?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The study aimed to compare the effectiveness and safety of ultrasound-guided microwave ablation (MWA) and percutaneous sclerotherapy (PS) for the treatment of large hepatic hemangioma (LHH).
Methods
This retrospective study included 96 patients who underwent MWA (n = 54) and PS (n = 42) as first-line treatment for LHH in three tertiary hospitals from January 2016 to December 2021. Primary outcomes were technique efficacy rate (volume reduction rate [VRR] > 50% at 12 months), symptom relief rate at 12 months and local tumor progression (LTP). Secondary outcomes included procedure time, major complications, treatment sessions, cost and one-, two-, three-year VRR.
Results
During a median follow-up of 36 months, the MWA group showed a higher technique efficacy rate (100% vs. 90.4%, p = .018) and symptom relief rate (100% vs. 80%, p = .123) than the PS group. The MWA group had fewer treatment sessions, higher one-, two- and three-year VRR, lower LTP rate (all p < .05), longer procedure time and higher treatment costs than the PS group (both p < .001). MWA shared a comparable major complications rate (1.8% vs. 2.4%, p = .432) with PS. After multivariate analysis, the lesion’s heterogeneity and maximum diameter >8.1 cm were independent risk factors for LTP (all p < .05). In the PS group, lesions with a cumulative dose of bleomycin > 0.115 mg/cm3 had a lower risk of LTP (p = .006).
Conclusions
Both MWA and PS treatments for large hepatic hemangioma are safe and effective, with MWA being superior in terms of efficacy.
Introduction
Hepatic hemangioma is the most common benign liver lesion, accounting for up to 70% of all benign liver lesions, with a prevalence of 0.4–20% in the population [Citation1–5]. The majority of hemangiomas are asymptomatic and remain stable over time, therefore surgical intervention is not usually needed, and the conservative approach is advocated [Citation1,Citation2]. However, those with a diameter larger than 5 cm, also known as large hepatic hemangioma (LHH), accompanied by continuous growth or distinct symptoms, necessitate active invasive management [Citation1,Citation2,Citation6].
Invasive management of symptomatic LHH primarily involves surgical resection (SR) and interventional radiology (IR) [Citation7]. Despite the well-established efficacy of SR as a conventional treatment method, it is associated with normal liver parenchyma loss and a high risk of complications, particularly severe bleeding [Citation1–3,Citation7–10].
Over the past decade, IR has emerged as a promising alternative to SR for the treatment of LHHs. This approach includes trans-arterial embolization (TAE), image-guided percutaneous thermal ablation and percutaneous sclerotherapy (PS) [Citation7]. However, TAE treatment is not considered curative for hemangiomas, as recurrence is common due to vascular recanalization and may result in severe complications such as ectopic embolization and sclerosing cholangitis [Citation6,Citation11–13]. Thermal ablation has gained popularity as a local treatment for liver tumors [Citation14,Citation15]. The primary ablation techniques are radiofrequency ablation (RFA) and microwave ablation (MWA). MWA is deemed more effective than RFA in treating LHHs because it creates a precise ablation boundary, is less limited by the heat sink and induces a faster temperature rise. Percutaneous injection of bleomycin is confirmed as the standard treatment for subcutaneous low-flow vascular malformations [Citation16]. Although PS has only been reported in a limited number of studies for the treatment of LHHs [Citation6,Citation17], it is theoretically less invasive than ablation. Nonetheless, no studies have compared the specific advantages and disadvantages of image-guided percutaneous microwave ablation and PS with bleomycin.
Our study aims to compare the effectiveness and safety of ultrasound (US)-guided percutaneous MWA versus PS with bleomycin for treating LHHs based on three-center data.
Materials and methods
Patients
This retrospective cohort study involved the utilization of electronic clinical record systems to gather data on all consecutive patients who underwent US-guided PMWA or PS as first-line treatments in three tertiary hospitals in China from January 2016 to December 2021. The study protocol was approved by the ethics committee of each center (registered as a multicenter cohort study, numbered: S2019-348-01); and adhered to the principles outlined in the 1975 Helsinki Declaration. Due to the retrospective nature of the study, patients’ informed consent was waived.
The diagnosis of LHH was based on contrast-enhanced examinations (contrast-enhanced ultrasonography [CEUS], CT, or MRI) showing typical peripheral nodular enhancement followed by centripetal filling in the arterial phase and hyperenhancement in the delay phase, with at least one diameter larger than 5 cm.
