furthermore, the nomograms were calibrated using the validation cohort of patients (n = 81), the baseline characteristics of the patients in the validation cohort in comparison to the experimental group (n = 192) is as summarized in Table S1 and the predicting curves are as summarized in Figure S1. By utilizing this model, patients’ baseline indices can be scored, facilitating the evaluation and prediction of their prognosis. Consequently, informed clinical recommendations can be offered, thereby furnishing a foundational framework for the discerning selection of appropriate treatment strategies.
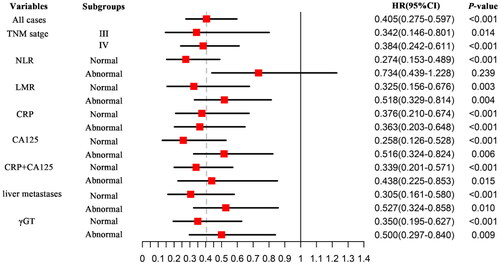
Risk stratification analysis of HIFU treatment on OS in patients with pancreatic cancer
Nomogram and Cox regression analysis showed that receiving regimen A was an independent protective factor for OS in patients with pancreatic cancer. The predictive value of the group HIFU-priority treatment regimen was independent of all other monitored clinical variables. Under the same conditions of other indicators, in patients with normal tumor marker CA125, the treatment effect of the HIFU-priority regimen was the best. Patients with pancreatic cancer with normal inflammation-related indicators such as NLR, LMR, and CRP who choose to receive regimen HIFU-priority can improve their survival and prognosis ().
Discussion
The investigation embarked on a comprehensive exploration of a sequential therapeutic paradigm, effectively amalgamating High-Intensity Focused Ultrasound (HIFU) and chemotherapy. In tandem, it strategically integrated inflammatory markers, tumor markers, and metabolic enzymes, all with the overarching goal of formulating a prognostic model tailored to predicting survival outcomes in individuals grappling with inoperable pancreatic cancer. The consequential revelation underscored a tangible elevation in overall survival rates, particularly when prioritizing the precedence of chemotherapy prior to subsequent HIFU administration, as opposed to the reversed sequence. Notably, distinct independent prognostic factors that contributed to survival gains were pinpointed, including the amalgamation of C-reactive protein (CRP) and carbohydrate antigen 125 (CA125), as well as the variances in gamma-glutamyl transferase (γGT) indicators. Building upon these intriguing insights, the research team successfully conceived a Nomogram model, spotlighting the optimal clinical efficacy of prioritizing HIFU treatment in cases of pancreatic cancer patients exhibiting normal CA125 tumor marker levels. Furthermore, the study shed light on the significance of embracing the treatment protocol in patients showcasing normative inflammation-associated markers like Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), and CRP, thereby further bolstering overall survival. Cumulatively, these compelling findings firmly reiterate the pivotal role of HIFU within the holistic landscape of pancreatic cancer management, particularly when dealing with inoperable cases. Notably, these conclusions harmoniously align with prior documented instances of clinical benefits post HIFU treatment at the treatment center affiliated with the research group, thereby effectively corroborating previously established insights [Citation15]. Beyond its conventional counterparts, this study also illuminated a noticeable variance in overall survival outcomes among inoperable pancreatic cancer patients, a disparity contingent upon the sequential administration of HIFU and chemotherapy. This intriguing observation beckons attention to the prospect that pre-chemotherapy thermal ablation might play an instrumental role in deactivating actively proliferating tumor tissue, subsequently potentiating the efficacy of subsequent chemotherapy interventions.
Pancreatic ductal adenocarcinoma, with its rapid progression, elevated metastatic recurrence rates, and swift acquisition of drug resistance, emerges as a formidable adversary in the realm of oncology. The stark reality lies in a meager 5-year survival rate of less than 5% [Citation16, Citation17]. The prevailing clinical landscape typically presents patients at advanced stages, be it locally advanced or brimming with distant metastases at the time of diagnosis. As a result, therapeutic interventions predominantly pivot around chemotherapy-based strategies, given the inherent limitations of surgical intervention. However, the intricate microenvironment that encapsulates pancreatic cancer, characterized by compromised blood supply and an abundance of matrix constituents, substantially compromises the efficacy of chemotherapy, thereby predisposing it to the emergence of drug resistance. This is where local thermal ablation has demonstrated its mettle, effectively reducing the tumor load within the coagulated and thermally compromised zone of the tumor tissue, known as the high-temperature region. Simultaneously, the peripheral zone surrounding the ablation site, known as the sub-high-temperature ring region, triggers a consequential inflammatory response that spurs vascular expansion, enhances vascular endothelial space, disrupts the integrity of tumor blood vessels, and augments microvessel permeability. Furthermore, the heightened temperature confers increased cell membrane permeability, effectively facilitating drug penetration and uptake, consequently amplifying chemotherapy’s therapeutic impact. This intricate interplay may well account for the clinical benefits observed when fuzing thermal ablation with chemotherapy [Citation18]. Adding to this, certain investigations have highlighted the intriguing concept of the "heating ratio," wherein the potency of most chemotherapy agents scales up within the temperature bracket of 40.5 to 43.0 °C. This intriguing phenomenon posits the notion that pairing chemotherapy subsequent to thermal ablation could potentially yield an intensified cytotoxic impact of chemotherapy agents [Citation19].
