Abstract
Objective
To investigate the factors which may cause thermal injury of abdominal skin in patients with uterine fibroids (UFs) who underwent ultrasound-guided focused ultrasound ablation surgery (FUAS).
Method
A total of 123 patients were enrolled in the injury group. In contrast, 246 patients without thermal injury were assigned to the non-injury group. The relationship between patient and treatment parameters and injury were explored using univariate analysis and multiple logistic regression analyses. In addition, the factors influencing the degree of thermal injury were analyzed using Kruskal–Wallis H.
Results
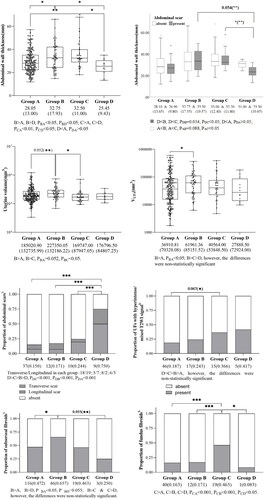
(1) Abdominal scars (p = .007, OR = 2.187, 95% CI: 1.242–3.849), abdominal wall thickness (p < .001, OR = 1.042, 95% CI: 1.019–1.067), fundus fibroids (p = .038, OR = 1.790, 95% CI: 1.033–3.100), UFs with hyperintense/mixed T2-weighted imaging (T2WI) signals (p = .022, OR = 1.843, 95% CI: 1.091–3.115), average sonication power (AP) (p = .025, OR = 1.021, 95% CI: 1.003–1.039), and treatment time (TT) (p < .001, OR = 1.017, 95% CI: 1.011–1.023) were independent risk factors for thermal injury, while treatment volume (TV) (p = .002, OR = 0.775, 95% CI: 0.661–0.909) was a protective factor for injury. (2) Four groups were subdivided according to the degree of thermal injury(Group A: without skin injury. Group B: with changed T2WI signal in the abdominal wall, Group C: mild skin injury, Group D: severe skin injury), comparison of each with every other showed that the abdominal wall in Groups A and D was thinner than Groups B and C, with statistically significant differences (PAB<0.05, PAC<0.01, PDC<0.05, PDB<0.05); Group A was slightly thicker than D, however, without statistical difference. The ratio of sonication time (ST) to TV in Group A was the lowest of all (PAB, PAC, PAD all < 0.05). And as the level of thermal injury rose, the ratio gradually increased, however, without statistical difference.
Conclusions
Based on our limited results, the following conclusion was made. (1) Abdominal scars, abdominal wall thickness, fundus fibroids, UFs with T2WI hyperintense/mixed signals, AP and TT were independent risk factor. (2) Neither too thick nor too thin abdominal walls would be recommended, as both might increase the risk of skin injury. (3) Noticeably, the risk of skin injury might increase considerably when the ST was longer and the sonication area was more fixed.
Introduction
UFs are the most common gynecological tumor in women of childbearing age, which seriously affects their health and quality of life. With the improvement of living standards and optimization of treatment strategies, the demand for treatment of UFs has gradually evolved historically from surgical techniques to minimally invasive or noninvasive one to avoid the risks of pelvic adhesions, long hospital stays or general anesthesia. Therefore, FUAS, a noninvasive technique, has been widely used in the ablation of UFs and become an option for young patients who have a strong desire to keep their uterus. The basic principle in treating UFs involves the conversion of ultrasound (US) energy into thermal energy at the target area, with consequent induction of tissue necrosis. Moreover, the safety and efficacy of FUAS treatment have been demonstrated in many studies [Citation1–4].