The inclusion criteria for the study were: (a) a definite diagnosis of hepatic hemangioma, with a maximum diameter larger than 5 cm, (b) patients displaying possible hemangioma-related symptoms (pain or fullness in the liver area) or progressive enlargement of the hemangioma (enlarged more than 0.5 cm within one year), (c) patients classified as Child-Pugh class A in terms of liver function status and (d) patients who declined surgical resection.
Patients with any of the following characteristics were excluded from the study: (a) previous acceptance of other treatments for LHH; (b) presence of any other possible causes of symptoms; (c) pulmonary fibrosis detected in preprocedural CT scans and (d) irregular follow-up.
A total of 108 patients met the inclusion criteria, of whom those who received TAE treatment before or had irregular follow-up were excluded. Finally, 96 patients were included in the data analysis, comprising 54 patients who underwent PMWA and 42 patients who underwent PS ().
Treatment procedures
All PMWA and PS procedures were performed under ultrasound guidance using either the Philips iU22 Ultrasound System (Philips Healthcare), Sequoia 512 (Siemens Medical), or Mindray M9 System (Mindray Medical International). The procedures were conducted by a pool of eight radiologists (L.P., Y.X.L., Y.J., C.Z.G., H.Z.Y, Z.Q.Y, Q.T.G., F.S.Y.) from the three centers, all of whom had over 10 years of experience with interventional ultrasound.
The pre-procedure evaluations, procedures and follow-up assessments were carried out utilizing 3.5–10 MHz convex array probes with the aforementioned US systems.
US-guided percutaneous microwave ablation
The MWA procedure was performed in the operating room, with the patient under general anesthesia. The KY-2000 2450 MHz microwave system, which was equipped with two 15-gauge cooled-tip antennae manufactured by Kangyou Medical, was used for the ablation.
During the ablation, the feeding artery of the LHH, confirmed by CEUS, was ablated first to potentially reduce the heat-sink effect in MWA. Next, an 18 G PTC needle (Leapmed Healthcare Corporation, Suzhou, China) was inserted into the LHH to aspirate blood from the hemangioma. Two microwave antennae were subsequently inserted into the deep side of the lesion to generate multiple overlapping ablation zones from deep to shallow until the LHH was replaced by a transient hyperechoic area. When withdrawing the antennae, the needle tracks were routinely cauterized to avoid bleeding. Hydro-dissection technique was utilized to prevent damage to vital structures outside the liver when ablating hemangiomas located in the left hepatic lobe, such as the gastrointestinal tract and pericardium. Additionally, thermal monitoring technique was employed to safeguard crucial structures within the liver. Detailed procedures are provided in Supplementary Appendix S1–S2.
Contrast-enhanced CT was performed one to three days after ablation to evaluate the gross extent of coagulation necrosis. The technical success of MWA was defined as the lesion being treated according to the protocol and the diameter of residual tissue adjacent to dangerous structures being less than 1 cm on contrast-enhanced CT.
US-guided percutaneous sclerotherapy
The PS procedure was performed in the operating room with patients under conscious sedation and local anesthesia. To prepare the injectable solution, 15 mg of bleomycin (Bleomycin Hydrochloride for Injection; Hisun Pfizer Pharmaceuticals Co.) was diluted in 5 ml of saline solution.
21 G PTC needle (Leapmed Healthcare Corporation, Suzhou, China) guided by ultrasound was inserted into the deep side of the lesion through 2–3 cm of normal liver parenchyma. The bleomycin solution was then injected gradually within the lesion at multiple points, starting from deep to shallow. The dosage of the bleomycin solution was adjusted based on the size of the lesion. During the initial sclerotherapy session, lesions with a maximum diameter of 5–10 cm received an injection of 5 ml of the bleomycin solution (containing 15 mg of bleomycin), while lesions with a maximum diameter of 10–15 cm received 10 ml of the bleomycin solution (containing 30 mg of bleomycin). Manual compression was applied at the injection site for 5 min at the end of the procedure. For subsequent sclerotherapy sessions, regardless of size, all lesions were injected with 5 ml of the bleomycin solution (containing 15 mg of bleomycin).
In the PS group, technical success was defined as the successful injection of the bleomycin solution after confirming the absence of a connection with the vascular or biliary system.