High-Intensity Focused Ultrasound (HIFU) emerges as a cutting-edge therapeutic approach, harnessing the prowess of ultrasonic energy directed externally onto internal tumor tissue. This orchestrated deployment culminates in the elevation of tissue temperatures beyond 65 °C in a mere span of 1 to 3 s, precisely within the treatment’s focal zone. The resultant temperature elevation prompts the coagulation and subsequent necrosis of tissue cells, all the while impeccably preserving the structural integrity of tissue outside the specifically targeted region [Citation20]. Beyond the rudimentary thermal effect, burgeoning insights underscore the multifaceted mechanism that underscores HIFU's therapeutic efficacy. This therapeutic mechanism draws upon the cavitation effect and mechanical impetus instilled by ultrasound waves, effectively culminating in the inactivation of tumors [Citation21]. In the contemporary clinical context, HIFU predominantly capitalizes on its thermal effect to orchestrate the annihilation of tumors. When compared to its thermal ablation counterparts such as radiofrequency, microwave, and irreversible electroporation, HIFU stands apart as a noninvasive strategy. Remarkably, it safeguards blood vessels with diameters exceeding 0.2 mm, rendering it a suitable intervention for individuals grappling with locally advanced and metastatic pancreatic cancer. Moreover, in instances where patients’ overall health precludes surgical options, HIFU holds the promise of localized pain relief, an improved quality of life, and crucially, avoidance of severe postoperative complications [Citation22, Citation23]. Notably, for palliative interventions involving voluminous tumors or multiple lesions, HIFU can be iteratively employed sans any uptick in the complication rate [Citation24].
Pancreatic cancer’s distinctive traits encompass its rapid progression, high vulnerability to metastatic resurgence, and accelerated acquisition of drug resistance. Notwithstanding the relentless advancements in pancreatic cancer research and therapeutics, the deficiency of robust biomarkers and optimal therapeutic strategies persistently dog the landscape of its diagnosis and management [Citation25, Citation26]. This underscores the significance of establishing a multifaceted evaluation framework and unearthing tumor markers that bear influence on cancer’s inception and progression. While CA19-9 has indeed carved a niche as a clinically valuable indicator for pancreatic cancer, its utility is marred by the specter of both false positives and negatives. These discrepancies, especially where negative CA19-9 values lead to delayed or missed diagnoses, underscore the need for alternatives [Citation27]. Enter CA125, which surfaces as a compelling candidate due to its direct involvement in pancreatic carcinogenesis, offering potential utility as both a diagnostic and prognostic biomarker for pancreatic cancer [Citation28–30]. In fact, researchers have unearthed its capacity to serve as a T cell activation-specific antigen and a target in pancreatic ductal adenocarcinoma, thereby potentially influencing immunotherapeutic paradigms [Citation31]. Moreover, the genesis and progression of pancreatic cancer are irrevocably intertwined with inflammatory mediators residing within the cancer’s microenvironment [Citation32, Citation33]. This has fostered the development of therapeutic strategies aimed at this complex milieu, underpinned by multimodal approaches and targets integrated with biological interventions [Citation34, Citation35]. Inflammatory molecules orchestrate the dynamics of tumorigenesis, involving pivotal proteins like NF-κB and STAT3 [Citation36, Citation37]. CRP, predominantly synthesized within the liver and galvanized by escalated cytokine-driven inflammation, effectively mirrors the ongoing inflammatory landscape and concurrent tissue damage through its serum values [Citation20, Citation38]. The synergistic amalgamation of CRP with CA125 effectively fuses inflammatory elements with immunogenic tumor markers, proffering a more comprehensive portrait of the serological shifts within the tumor’s microenvironment. Within the unique context of pancreatic cancer’s microenvironment, an array of immune cells perpetuate the inhibition of tumor immunity by impeding the activity of cytotoxic T lymphocytes, thereby propelling tumorigenesis [Citation21]. Notably, this study unearthed the heightened predictive potential of the CRP-CA125 combination in discerning subsets of pancreatic cancer patients poised for poorer prognosis.