Previous studies have shown that the lowest temperature being able to cause skin burn is 44 °C [Citation5,Citation6], whereas the temperature at the target area could reach 60–100 °C. Skin exposure to 60 °C for 10 s can cause a full-thickness burn [Citation7]. Due to the complexity of human anatomy, non-homogeneity of tissues and organs, and inter-individual variability, US propagates non-linearly in the body, and different tissues absorb ultrasound energy in various ways [Citation8]. Therefore, thermal injury to skin located in the US pathway is one of the most common adverse effects of FUAS, with an incidence of 0.5%–15.0% [Citation1,Citation4,Citation9]. The skin injuries might appear pink to red, red with blisters and being wet, yellow/white and being dry, or white/black/brown. In clinical practice, it has been observed that some burn injuries were superficial, requiring no treatment or only wound care, and could heal spontaneously in several days. Some injuries, however, were deep and could not heal with wound care alone, or even gradually worsened several days after FUAS, showing a delayed onset that was difficult to heal within a short period of time and often required elective surgical excision, thus resulting in longer hospital stays and a worse experience of noninvasive treatment [Citation1]. Perhaps there was a difference in the characterization of these two injuries? Anyway, skin injury may not only interrupt FUAS, but also reduce the chances for patients to choose FUAS again. Therefore, it deserves more detailed attention. Previous studies of skin thermal injury were based mostly on T2WI signal changes in the abdominal wall after FUAS [Citation10,Citation11]. The aim of the present study was to further analyze the factors associated with thermal injury of abdominal skin in FUAS, as well as illustrate the clinical characteristics related to the different levels of skin thermal injury. The results obtained may help to improve assessment procedures prior to performing FUAS, as well as to better predict skin injury risk factors.
Materials and methods
A nested case-control study was conducted using the real-world data from the electronic health record of Suining Central Hospital of Sichuan province. This analysis was approved by the ethics committee at our institute (LLSLH-20220112). Patient data were anonymized and protected according to national standards.
Patients
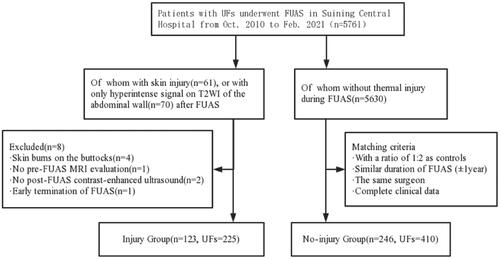
From November 2010 to February 2021, a total of 5761 patients with UFs underwent FUAS in Suining Central Hospital of Sichuan province. To establish the case-control dataset, we extracted patients who sustained abdominal thermal injury after FUAS for UFs, including skin burns and abdominal walls with changed T2WI signals, to constitute the injury group. The exclusion criteria of being in the injury group were: (1) skin burns on the buttocks; (2) no pre-FUAS MRI evaluation; (3) no post-FUAS contrast-enhanced ultrasound; (4) discontinued FUAS due to skin injury. The 1:2 case-control matching was performed by selecting patients from the same cohort who had UFs and underwent FUAS. The matching criteria included a similar duration of FUAS (±1 year) and the same surgeon as each injured patient. All patients in the case-control dataset should have complete clinical data.
A total of 123 patients were involved in the injury group, which compromised 53 cases of skin burns and 70 cases of abdominal walls with changed T2WI signals. Meanwhile, 246 patients with no thermal injury were included in the non-injury group. Thermal injuries include skin thermal injury, which was assessed via the diagnostic criteria for skin burns [Citation12], and the changed T2WI signal in the abdominal wall, which was assessed via measurement of signal intensity on T2WI ().
Focused ultrasound ablation surgery
All patients received bowel and abdominal skin preparation before FUAS. Bowel preparation included an easily digestible and no-gas-producing diet for 3 d, and a cleansing enema in the morning of the surgery. Abdominal skin preparation involved shaving, cleaning with alcohol solution and also degreasing and degassing from the umbilicus to the upper margin of the pubic symphysis. Then, a urinary catheter was inserted and filled with degassed normal saline into the bladder, in order to regulate the bladder volume, thus optimizing the therapeutic acoustic pathway before FUAS.
Ultrasound-guided FUAS was performed using a Focused Ultrasound Tumor Therapeutic System (Model-JC200, Chongqing Haifu Medical Technology Co. Ltd., Chongqing, China). The treatment procedure in our institution was previously reported [Citation2,Citation4,Citation10]. The focused spot was kept at least 1 cm away from the margin of the fibroids and the therapeutic acoustic power ranged from 0 to 400 W. The therapeutic response was evaluated by the non-perfused volume (NPV) performing on contrast enhanced ultrasound (CEUS). The skin in the US pathway was examined immediately after the procedure and skin redness, blisters, burst and subcutaneous nodules recorded. Other adverse effects were also recorded. Furthermore, the severity of any complications was documented according to the Society of Interventional Radiology (SIR) classification system [Citation1].