In both the MWA and PS groups, during a single treatment session, patients with multiple lesions larger than 5 cm were simultaneously treated. This simultaneous treatment approach aimed to reduce treatment and hospitalization time while ensuring comprehensive treatment of each lesion larger than 5 cm. Repeat treatment in both groups was decided based on changes in the lesion’s volume and improvements in the patient’s symptoms during follow-up.
Follow-up
The US presentations were recorded and evaluated at 3, 6 and 12 months after treatment and 12-month intervals thereafter.
For each lesion, the maximal diameter (a) and two orthogonal diameters (b and c) were recorded on the US examination. The lesion volume was estimated using the ellipsoid formulation: The volume reduction ratio (VRR) was formulated as follows:
Study outcomes
The primary outcomes of the study included technique efficacy, symptom relief at 12 months and local tumor progression (LTP). For both treatment groups, technique efficacy was defined as a lesion VRR greater than 50% at 12 months. LTP was defined as the appearance of tumor foci at the periphery of the ablation zone [Citation18]. Due to the benign nature of LHHs, in our study, we confirmed LTP by employing CEUS to detect tumor foci with blood supply and by observing a ≥ 20% increase in the maximum diameter of lesions when compared to the smallest post-treatment lesion size as the reference point. Symptom relief was also evaluated as a primary outcome, as it was one of the intended goals of the treatment. To evaluate symptom relief outcomes, symptoms of abdominal pain or fullness were evaluated 12 months after treatment.
The secondary outcomes included procedure time, major complications, treatment sessions, cost and one-, two-, three-year VRR. According to the reporting standards of the Society of Interventional Radiology [Citation19], a major complication is defined as one of the following classes: class C—requires therapy and involves minor hospitalization (<48 h); class D—requires major therapy, results in an unplanned increase in the level of care and leads to prolonged hospitalization (>48 h); class E—results in permanent adverse sequelae; class F—results in death. The procedure time was defined as from the beginning of skin disinfection until the patient left the operating room. Treatment sessions were recorded to assess the frequency of treatment required for each patient. The costs included the preoperative examination, treatment procedure, anesthesia fees and hospital bed, nursing and postoperative medication fees if needed. Considering that some patients in the PS group had multiple sessions of treatment, the cost was calculated as the sum of multiple treatments.
Statistical analysis
Data analysis was performed using statistical software (SPSS 25 for Windows; IBM, Armonk, New York), EmpowerStats software (www.empowerstats.com, X&Y Solutions, Inc.) and GraphPad Prism 9 (GraphPad Software Inc.). Continuous variables were presented as mean ± standard deviation (SD) or median (25%–75% interquartile range [IQR]), while categorical variables were expressed as frequency (percentage). The t-test or Wilcoxon rank-sum test was used for continuous variables, and the chi-squared test or Fisher’s exact test was used for categorical variables. Technique efficacy, symptom relief, treatment sessions, procedure time, complications and costs were calculated based on the number of patients, while the lesion size and volume changes as well as LTP were calculated based on the number of lesions.
To analyze the changes in lesion size and volume within 12 months, a generalized estimating equation model for repeated measurements was used. A logistic regression model was employed to evaluate the associations between multiple clinical variables and LTP. Variables with a p value <.1 in univariable analyses were included in the multivariable models. Smooth curve fitting and threshold-effect analysis were utilized to determine the maximum simulated likelihood of maximum diameter on LTP. The cutoff value of the cumulative dose of bleomycin in relation to LTP in the PS group was identified using the receiver operating characteristic (ROC) curve. All tests were two-sided, and statistical significance was defined as p < .05.
Results
Patients and lesion characteristics
The baseline characteristics of the patients and lesions are presented in . The maximum diameter and median volume of lesions in the MWA group were compared with those in the PS group, resulting in 7.8 ± 1.9 cm versus 7.6 ± 2.1 cm (p = .586) and 176.6 (94.4–233.5) cm3 versus 173.3 (68.6–174.8) cm3 (p = .328), respectively. There were no significant differences observed in any of the baseline parameters, including age, gender, lesion number, location, echogenicity, shape, homogeneity, vascularity and reasons for treatment between the two groups.
Table 1. Baseline demographics and lesions’ characteristics.
