Abstract
Histotripsy is the first noninvasive, non-ionizing, and non-thermal ablation technique that mechanically fractionates target tissue into acellular homogenate via controlled acoustic cavitation. Histotripsy has been evaluated for various preclinical applications requiring noninvasive tissue removal including cancer, brain surgery, blood clot and hematoma liquefaction, and correction of neonatal congenital heart defects. Promising preclinical results including local tumor suppression, improved survival outcomes, local and systemic anti-tumor immune responses, and histotripsy-induced abscopal effects have been reported in various animal tumor models. Histotripsy is also being investigated in veterinary patients with spontaneously arising tumors. Research is underway to combine histotripsy with immunotherapy and chemotherapy to improve therapeutic outcomes. In addition to preclinical cancer research, human clinical trials are ongoing for the treatment of liver tumors and renal tumors. Histotripsy has been recently approved by the FDA for noninvasive treatment of liver tumors. This review highlights key learnings from in vivo shock-scattering histotripsy, intrinsic threshold histotripsy, and boiling histotripsy cancer studies treating cancers of different anatomic locations and discusses the major considerations in planning in vivo histotripsy studies regarding instrumentation, tumor model, study design, treatment dose, and post-treatment tumor monitoring.
Introduction
Histotripsy is a noninvasive, non-ionizing, mechanical ablation technique using focused ultrasound to generate cavitation bubbles for targeting and destroying tissue. First published in 2004 by the University of Michigan group, the name ‘histotripsy’ was derived from the combination of the Greek words ‘histo’, which means soft tissue, and ‘tripsy’ which means breakdown [Citation1]. The histotripsy cavitation bubbles are produced by depositing high-intensity ultrasound pulses into the target tissue, via three distinct approaches: 1) shock-scattering histotripsy, 2) intrinsic threshold histotripsy, and 3) boiling histotripsy. The original shock-scattering histotripsy approach uses ultrasound pulses of 3–20 cycles where the peak negative amplitude is below the intrinsic threshold, which is a tissue-specific pressure value dependent on the chemical components of the target tissue. For example, the intrinsic threshold is the peak negative pressure of 26–28 MPa in water-based tissue and 14 MPa in fat-based tissue [Citation2]. Due to the inherent gaseous nature of tissues, there exists a probabilistic chance of generating an initial individual microbubble within the initial 1–2 cycles of the ultrasound pulse. The subsequent ultrasound pulses create non-linear shockwaves with high-frequency harmonic components, compressing the positive phase of an ultrasound pulse and increasing its peak amplitude as it travels toward the focus [Citation3]. When this positive phase shock-front reflects off the initial microbubble, phase inversion results in a high peak negative pressure as the wave travels back, interfering with the incoming negative pressure phase and generating a cavitation cloud where the intrinsic threshold is exceeded [Citation4, Citation5]. In comparison, the intrinsic threshold histotripsy technique uses ≤2 cycle ultrasound pulses with a single negative pressure phase, such that the peak negative amplitude exceeds a cavitation threshold intrinsic to the target tissue and generates cavitation bubbles [Citation2, Citation6]. In both shock-scattering histotripsy and intrinsic threshold histotripsy approaches, the rapid expansion and collapse of histotripsy cavitation bubbles creates high strain that fractionates the cellular structures in the target tissue into an acellular homogenate [Citation4, Citation5]. The histotripsy homogenate is resorbed by the body within a few weeks, leaving behind marginal scar tissue at the site of ablation [Citation7–9]. The third approach known as boiling histotripsy, initially developed at the University of Washington in 2010, uses millisecond length ultrasound pulses with peak negative pressure (10–15 MPa), lower than both intrinsic threshold histotripsy or shock-scattering histotripsy, to create localized shockwave heating and cellular disruption [Citation10, Citation11]. In this approach, a boiling vapor bubble is generated at the focus due to superheating by the incident high-frequency ultrasound shockwave energy [Citation10, Citation11]. The initial vapor bubble rapidly grows in size and creates an acoustic fountain consisting of tissue fragments directed into the acoustic cavity (termed acoustic atomization) [Citation12, Citation13]. This ultimately results in the mechanical liquefaction of surrounding cellular structures into acellular debris in an effect similar to shock-scattering histotripsy and intrinsic threshold histotripsy [Citation11]. In a nutshell, while the individual histotripsy approaches may differ in terms of pulsing schemes and instrumentation, all three histotripsy approaches cause mechanical tissue homogenization. Undesirable thermal effects are also avoided due to the low duty cycle of the histotripsy pulses, which promotes more efficient and homogenous ablation [Citation14].