Data collection
Data were extracted from the electronic medical records. Patient characteristics included age, pregnancy times, parturition times, abdominal scars, abdominal wall thickness, abdominal fat thickness, the posterior uterus, uterine volume (V), total volume of UFs (VUFs), No. of UFs, distance from the UF ventral side to skin, distance from the main UF dorsal side to the sacrum, UF location, UF type, and signal intensity on T2WI. In addition, there were following treatment parameters, such as sonication time (ST), treatment time (TT), average sonication power (AP), ultrasound energy (UE), treatment intensity (TI), energy efficiency factors (EEF), treatment volume (TV, i.e., the volume of US irradiation), and non-perfusion volume rate (NPVR). The data were defined as follows. The NPV, and volumes of UFs and uterus were calculated by an ellipsoid formula: 0.5233 × longitudinal diameter × anteroposterior diameter × transverse diameter. TI was defined as the sonication time per hour, TI(s/h) = ST/(TT/60). EEF referred to the ultrasound energy delivered for ablating 1 mm3 of the UFs lesion tissue, EEF(J/mm3) = ŋPt/1000v’ (ŋ = 0.7, represents focusing coefficient). NPVR denoted the NPV divided by VUFs, NPVR = NPV/VUFs×100%. The evaluation of UFs MRI data before FUAS was performed independently by two doctors with 10 and 8 years of experience in gynaecological tumor imaging, respectively. If the evaluation of the imaging features was inconsistent, a consensus was reached through discussion.
Statistical analysis
Normally distributed data were recorded as mean ± standard deviations (SD), and the t-test was performed between two groups, while ANOVA was used to compare group differences between multiple groups. Skewed distribution data were expressed as the median and IQR, and non-parametric tests were conducted. Categorical data were expressed as frequency and percentage and analyzed with the chi-square test (when 1<T < 5, the continuous corrected chi-square test was used). Besides, univariate analysis was made to compare the patient characteristics and treatment parameters between the two groups. The significant independent variables were evaluated as to their relationships with the abdominal thermal injury by using a binary logistic regression model. In particular, the sample size for this study was 15 times greater than the number of independent variables. In addition, 4 subgroups were generated according to the degree of thermal injury: Group A (no skin injury), Group B (changed T2WI signal in the abdominal wall), Group C (mild skin injury) and Group D (severe skin injury). Additionally, the Kruskal-Wallis H was used to compare the ranked data among multiple groups, while inter-group pairwise comparison was made by the Bonferroni method. Statistical analyses and graphs were produced using the SPSS versionb26.0 (IBM, Chicago, IL), and Graph Pad Prism software (version9.0; Graph Pad Software Inc). The confidence interval (CI) was set at 95%, and p < .05 was considered statistically significant.
Results
Basic information of patients with abdominal skin injury
The median age of all patients was 42 years (range 38–46). With the exclusion of skin burns on the buttocks, a total of 57 patients experienced abdominal skin burns during FUAS, resulting in an incidence of 0.99% (57/5,761) in our center. The postoperative MRI showed sheet- or strip-like hypersignal changes on the abdominal wall T2WI. There were 45 cases of mild skin injury, including superficial burn (I°) and superficial partial-thickness burn (S-II°), of which 10 cases were complicated by abdominal scars (8 transverse and 2 longitudinal). No special treatment or only local ice application was given to the mild skin injuries, most of which were healed within 3–14 d, with normal skin function and without scar retention. There were 12 cases of severe skin injury, including deep partial-thickness burn (D-II°) and A full-thickness burn (III°), of which 9 cases were complicated by abdominal scars (6 transverse and 3 longitudinal). Severe skin injuries required wound dressing change or even surgical treatment, whose incidence in our center was 0.21%. To be specific, regular wound dressing change was implemented for 9 D-II°Cases, where 4 cases were healed within 1 month and 5 cases healed within 3 months. The rest cases were cured after surgical excision of necrotic skin. Here, it should be noted that other postoperative adverse effects were all SIR A-B grades, including nausea, vomiting, vaginal drainage, paresthesia of lower limbs, urinary retention and urinalgia. No inter-group difference was discovered, and most of the symptoms were relieved spontaneously or after symptomatic treatment. Moreover, the incidence of skin injury and severe skin injury at different time periods was further compared. During the first five-year period, the incidence of skin injury and severe skin injury was 1.35% and 0.30% respectively, whereas, they decreased to 0.68% and 0.16%, accordingly during the second five-year period (, ).