Fine-needle aspiration (FNA), through-the-needle biopsy (TTNB), and Endoscopic Ultrasound (EUS) are established techniques for obtaining cytological and histological pathological results in the evaluation of pancreatic lesions [Citation39, Citation40]. However, it remains a critical question whether the integration of image-guided FNA and EUS-guided FNA can augment the diagnostic capabilities of high-intensity focused ultrasound (HIFU) in treating pancreatic cancer [Citation41]. Currently, there is a notable dearth of research specifically addressing this aspect, warranting comprehensive exploration. Such research endeavors hold the potential to enhance our ability to predict the effectiveness of HIFU therapy based on the imaging level and, in turn, improve the identification of suitable candidates for personalized HIFU treatment. This area thus merits further in-depth analysis to facilitate the prioritization of HIFU as a therapeutic modality in the clinical management of pancreatic cancer, ultimately leading to more tailored and effective treatment strategies for patients.
The confines of this retrospective study necessitate acknowledging its inherent limitations. Firstly, the single-center focus could potentially introduce selection bias into the patient cohort. Furthermore, the lack of extended follow-up, essential for the robust long-term clinical validation of the proposed model, raises concerns of potentially insufficient clinical validation data. Moreover, the study refrained from delving into a granular stratification of treatment cycles within the HIFU-second group of pancreatic cancer patients. This consideration becomes critical as it could tangibly impact the precision of the results. Addressing these limitations and bolstering the reliability of the findings necessitates an urgent shift toward prospective, multi-center, clinical randomized controlled studies that can independently corroborate and endorse the outcomes of this research endeavor.
Conclusion
In summary, the prioritized integration of HIFU treatment alongside standard chemotherapy offers a substantial extension of survival for individuals afflicted with inoperable pancreatic cancer. Furthermore, the pretreatment serum indicators CRP combined with CA125 stand out as independent prognostic determinants for the survival outcome in this patient group. The formulated Nomogram model demonstrates the ability to prognosticate the overall survival of inoperable pancreatic cancer patients with a commendable balance of sensitivity and specificity. Beyond the conventional staging framework, which incorporates factors of inflammation and immunogenic tumor markers, our nomogram represents a valuable instrument empowering clinicians to strategize treatment plans, foster personalized therapeutic approaches, and opt for optimal disease management strategies.
Ethical approval
This retrospective study was approved by the Ethics Committee of the Shanghai Cancer Center (approval no. 1612167-18) and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study involves subject-specific medical information obtained from hospitalized patients in the hospital after 2017. Patients are required to sign relevant documents upon admission, consenting to the use of their medical information during their hospital stay for scientific research purposes.
Author contribution
S.D: conceptualization, methodology, software, data curation, visualization, investigation, and writing – original draft preparation; A.Z.: data curation, methodology, software, and writing – original draft preparation; H.Z.: software, data curation, visualization, and investigation; K.W.: software, data curation, and visualization; C.C. and Z.M.: conceptualization, methodology, supervision, and writing – reviewing and editing.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data and material will be made available on request.
Additional information
Funding
Reference
- Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol. 2017;116(1):25–34. doi: 10.1002/jso.24623.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654.
- Hatashima A, Arango MJ, Reardon J, et al. First-line gemcitabine plus nab-paclitaxel versus FOLFIRINOX for metastatic pancreatic cancer in a real-world population. Future Oncol. 2022;18(20):2521–2532. doi: 10.2217/fon-2021-1367.
- Goral V. Pancreatic cancer: pathogenesis and diagnosis. Asian Pac J Cancer Prev. 2015;16(14):5619–5624. doi: 10.7314/apjcp.2015.16.14.5619.
- Adamska A, Domenichini A, Falasca M. Pancreatic ductal adenocarcinoma: current and evolving therapies. Int J Mol Sci. 2017;18(7):1338. doi: 10.3390/ijms18071338.
- Ning Z, Xie J, Chen Q, et al. HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients. Onco Targets Ther. 2019;12:1021–1029. doi: 10.2147/OTT.S185424.
- Wang K, Chen L, Meng Z, et al. High intensity focused ultrasound treatment for patients with advanced pancreatic cancer: a preliminary dosimetric analysis. Int J Hyperthermia. 2012;28(7):645–652. doi: 10.3109/02656736.2012.713541.
- Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced pancreatic cancer: high-Intensity focused ultrasound (HIFU) and other local ablative therapies. Rofo. 2019;191(3):216–227. doi: 10.1055/a-0820-5564.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2019;40(5):625–637. doi: 10.1055/a-0591-3386.
- Thudium M, Bette B, Tonguc T, et al. Multidisciplinary management and outcome in pancreatic cancer patients treated with high-intensity focused ultrasound. Int J Hyperthermia. 2020;37(1):456–462. doi: 10.1080/02656736.2020.1762006.