Treatment parameters and complications
The technical success rates for both the MWA and PS groups were 100%. Treatment parameters and complications are presented in . In the MWA group, the mean ablation time was 2000 ± 820 s, and the mean ablation energy was 113.8 ± 52.5 kJ. All lesions in the MWA group were treated in a single session, whereas the PS group required a mean of 1.37 ± 0.69 sessions (p < .0001). Compared to the MWA group, the PS group required more treatment sessions, had shorter procedure times and incurred lower treatment costs (all p < .001) ().
Table 2. Treatment variables and complications of MWA and PS groups.
In the MWA group, one patient experienced a class D complication, namely, acute kidney injury (AKI). Laboratory results showed creatinine of 353 mmol/L and urea of 15.1 mmol/L on the third day after ablation. After undergoing hemodialysis, the patient’s renal function gradually recovered, and dialysis was discontinued on the 12th day. In the PS group, intraperitoneal hemorrhage (class C) was observed in one patient. The patient experienced significant abdominal pain immediately after the procedure. Hemoglobin levels decreased from 170 g/L to 137 g/L within 24 h, and 5.5 cm of free liquid was detected in the abdominal cavity via ultrasound. The patient was discharged on the fourth day after receiving medical treatment.
Follow-up outcomes
The median follow-up time for the MWA and PS groups was 36 (IQR 27–48) months and 36 (IQR 24–48) months, respectively (p = .864).
Technique efficacy and symptom relief
The technique efficacy rate of MWA for LHHs was higher than that of PS (100% vs 90.4%, p = .018) () (). The initial maximal diameters of the four failed lesions in the PS group were 14.4, 12.7, 9.8 and 8.1 cm; and the VRRs at 12 months were −18.5%, −4.2%, 49.8% and 9.5%, respectively. All patients in the MWA group experienced an improvement in their symptoms at 12 months; 16 of 19 (84.2%) had complete relief and 3 (15.8%) had partial relief. And in the PS group, 9 of 15 (60.0%) patients had complete relief, 3 of 15 (20%) had partial relief and 3 (20%) patients with persistent symptoms. Although there was a variation in the rate of symptom relief between the two groups (100% vs 80.0%), the difference did not reach statistical significance (p = .123) ().
Figure 2. Ultrasonographic images in a 41-year-old man with LHH received single-session percutaneous microwave ablation. (A) A homogeneous hyperechoic lesion with an initial size of 8.8 cm × 7.6cm × 6.7 cm was detected in the left lobe of the liver. (B) After MWA, no enhancement was observed in the lesion area on CEUS. (C) Size of the ablation area was 3.5 cm × 3.2 cm ×2.9 cm at 12 months after treatment.
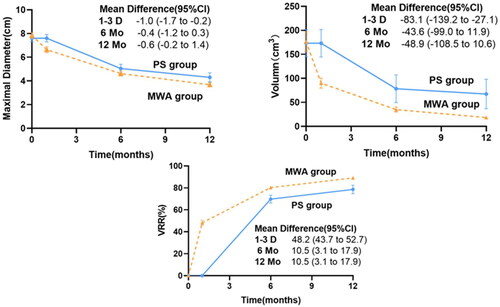
Figure 3. Ultrasonographic images of a 38-year-old female patient with large hepatic hemangioma who received single-session percutaneous sclerotherapy with 15 mg bleomycin. (A) A homogeneous hyperechoic lesion with an initial size of 6.0 cm × 4.3 cm × 3.5 cm was detected in the right lobe of the liver. (B) Size of the sclerotherapy area was 3.6 cm × 2.6 cm × 1.7 cm at 12 months after treatment.
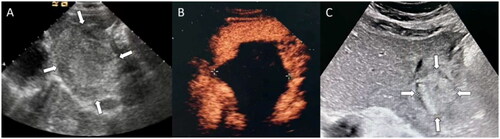
Figure 4. Ultrasonographic images of a 39-year-old male patient with large hepatic hemangioma who received two sessions of percutaneous sclerotherapy with cumulative 30 mg bleomycin. (A) A inhomogeneous hyperechoic lesion with an initial size of 12.7 cm × 10.8 cm × 10 cm was detected in the right lobe of the liver. (B) Size of the sclerotherapy area was 13 cm × 11.7 cm × 9.4 cm at 12 months after treatment.