Figure 2. A 46-year-old woman with UFs but without abdominal scar: (A1) superficial burn (I°) of skin with erythema and edema measuring about 15 cm × 10cm after FUAS immediately; sagittal view of T2WI, (A1) no abnormal signals in the abdominal wall before FUAS; (A3) hyperintense signals after FUAS (indicated by the arrowhead); a 33-year-old woman with UFs and an abdominal scar: (B) superficial partial-thickness burn (S-II°) of skin with blisters measuring about 2 cm × 2cm 1 day after FUAS (indicated by the arrowhead); a 47-year-old woman with UFs but without abdominal scar:(C) deep partial-thickness burn (D-II°) of skin with cheesy, white-to-red appearance measuring about 3 cm × 3cm after FUAS immediately (indicated by the arrowhead); a 31-year-old woman with UFs and an abdominal scar: (D) full-thickness burn (III°) of skin with grayish and wrinkled appearance measuring about 1.5 cm × 1cm after FUAS immediately (indicated by the arrowhead).
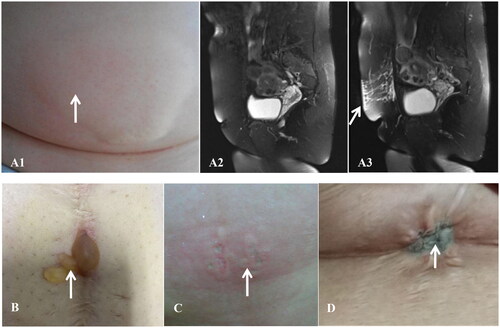
Table 1. Patient with UFs suffered skin injury during FUA at different time periods.
Correlations among patient traits, treatment parameters and skin thermal injury
Univariate analysis revealed significant differences between injured and control patients in factors such as the abdominal scar, abdominal wall thickness, abdominal fat thickness, VUFs, fundus fibroids and hyperintense/mixed T2WI signals (all p < .05). In terms of treatment parameters, differences were also found in ST, TT, AP, UE, TI, TV and EEF. However, no differences were identified in the posterior uterus and posterior fibroids ().
Table 2. Univariate analysis of variables associated with thermal injury.
Risk factors for skin thermal injury
The multicollinearity test showed collinearity among the two sets of variables: abdominal wall thickness and abdominal fat thickness, as well as TT and ST. Thus, abdominal fat thickness and ST were excluded in the multivariable analysis. The final binary logistic regression with thermal injury as the dependent variable evaluated patient characteristics/treatment parameters that displayed significant between-group difference in univariable analysis. The preoperative independent risk factors for thermal injury involved the abdominal scar (p = .007, OR = 2.187, 95% CI: 1.242–3.849), abdominal wall thickness (p < .001, OR = 1.042, 95% CI: 1.019–1.067), the fundal fibroid (p = .038, OR = 1.790, 95% CI: 1.033–3.100) and the high/mixed fibroid T2WI signal (p = .022, OR = 1.843, 95% CI: 1.091–3.115) (). The intraoperative independent risk factors were AP (p = .025, OR = 1.021, 95% CI: 1.003–1.039) and TT (p < .001, OR = 1.017, 95% CI: 1.011–1.023), while the intraoperative protective factor was TV (p = .002, OR = 0.775, 95% CI: 0.661–0.909) ().
Table 3. Multivariable binary logistic regression analysis to evaluate the correlation of thermal injury with the significant patient characteristics of univariate analysis.
Table 4. Multivariable binary logistic regression analysis to evaluate the correlation of the thermal injury with the significant treatment parameters according to univariate analysis.