Early histotripsy studies focused on establishing the safety and feasibility of targeting normal tissue in vivo and demonstrated uniform tissue homogenization with vessel-sparing effects [Citation8, Citation15–17]. Recently, researchers have been investigating modified histotripsy approaches in vitro, some of which utilize enhanced ultrasound pulsing schemes [Citation18–25]. Histotripsy has been preclinically investigated for cancer and other applications requiring noninvasive tissue removal including brain surgery, blood clot and hematoma liquefaction, neonatal congenital heart disease, valvular diseases, and treatment of tendon injuries [Citation26–35]. Histotripsy has also been evaluated for safety and feasibility in early-stage human clinical trials for benign prostatic hyperplasia, liver cancer, and calcified valve stenosis [Citation36–39]. Of all the potential clinical applications for histotripsy, cancer is one of the most promising applications with generally positive therapeutic outcomes and minimal side effects being reported from preclinical investigations so far. Notably, in the past two decades, histotripsy cancer research has rapidly evolved with the development of dedicated instrumentation and treatment dosing strategies based on the type and location of cancer. Unfortunately, the core differences between the histotripsy approaches and equipment make the direct comparison of results across studies complicated.
This review attempts to consolidate the vast insights from in vivo preclinical histotripsy cancer studies, which have utilized intrinsic threshold, shock-scattering, and boiling histotripsy approaches (). It is broadly divided into two sections. The first section summarizes the key learnings from histotripsy studies targeting different types of cancers in vivo. The second section highlights the various considerations and challenges in planning these studies with respect to instrumentation, tumor model, study design, histotripsy treatment dose, and post-treatment tumor monitoring.
Figure 1. Preclinical investigation of histotripsy for cancer treatment. Histotripsy is a noninvasive, non-ionizing, and non-thermal ablation technique that mechanically fractionates target tissue into acellular homogenate via controlled acoustic cavitation. Intrinsic threshold histotripsy, shock-scattering histotripsy, and boiling histotripsy approaches are being investigated preclinically for treating cancers of various anatomic locations in large and small animal tumor models. This review discusses the important findings from in vivo histotripsy cancer studies reporting on the safety, feasibility, survival outcomes, characterization of post-treatment tumor response, and immune effects.
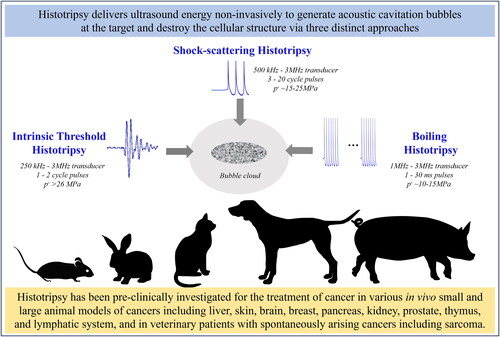
Key findings from in vivo tumor model studies
Intrinsic threshold histotripsy, shock-scattering histotripsy, and boiling histotripsy are being investigated preclinically for treating cancers of various anatomic locations in large and small animal tumor models. Tumor models are animal models with implanted or spontaneously occurring tumors used for studying cancer by mimicking the neoplastic development in humans. Studying the safety, feasibility, and survival outcomes, and characterizing immune effects and tumor responses after stand-alone histotripsy and combinatorial approaches with immune checkpoint inhibitors are the common themes in this research area. Additionally, some studies have reported results from naturally occurring spontaneous tumors in canine and feline veterinary patients. To date, histotripsy has been investigated for the treatment of liver, skin, brain, pancreatic, breast, sarcoma, renal, prostate, thymoma, and lymphoma cancer in animals. The main results from the preclinical histotripsy studies are summarized in this section, classified based on the type of cancer treated. The acoustic parameters reported in each study and the study highlights are summarized in .
Table 1. Histotripsy treatment parameters and study highlights.