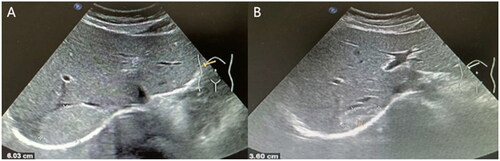
Table 3. The primary outcomes of MWA and PS groups.
Lesion size and volume change
Changes in mean maximal diameter, volume and VRR of therapy area at 1–3 days, 6 months and 12 months after MWA or PS were shown in . At the follow-up of 6 and 12 months, the average maximum diameter and volume of the two groups decreased significantly and the VRR in both groups increased significantly. The difference in VRR between the two groups was statistically significant (p = .000) () (). Compared with PS, MWA also achieved higher mean VRR at 24 months (91.6% vs 81.1%, p = .001) and VRR at 36 months (92.4% vs 80.6%, p < .0001).
Figure 5. Lesions’ changes of maximal diameter, volume and VRR at each follow-up through 12 months. At baseline, the data points represent the observed means in the MWA group and the PS group, whereas the data points on the plot lines represent the estimated means based on a generalized estimating equations model after adjustment for the baseline value. The I bars denote 95% confidence intervals. (A) Trend of adjusted mean maximal diameter in the MWA and PS groups. (B) Trend of adjusted mean volume in the two groups. (C) Trend of adjusted mean VRR in the two groups. VRR: volume reduction ratio; CI: confidence intervals.
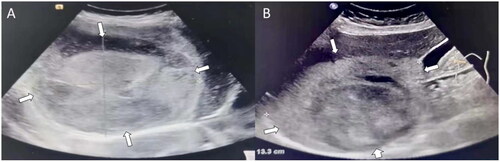
Table 4. Changes in the maximal diameter, volume and VRR of the lesions after treatment at each follow-up through 12 months of MWA and PS groups.
Local tumor progression
During the follow-up period, six lesions in the PS group developed LTP within a median time of 36 months (IQR, 9.75–48 months), while only one lesion in the MWA group developed LTP at the 24th month after treatment (14.3% vs 1.9%, p = .075) (). We used smooth curve fitting and threshold effect analysis to find out the maximum simulated likelihood value of the maximum diameter (8.1 cm) on LTP (Figure S1 and Table S1) and included it in the univariate risk factor analysis. After univariate risk factor analysis, homogeneity (p = .005), maximal diameter (p = .013) and treatment group (p = .053) were included in the multivariable analysis of risk factors. The risk of LTP significantly increases when the maximum diameter is larger than 8.1 cm (OR:19.14; 95%CI: 2.19 − 166.96, p = .007) (). Since the dose of drug injection differs in different lesions in the PS group, we further analyzed whether the cumulative dose of bleomycin of lesions (mg/cm3) was related to the LTP. The ROC curve is shown in Figure S2. LHHs with a cumulative dose of bleomycin of lesions > 0.115 mg/cm3 had a lower risk of LTP (OR, 0.1; 95%CI: 0.0, 0.5, p = .006).
Table 5. Univariable and multivariable logistic regression analysis of risk factors for local tumor progression.
Discussion
Guidelines for the treatment of hepatic hemangiomas recommend individualized treatment plans based on the principles of minimizing trauma and achieving the most satisfactory treatment outcome. Surgical resection is considered the most effective treatment for LHHs, but it entails the loss of hepatic parenchyma and carries a high risk of intraoperative and postoperative bleeding [Citation1–3]. Two emerging minimally invasive therapeutic modalities, namely, US-guided microwave ablation (MWA) and percutaneous sclerotherapy (PS), have gained attention [Citation7]. While PS with intralesional bleomycin injection has been established as the standard treatment for low-flow venous malformations [Citation16, Citation20,Citation21], the effect of PS with bleomycin alone on liver hemangiomas (LHHs) remains unknown. Additionally, there is a lack of data providing a direct comparison between MWA and PS in treating LHHs.
Our results suggest that both MWA and PS can be considered as minimally invasive treatment options for LHHs, offering effective and safe outcomes, and each has advantages and drawbacks.