Associations between patient traits/treatment parameters and severity of skin thermal injury
According to the severity of the thermal injury, the patients were classified into 4 groups, namely Group A (246 cases without injury), Group B (70 patients with changed T2WI signal in the abdominal wall), Group C (41 cases with mild skin injury) and Group D (12 cases with severe skin injury).
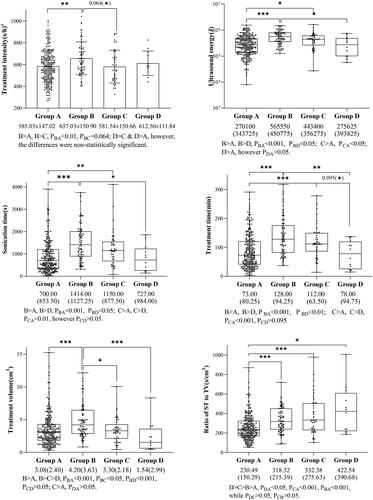
Comparison of the groups with each other indicated that the abdominal wall in Groups A and D was thinner than that in Groups B and C, with statistically significant differences; however, attention should be drawn to the fact that Group D, i.e., the severe skin injury group, had a thinner abdominal wall instead (PAB<0.05, PAC<0.01, PDC<0.05, PDB<0.05). It was the same with subcutaneous fat thickness. The proportion of abdominal scar in Group D was the highest, and the difference with the rest of the groups was statistically significant (PDA, PDB, PDC all < 0.001). Differences in abdominal wall thickness and scar were further analyzed between groups. Within-group comparisons showed that abdominal wall thickness was thinner in Group D combined with scar than without scar, whereas there was no statistically significant difference within the other groups. Between-group comparisons showed that abdominal wall thickness was thinner in Group D combined with a scar than in the other groups(PDC<0.05, PDB=0.054). Interestingly, with the aggravation of skin thermal injury, the proportions of transverse scars gradually increased. In addition, the proportion of subserous fibroids in Group B was higher than that in any other group, while only the differences between Groups B and A were statistically significant (PBA < 0.05). Similarly, the proportion of fundus fibroids in Group C was higher than that in any other group, with statistically significant differences (PCA<0.001, PCB<0.001, PCD<0.05). In terms of ST or TT, and TV, both them experienced a decreasing trend with the aggravation of skin injury, and the differences between the groups were detailed in . It should be particularly found that the ratio of ST to TV was analyzed as a composite index, which was defined as the sonication time given to per 1 cm3, showing that as the level of thermal injury increased, the ratio gradually increased. The difference between Group A and the rest groups was statistically significant, but no statistical difference was found among Groups B, C, and D (PAB<0.001, PAC<0.001, PAD<0.05). Comparison of all results between groups was presented in and .
Table 5. Evaluation of the relationship between the degree of thermal injury and patient characteristics/treatment parameters according to Kruskal-wallis test.
The Kruskal–Wallis H was used to compare ranked data between multiple groups. The images were labeled with adjusted p values, and those in brackets were pre-adjusted p values. ***: p < .001, **: p < .01, *: p < .05. Unless otherwise noted, values are median and inter-quartile range. aNormally distribution data were expressed as mean ± standard deviation. bQualitative data were expressed as frequency and percentage. UFs: uterine fibroids; VUFs: total volume of UFs treated; T2WI: T2 Weighted Image; ST: sonication time; TV: treatment volume; ratio of ST to TV: a composite index, defined as the sonication time per 1 cm3
Discussion
In this retrospective study, it was found that hyperintense T2WI signal changes were present on the abdominal wall at the early stage of thermal injury, without evident skin burn manifestations. With the accumulation of ultrasound energy in the abdominal wall, the skin thermal injury was gradually aggravated, which might be manifested as erythema, blisters, and even sallowness and leathery transformation. Mild skin injuries do not require treatment or simply require ice compress, and usually can heal spontaneously within 2 weeks. In the meanwhile, severe skin injuries can heal spontaneously for up to 3 months, or require surgical excision, which not only extends the patients’ hospital stay, but also reduces the satisfaction of noninvasive treatment. The univariate analysis in this study revealed that skin injury was closely associated with the following preoperative features: abdominal scars, abdominal fat thickness, abdominal wall thickness, VUFs, fundal fibroids, and UFs with hyperintense/mixed T2WI signals. The intraoperative related factors included AP, TT, ST, TI, EEF, TV and UE. As revealed by the multivariate analysis following the exclusion of collinear indicators, the abdominal scar, abdominal wall thickness, fundus fibroids, UFs with hyperintense/mixed T2WI signals, AP and TT constituted the risk factors for skin injury, while the TV manifested a protective effect, a consistent finding with previous reports [Citation10,Citation11]. Meanwhile, our study was the first to analyze the associations between patient traits/treatment parameters and the severity of skin thermal injury. The results demonstrated that in the severe skin injury group, the abdominal wall or subcutaneous fat thickness was thinner, and the proportions of the abdominal scar and UFs with hyperintense T2WI signals were higher, but the ST or TT was shorter, and the composite index, the ratio of ST to TV increased gradually with the aggravation of the skin injury.