Liver cancer
The liver was one of the first oncological targets investigated for histotripsy. Recently, histotripsy has been approved by the FDA to treat liver tumors in humans, supported by promising results from animal studies and human clinical trials. In the first-ever histotripsy tumor survival study in rodents, Worlikar et al. showed that intrinsic threshold histotripsy ablation of subcutaneous human-derived hepatocellular carcinoma (HCC) Hep3B tumors in immunocompromised NSG and NOD-scid mouse models resulted in complete tumor tissue fractionation with tumor burden reduction effects and improved survival compared to controls [Citation40]. However, residual viable tumor cells at the tumor boundary due to lack of treatment margin could have contributed to the eventual tumor recurrence [Citation40]. This was also one of the earliest studies to characterize the evolution of radiological appearances of the tumor in response to histotripsy [Citation40]. Hendricks-Wenger et al. carried out an intrinsic threshold histotripsy dosimetry study evaluating the response of different histotripsy doses (histotripsy dose is arbitrarily defined as the number of pulses/point delivered within the target volume) using subcutaneous human-derived cholangiocarcinoma tumors in immunocompromised NSG mice [Citation41]. Each of the doses investigated (250, 500, and 1000 pulses/point) showed complete tumor cell disruption with no intact tumor cells found at the center of the ablation zone [Citation41]. Interestingly, an increased number of histotripsy pulses/point (1000) resulted in a sharper boundary between the ablated region and intact tissue, whereas intact cells were observed within the boundary region for lower doses (250 and 500) [Citation41]. A survival study by Hendricks-Wenger et al. used the same tumor model and intrinsic threshold histotripsy with a dose of 500 pulses/point [Citation42]. Improved tumor progression-free and overall survival were observed in histotripsy-treated versus control mice [Citation42]. This study also reported significant skin disruption and damage to intestinal tissues when tumors were targeted with an additional 1–2 mm margin [Citation42]. A study by Qu et al. compared the response of stand-alone unilateral intrinsic threshold histotripsy ablation, stand-alone checkpoint inhibition therapy (anti-CTLA-4 monoclonal antibody), and combination therapy of histotripsy and checkpoint inhibition to controls using C57BL/6 mice with subcutaneous bilateral hepatoma Hepa1-6 tumors [Citation43]. This was one of the first studies suggesting the possibility of inducing an abscopal effect in the untreated tumor with histotripsy in a poorly immunogenic tumor model [Citation43]. Of the four groups, combination therapy had the greatest local tumor suppression of the non-targeted contralateral tumor, followed by histotripsy [Citation43].
Recent orthotopic tumor studies by Worlikar et al. in immunocompetent Sprague Dawley rats demonstrated the feasibility of safely targeting liver tumors through the intact chest and abdominal wall [Citation7, Citation44]. In a study using the N1S1 orthotopic tumor model, it was shown that both partial (50–75% volume targeted) and complete (100% volume targeted) intrinsic threshold histotripsy ablation of the tumor region had the potential to cause complete regression of the tumor with no evidence of tumor recurrence by study endpoint [Citation7]. These results were supported by a follow-up study using the highly metastatic McA-RH7777 orthotopic tumor model, which showed that partial (50–75% volume) targeting of tumors with intrinsic threshold histotripsy caused both targeted and untargeted tumor regions to completely regress without an increased risk of metastasis or tumor recurrence compared to controls [Citation44]. Based on magnetic resonance (MR) imaging, tumor regression was observed beginning 1 week post-treatment, and complete regression was observed as early as 3 weeks post-treatment [Citation44]. In fact, histology revealed that the acellular homogenate was being resorbed and scar tissue was forming in the ablation zone with no evidence of intact tumor cells as early as one week post-treatment [Citation44]. This study also revealed that even partial histotripsy was able to stimulate an increased immune response as compared to controls receiving no intervention, possibly playing a role in the observed tumor regression [Citation44]. However, when the targeted tumor volume was minimal (<25%), immune cell infiltration was minimal and no benefits of local tumor regression were observed [Citation44].
Skin cancer
Melanoma animal tumor models have been used for histotripsy immune studies. In the first study investigating the immune response due to histotripsy, Qu et al. showed that intrinsic threshold histotripsy treatment of subcutaneous B16GP33 melanoma tumors in C57BL/6 mice resulted in an increased intratumoral immune infiltration of natural killer cells, dendritic cells, neutrophils, B cells, CD4+ T cells and CD8+ T cells, including antigen-specific CD8+ T cells [Citation43]. Additionally, regional and systemic tumor-specific CD8+ T cell responses and systemic immunostimulatory effects such as the release of tumor-specific antigens and HMGB1 (a damage-associated molecular pattern [DAMP] molecule) were observed, potentially contributing to local tumor control in histotripsy-treated mice [Citation43]. In a bilateral tumor model, the abscopal response was observed in the contralateral, untreated tumor when one of the tumors was ablated [Citation43]. This response was augmented when combined with checkpoint inhibition therapy (anti-CTLA-4 monoclonal antibody) [Citation43]. In a tumor model with tail-vein-induced pulmonary metastasis, histotripsy ablation of the primary subcutaneous tumor inhibited the development of distant pulmonary metastases [Citation43]. A follow-up study by Pepple et al. using bilateral B16F10 melanoma and Hepa1-6 subcutaneous murine tumor models suggested evidence for intrinsic threshold histotripsy-induced necroptosis as the immunogenic cell death mechanism in the treated tumor and ferroptosis as the cell death mechanism in the untreated contralateral tumor [Citation45]. In a tumor challenge experiment, vaccines generated from histotripsy tumor homogenates administered to naïve mice one day before tumor inoculation inhibited tumor growth compared to control animals and animals receiving vaccines with tumor lysates generated from freeze-thaw dissociation and radiation therapy [Citation45].