- Tao SF, Gu WH, Gu JC, et al. A retrospective case series of high-intensity focused ultrasound (HIFU) in combination with gemcitabine and oxaliplatin (gemox) on treating elderly middle and advanced pancreatic cancer. Onco Targets Ther. 2019;12:9735–9745. doi: 10.2147/OTT.S220299.
- Dell’Aquila E, Fulgenzi CAM, Minelli A, et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget. 2020;11(10):924–941. doi: 10.18632/oncotarget.27518.
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105–119.
- Luchini C, Brosens LAA, Wood LD, et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut. 2021;70(1):148–156. doi: 10.1136/gutjnl-2020-320726.
- Ning ZY, Cheng CS, Xie J, et al. A retrospective analysis of survival factors of high intensity focused ultrasound (HIFU) treatment for unresectable pancreatic cancer. Discov Med. 2016;21(118):435–445.
- Ishido K, Hakamada K, Kimura N, et al. Essential updates 2018/2019: current topics in the surgical treatment of pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2021;5(1):7–23. doi: 10.1002/ags3.12379.
- Satoi S. Surgical treatment of pancreatic ductal adenocarcinoma. Cancers (Basel). 2021;13(16):4015. doi: 10.3390/cancers13164015.
- Friedl J, Turner E, Alexander HR. Jr. Augmentation of endothelial cell monolayer permeability by hyperthermia but not tumor necrosis factor: evidence for disruption of vascular integrity via VE-cadherin down-regulation. Int J Oncol. 2003;23(3):611–616.
- Issels RD. Regional hyperthermia in high-risk soft tissue sarcomas. Curr Opin Oncol. 2008;20(4):438–443. doi: 10.1097/CCO.0b013e3283025e50.
- Salmiheimo A, Mustonen H, Stenman UH, et al. Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS One. 2016;11(9):e0163064. doi: 10.1371/journal.pone.0163064.
- Takahashi R, Macchini M, Sunagawa M, et al. Interleukin-1beta-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut. 2021;70(2):330–341.
- Marinova M, Rauch M, Mucke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26(11):4047–4056. doi: 10.1007/s00330-016-4239-0.
- Strunk HM, Lutzow C, Henseler J, et al. Mesenteric vessel patency following HIFU therapy in patients with locally invasive pancreatic cancer. Ultraschall Med. 2018;39(6):650–658. doi: 10.1055/s-0043-125391.
- Yuan Y, Shen H, Hu XY, et al. Multidisciplinary treatment with chemotherapy, targeted drug, and high-intensity focused ultrasound in advanced pancreatic carcinoma. Med Oncol. 2012;29(2):957–961. doi: 10.1007/s12032-011-9892-1.
- Zhong A, Cheng CS, Kai J, et al. Clinical significance of glucose to lymphocyte ratio (GLR) as a prognostic marker for patients with pancreatic cancer. Front Oncol. 2020;10:520330. doi: 10.3389/fonc.2020.520330.
- Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156(7):2024–2040. doi: 10.1053/j.gastro.2019.01.259.
- Guo M, Luo G, Lu R, et al. Distribution of lewis and secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS Open Bio. 2017;7(11):1660–1671. doi: 10.1002/2211-5463.12278.
- Jiang K, Tan E, Sayegh Z, et al. Cancer antigen 125 (CA125, MUC16) protein expression in the diagnosis and progression of pancreatic ductal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2017;25(9):620–623. doi: 10.1097/PAI.0000000000000368.
- Luo G, Liu C, Guo M, et al. Potential biomarkers in lewis negative patients with pancreatic cancer. Ann Surg. 2017;265(4):800–805. doi: 10.1097/SLA.0000000000001741.
- Luo G, Xiao Z, Long J, et al. CA125 is superior to CA19-9 in predicting the resectability of pancreatic cancer. J Gastrointest Surg. 2013;17(12):2092–2098. doi: 10.1007/s11605-013-2389-9.
- Balachandran VP, Łuksza M, Zhao JN, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551(7681):512–516. doi: 10.1038/nature24462.
- Farrow B, Sugiyama Y, Chen A, et al. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239(6):763–771. doi: 10.1097/01.sla.0000128681.76786.07.
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611.
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi: 10.1038/s41571-020-0363-5.
- Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. doi: 10.1186/s12943-018-0858-1.
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014.
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921.
- Facciorusso A, Bajwa HS, Menon K, et al. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: a meta-analysis. Endosc Ultrasound. 2020;9(3):167–174. doi: 10.4103/eus.eus_4_19.
- Facciorusso A, Kovacevic B, Yang D, et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: a recursive partitioning analysis. Endoscopy. 2022;54(12):1158–1168. doi: 10.1055/a-1831-5385.
- Lisotti A, Napoleon B, Facciorusso A, et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: systematic review and meta-analysis. Gastrointest Endosc. 2021;94(5):881–889.e5. doi: 10.1016/j.gie.2021.06.028.