Ultrasound pathway related factors
The thickness of the abdominal wall or subcutaneous fat directly reflects the abdominal wall fat content of patients, which is a structure inevitably passed through by the US and an associated factor for skin thermal injury. Noticeably, we were first to focus on the associations of the thin abdominal wall or subcutaneous fat with the risk of skin injury during FUAS. Totally 4 groups were sub-classified depending on the severity of thermal injury, which revealed that when the abdomen was thicker, the risk of abnormal T2WI signal changes on the abdominal wall or mild skin injury increased during FUAS. Contrastively, in case the abdomen was thinner, the risk of severe skin injury increased, especially when being complicated by other high-risk factors. For example, the results of further analysis in this study showed that the abdominal wall thickness was thinner in patients with scars in Group D. Accordingly, it was speculated that those with thinner abdominal walls and combined scars were more prone to severe injuries. Thus, there may be a suitable range of abdominal wall thickness in the acoustic channel for FUAS, and the risk of skin injury is low when treatment is accomplished within this range. Due to the lack of blood supply in adipose tissues, heat dissipation was hindered; the abdominal wall thickened excessively to the focal distance; attenuation of ultrasonic energy increased, and the time required for treating identical lesions increased. Moreover, to acquire clearer ultrasonograms during treatment, the skin needed to be properly compressed to shorten the distance, and thus the fat ischemia was aggravated and the risk of injury increased. Contrastively, the thin abdominal wall led to a low tolerance to UE or TI. When being complicated by other high-risk factors, such as abdominal scars or UFs with hyperintense/mixed T2WI signals, the risk of severe skin injury was still high even during short irradiation time. Since abdominal walls with abnormal T2WI signals were an early indication of skin thermal injury, intraoperative monitoring of MRI or US signals on abdominal walls may provide a basis for intraoperative early warning of skin injury. Nonetheless, more in-depth studies are required to verify this assumption. Hence, the body mass index (BMI) and abdominal wall/subcutaneous fat thickness of patients before treatment should be evaluated. When patients are obese or thin, the risk of skin damage may rise. According to the current guideline [Citation3], FUAS would not be recommended when the patient’s BMI is more than 29.9 kg/m2. Meanwhile, excessive or prolonged compression of abdominal walls should be avoided during the operation, as well as assisting the skin to dissipate heat to reduce the risk of injury.
The abdominal scar was an independent risk factor for skin injury, and the cicatrix proportion increased with the aggravation of skin injury, which was in line with previous studies. Since the cicatrices differ from normal skin in density and structure inside the US pathway, the US was more likely to be reflected, refracted and scattered to deposit energy there [Citation13–15]. Moreover, cicatrix tissues lack blood supply, which became even worse under extrusion and was thus detrimental to heat dissipation. Scars were not sensitive to burning pain, so that timely feedback from patients was impossible, all of which increased the risk of skin injury. In this study, there were 68 cases of abdominal scars, with skin injury incidences of 14/37 for transverse scars and 5/31 for longitudinal scars. Clearly, transverse scars accounted for a higher proportion of skin injuries, because the US focused on the body in a cone shape, and treated each sagittal plane sequentially along the transverse diameter of UFs in space. While the transverse scar was parallel to the UFs’ transverse diameter, the US could hardly avoid the scar. Contrastively, as longitudinal scars were mainly centered, ultrasonic passage through scars could be reduced or avoided by adjusting the incidence angle or even skipping the corresponding treatment layers. Recently, Keserci B et al. have found that scar patches covering scars could block the US conduction in scars and lower the risk of skin injury, without compromising the efficacy of treating UFs. Yin Na et al. discovered that scars in the US pathway causing width <10mm acoustic attenuation had no obvious influence on the efficacy and safety of FUAS treating UFs. However, for those with a history of abdominal wall liposuction, FUAS is not suitable due to the increased risk of skin injury attributed to the extensive subcutaneous cicatrices [Citation16].