The primary challenge associated with the use of MWA and PS treatment for LHHs was ensuring their safety. In the MWA group, one case (2.5%) experienced acute kidney injury (AKI), a major complication, shortly after the procedure. This could potentially be attributed to heat-induced intravascular hemolysis. To mitigate the risk of AKI, we refined our technique by administering intraoperative quick infusion, followed by intravenous use of sodium bicarbonate solution and furosemide. Subsequent patients did not encounter AKI. Other studies on MWA treatment have reported similar or lower incidences of acute renal injury [Citation11,Citation12]. In the PS group, one case (3.3%) exhibited intraperitoneal hemorrhage, another major complication, which was managed conservatively. Besides, bleomycin as an anti-tumor agent is associated with the risk of pulmonary fibrosis when administered in large cumulative doses (>400 mg during the lifetime or a single 30-mg dose) [Citation22]. In our study, the cumulative bleomycin dose for LHH patients was 15–75 mg, and the single-session dose was ≤30 mg. All patients in the PS group underwent a chest CT examination 12 months after treatment, and no pulmonary fibrosis was observed. A meta-analysis [Citation23] of venous malformations treated with bleomycin also showed that intralesional bleomycin injections did not increase the risk of pulmonary fibrosis, indicating the safety of injecting small doses of bleomycin into hepatic hemangiomas.
Secondary, the two treatment methods have different mechanisms. MWA directly cuts off the blood supply by damaging the blood vessels and fibrous tissue in the hemangioma. On the other hand, PS with bleomycin causes long-term damage to the blood vessels, leading to tissue ischemia and necrosis. To compare their effectiveness, we looked at symptom relief and volume reduction after 12 months and LTP in the long term. At the 12-month follow-up, PS showed lower effectiveness, with fewer symptoms relieved and a higher rate of LTP compared to MWA. In the PS group, the average volume reduction rate after 1.37 ± 0.69 PS sessions with bleomycin was 78.6%. This is similar to a study by Yazdi et al. where they achieved a mean volume reduction rate of 76.0% after 12 months for LHH patients using single-session PS with bleomycin and ethiodized oil [Citation6].
Our study also found that LHHs treated with cumulative bleomycin doses less than 0.115 mg/cm3 had a higher risk of LTP. Therefore, multiple PS sessions with sufficient medication were necessary for effective treatment of LHHs.
Trans-arterial embolization (TAE) is another interventional radiology technique used in the treatment of LHHs. In a recent review article [Citation24], four studies conducted volume analyses after treating LHHs with bleomycin and ethiodized oil through arterial embolization. The results showed an average volume reduction rate ranging from 48.0% to 77.6%. Among these four studies, 13% to 32% of patients required a second embolization due to persistent symptoms or insufficient reduction in lesion size. Major complications (Grade C-D) were reported in three of the studies, including a decrease in hemoglobin levels, ischemic cholecystitis and post-embolization syndrome, with an incidence rate of 3.8% to 12%. In our current study, we did not encounter complications similar to those associated with TAE. However, the volume reduction rate and the need for multiple treatments in the PS group were comparable to those observed with TAE. Both treatment approaches have their benefits. PS treatment is performed under local anesthesia and does not require hospitalization, in contrast to the five-day median hospitalization period [IQR, five to seven days] for MWA and can be conducted at a lower cost, which are its inevitable advantages. However, multiple sessions are required to ensure an appropriate treatment effect. In this regard, the technical effectiveness of single-session MWA is significant, as it can reduce the cost of multiple treatment sessions and the psychological pressure associated with repeated monitoring.
Our study had several limitations. First, although the baseline demographic and lesion characteristics of the groups were comparable, the selection bias caused by the relatively small sample size may have influenced the research findings. Second, because of the short follow-up period, the observation of LTP may have been underestimated, especially for the PS group, and the degree of LTP was worth clarifying. More patients with longer follow-up will be necessary to validate whether these findings are durable. Furthermore, prospective studies are required to confirm the findings of this study.
In conclusion, as minimally invasive techniques, US-guided MWA and PS are optional modalities that can yield safe results for the treatment of LHH. MWA shows better treatment effectiveness with fewer treatment sessions.
Supplementary Material
Download PDF (754 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author Jie Yu upon reasonable request. Due to their containing information that could compromise the privacy of research participants, the data are not publicly available.
Additional information
Funding
References
- Belghiti J, Cauchy F, Paradis V, et al. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014;11(12):737–749. doi: 10.1038/nrgastro.2014.151.
- European Association for the Study of the Liver (EASL). EASL clinical practice guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386–398. doi: 10.1016/j.jhep.2016.04.001.
- Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109(9):1328–1347; quiz 1348. doi: 10.1038/ajg.2014.213.