Uterine fibroids and treatment related factors
The data demonstrated that UFs with hyperintense/mixed T2WI signals were related to skin injury, and the proportion of T2WI hyperintense/mixed signal fibroids gradually increased with the aggravation of skin injury, although no inter-group statistical significance was observed. Since this kind of UFs was difficult to ablate, a satisfactory NPVR was generally attained by increasing the TI, which correspondingly led to prolonged TT and ST. In this way, both types of energy were deposited in the abdominal wall during US irradiation, and the skin remained in a compressed state during intermittent irradiation, which were likely to significantly increase the risk of skin injury, especially when the US was irradiated in the same area for a long time. The analysis of TV in our study revealed a protective effect on skin injury, and from further analysis of the composite index, ST/TV (i.e., ratio of ST to TV), its positive correlation with the severity of skin injury was found, which supported the above speculation and was the first time to explain the interaction between random two of ST, TV and skin injury by a composite index. An increased risk of skin injury among patients with fundus or subserous fibroids was also pointed out, which was probably attributed to their relative closeness to the abdominal wall, increasing conduction of heat to the wall. Nonetheless, the injuries were likely to be mild. Furthermore, in this study, it was presumed that patients with UFs close to the abdominal wall had more pronounced abdominal responses by heat conduction stimulation, which reminded the operators of relaxing the skin. Therefore, regular skin relaxation was particularly vital for lowering the risk of skin injury.
Other factors
During the early implementation of FUAS in our center, the incidence of skin injury was rather high, but it has gradually decreased in recent years owing to the accumulation of experience. During the recent years, the skin injury incidence has been 0.68%, of which severe skin injuries requiring intervention account for 0.16%. Compared to surgery, the incidences of abdominal wall incision fat liquefaction after transabdominal and laparoscopic myomectomies are approximately 0.5–5% and 0.3–1.25%, respectively [Citation2,Citation4,Citation11]. Moreover, the incidences of other adverse effects after FUAS are all lower. The median ablation rate of UFs in this study exceeded 80%, which was similar to the previous reports [Citation1,Citation2]. It has been confirmed that the symptom relief and quality of life improvement of patients after FUAS were better than those 6 months after myomectomy postoperatively, while no statistical differences were found in the efficacy, symptom recurrence or retreatment rate between the two treatments in long-term follow-up [Citation4,Citation17]. Notably, the efficacy and safety of FUAS were comparable to those of myomectomy. With the advancement of technology and the accumulation of ablation experience, the incidence of skin injury would be reduced effectively.
Limitations
However, our study had the following limitations. (1) Case-control design and retrospective data, cannot fully reflect the various risk factors for skin injury. (2) The incidence of moderate/severe injury is low. However, all changes related to potential injury were involved, thus providing guidance for future practice. (3) The preoperative factors and treatment factors may have causal effect, and thus further analysis through prospective studies with larger sample sizes, refined indicators and objective monitoring techniques in combination with animal experiments is required.
Conclusion
In conclusion, skin thermal injury might be multi-dimensionally associated and interactive with the ablation experience, abdominal wall structure, skin compression, heat transfer, difficulty of ablation, intraoperative treatment parameters, as well as influence of patient response on treatment rhythm. For patients with abdominal scars, preoperative use of a scar scale and ultrasonic attenuation would be recommended for evaluating scar status. For those who were obese or thin, especially in combination with multiple risk factors, accurately assessing BMI, abdominal walls and fat thickness would be necessary. Intraoperatively, for UFs in the anterior position, attention should be paid to the risk of heat conduction. For UFs with abundant blood supply and ablation difficulty, pretreatment, e.g., gonadotropin-releasing hormone analogues, could be performed to the lower blood supply and shrink volume, thereby reducing the treatment difficulty [Citation18]. In the meantime, intraoperative control of treatment intensity and sonication time in identical area, careful attention to the patient skin reactions, as well as regular skin relaxation were effective in lowering the risk of skin injury. Moreover, with the standardization of treatment procedures, advances in technology, and optimization of monitoring equipment and programs, the complications would be further decreased, and FUAS would really achieve noninvasive treatment.
Acknowledgments
The authors gratefully acknowledge the support of the continuing education and training center for non-invasive and micro-invasive medicine of Chongqing Medical University/Suining Central Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors support data transparency.
Additional information
Funding
Reference
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61. doi: 10.1080/02656736.2018.1473894.
- Gong X, Zhang X, Liu D, et al. Evaluation of physician experience in achieving non-perfused volume ratio of high-intensity focused ultrasound ablation for uterine fibroids: a multicentre study. J Int Med Res. 2022;50(5):3000605221102087. doi: 10.1177/03000605221102087.
- Association MITGoMACoCME. China consensus on MR-guided focused ultrasound for uterine fibroids. Med J PUMCH. 2020;11(5):571–579.
- Tempest N, Hapangama D. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2017;125(3):354–364.
- Martin NA, Falder S. A review of the evidence for threshold of burn injury. Burns. 2017;43(8):1624–1639. doi: 10.1016/j.burns.2017.04.003.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294. doi: 10.1080/0265673031000119006.
- Tolles J. Emergency department management of patients with thermal burns. Emerg Med Pract. 2018;20(2):1–24.
- Kyriakou Z, Corral-Baques MI, Amat A, et al. HIFU-Induced cavitation and heating in ex vivo porcine subcutaneous fat. Ultrasound Med Biol. 2011;37(4):568–579. doi: 10.1016/j.ultrasmedbio.2011.01.001.
- Sehmbi AS, Froghi S, Oliveira de Andrade M, et al. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB. 2021;23(2):187–196. 2020 doi: 10.1016/j.hpb.2020.06.013.
- Junshu L, Mingxia Z, Yong W, et al. Impact factors of T2WI appearances of abdominal wall after high intensity focused ultrasound for treating uterus myoma. Chin J Interv Imaging Ther. 2022;19(9):560–564.
- Yin N, Hu L, Xiao Z-B, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34(8):1298–1303. doi: 10.1080/02656736.2018.1433880.
- Warby R, Maani CV. Burn classification. In: Warby R, Maani CV, editors. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Chinese Association of Plastics and Aesthetics Scar Medicine Branch. National expert consensus on early management of scars (2020 version). Zhonghua Shao Shang Za Zhi. 2021;37(2):113–125.
- Grisey A, Heidmann M, Letort V, et al. Influence of skin and subcutaneous tissue on high-intensity focused ultrasound beam: experimental quantification and numerical modeling. Ultrasound Med Biol. 2016;42(10):2457–2465. doi: 10.1016/j.ultrasmedbio.2016.06.013.
- Park JH, Lim SD, Oh SH, et al. High-intensity focused ultrasound treatment for skin: ex vivo evaluation. Skin Res Technol. 2017;23(3):384–391. doi: 10.1111/srt.12347.
- Zhao W-P, Chen J-Y, Chen W-Z. Effect of abdominal liposuction on sonographically guided high-intensity focused ultrasound ablation. J Ultrasound Med. 2014;33(9):1539–1544. doi: 10.7863/ultra.33.9.1539.
- Yuan B, Qin X, Xi J. The comparison of life quality between ultrasound-Guided high-intensity focused ultrasound and laparoscopic myomectomy for the treatment of uterine fibroids. Comput Math Methods Med. 2022;2022:9604915–9604915. doi: 10.1155/2022/9604915.
- Yang S, Kong F, Hou R, et al. Ultrasound guided high-intensity focused ultrasound combined with gonadotropin releasing hormone analogue (GnRHa) ablating uterine leiomyoma with homogeneous hyperintensity on T2 weighted MR imaging. Br J Radiol. 2017;90(1073):20160760. doi: 10.1259/bjr.20160760.