- Pompili M, Ardito F, Brunetti E, et al. Benign liver lesions 2022: guideline for clinical practice of associazione italiana studio del fegato (AISF), società italiana di radiologia medica e interventistica (SIRM), società italiana di chirurgia (SIC), società italiana di ultrasonologia in medicina e biologia (SIUMB), associazione italiana di chirurgia Epatobilio-Pancreatica (AICEP), società italiana trapianti d‘Organo (SITO), società italiana di anatomia patologica e citologia diagnostica (SIAPEC-IAP) - Part II - solid lesions. Dig Liver Dis. 2022;54(12):1614–1622. doi: 10.1016/j.dld.2022.08.031.
- Li X, An C, Liu F, et al. The value of 3d visualization operative planning system in ultrasound-guided percutaneous microwave ablation for large hepatic hemangiomas: a clinical comparative study. BMC Cancer. 2019;19(1):550. doi: 10.1186/s12885-019-5682-5.
- Ayoobi Yazdi N, Mehrabinejad M-M, Dashti H, et al. Percutaneous sclerotherapy with bleomycin and ethiodized oil: a promising treatment in symptomatic giant liver hemangioma. Radiology. 2021;301(2):464–471. doi: 10.1148/radiol.2021204444.
- Dong W, Qiu B, Xu H, et al. Invasive management of symptomatic hepatic hemangioma. Eur J Gastroenterol Hepatol. 2019;31(9):1079–1084. doi: 10.1097/MEG.0000000000001413.
- Giuliante F, Ardito F, Vellone M, et al. Reappraisal of surgical indications and approach for liver hemangioma: single-center experience on 74 patients. Am J Surg. 2011;201(6):741–748. doi: 10.1016/j.amjsurg.2010.03.007.
- Miura JT, Amini A, Schmocker R, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB. 2014;16(10):924–928. doi: 10.1111/hpb.12291.
- Clarke DL, Currie EJ, Madhavan KK, et al. Hepatic resection for benign non-cystic liver lesions. HPB. 2004;6(2):115–119. doi: 10.1080/13651820410026326.
- Shi Y, Song J, Ding M, et al. Microwave ablation versus transcatheter arterial embolization for large hepatic hemangiomas: clinical outcomes. Int J Hyperthermia. 2020;37(1):938–943. doi: 10.1080/02656736.2020.1766122.
- Liu F, Yu X, Liang P, et al. Ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous haemangiomas. Int J Hyperthermia. 2018;34(7):1061–1066. doi: 10.1080/02656736.2017.1392045.
- Jin S, Shi X, Sun X, et al. Sclerosing cholangitis secondary to bleomycin-iodinated embolization for liver hemangioma. World J Gastroenterol. 2014;20(46):17680–17685. doi: 10.3748/wjg.v20.i46.17680.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018.
- Park S, Tak W, Jung M, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54(3):559–565. doi: 10.1016/j.jhep.2010.07.024.
- Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15(5):431–445. doi: 10.1097/01.rvi.0000124949.24134.cf.
- Ayoobi Yazdi N, Dashti H, Batavani N, et al. Percutaneous sclerotherapy for giant symptomatic liver hemangiomas: a pilot study. J Vasc Interv Radiol. 2018;29(2):233–236. doi: 10.1016/j.jvir.2017.10.009.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260. doi: 10.1148/radiol.14132958.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e.
- Nevesny F, Chevallier O, Falvo N, et al. Bleomycin for percutaneous sclerotherapy of venous and lymphatic malformations: a retrospective study of safety, efficacy and mid-term outcomes in 26 patients. J Clin Med. 2021;10(6):1302. doi: 10.3390/jcm10061302.
- Muir T, Kirsten M, Fourie P, et al. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004;19(12):766–773. doi: 10.1007/s00383-003-1058-6.
- O'Sullivan JM, Huddart RA, Norman AR, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14(1):91–96. doi: 10.1093/annonc/mdg020.
- Horbach S, Rigter I, Smitt J, et al. Intralesional bleomycin injections for vascular malformations: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(1):244–256. doi: 10.1097/PRS.0000000000001924.
- Furumaya A, van Rosmalen BV, Takkenberg RB, et al. Transarterial (chemo-)embolization and lipiodolization for hepatic haemangioma. Cardiovasc Intervent Radiol. 2019;42(6):800–811. doi: 10.1007/s00270-019-02169-x.