Singh et al. demonstrated that partial boiling histotripsy (40–50% tumor volume targeted) with anti-CD40 agonistic antibody was able to reprogram poorly immunogenic subcutaneous B16F10 melanoma tumors in C57BL/6 mice and sensitize them to checkpoint inhibition therapy (anti-CTLA-4 and anti-PD-1 monoclonal antibodies) [Citation46]. In a unilateral tumor model, boiling histotripsy with anti-CD40 demonstrated local tumor suppression with an increase in tumor-infiltrating T lymphocytes and enhanced the activation of CD8+ T cells [Citation46]. In the bilateral tumor model, the combination of all three therapies showed abscopal effects in the untreated tumor, leading to improved survival [Citation46].
Brain cancer
Transcranial histotripsy showed promise in treating non-cancerous brain tissue through intact ex vivo human skulls and in in vivo porcine models and was eventually investigated for noninvasive treatment of brain cancer [Citation26, Citation29, Citation47]. Choi et al. described a transcranial stereotactic intrinsic threshold histotripsy treatment system using MRI obtained prior to histotripsy for treatment planning and validated it using an orthotopic, murine glioblastoma model using C57BL/6 albino mice and the GL261 cell line [Citation48]. A follow-up study characterized the temporal evolution of radiological and histological changes following partial intrinsic threshold histotripsy ablation (50–75% tumor volume targeted, 50 pulses/point) delivered through the intact skull of GL261 tumor-bearing as well as naïve C57BL/6 mice [Citation49]. Minor localized bleeding was observed following histotripsy, which eventually resolved within a week [Citation49]. Interestingly, this study also presented initial evidence for histotripsy-mediated transient blood-brain barrier opening [Citation49]. A preliminary investigation by Gerhardson et al. characterized the immunomodulatory effects following transcranial intrinsic threshold histotripsy ablation of orthotopic GL261 tumors in C57BL/6 mice with 50 pulses delivered to a single location [Citation50]. Notably, there was a significant decrease in myeloid-derived suppressor cells and an increase in IFN-γ [Citation50]. Untreated tumor cells not directly targeted by histotripsy appeared shrunken with pyknotic nuclei on histology [Citation50]. Recently, an intrinsic threshold histotripsy dosimetry study by Duclos et al. evaluated tumor response and bleeding in two orthotopic murine brain tumor models using GL261 and LL/2-Luc2 cell lines in C57BL/6 mice [Citation51]. Volumetric histotripsy ablation with fewer total pulses delivered (10–20) resulted in a 40–80% reduction in bioluminescence imaging intensity and >50% tumor cell death on histology, with minimal bleeding and unintended injury [Citation51]. In comparison, increasing the total pulses to >50 resulted in increased bleeding without a corresponding improvement in tumor cell death percentage [Citation51].
In a recent study, Iwanicki et al. investigated intrinsic threshold histotripsy response in a murine model of neuroblastoma using NGP-Luciferase cells injected intrarenally in NCr nude mice [Citation52]. Reduced tumor viability was observed in the targeted region on bioluminescence imaging [Citation52]. Histology revealed increased cellular apoptosis at the ablation zone periphery and in untargeted tumor regions, with a corresponding increase in tumor necrosis factor (TNF) α and cleaved caspase 3, and a decrease in pericytes, hypoxia, vascular endothelial growth factor (VEGF) A, and platelet-derived growth factor-B in the treated tumors [Citation52].
Eranki et al. evaluated partial boiling histotripsy ablation in combination with checkpoint inhibitors (anti-PD-L1 and anti-CTLA-4 monoclonal antibodies) in a subcutaneous murine Neuro2a neuroblastoma model in A/J mice [Citation53]. The observed systemic anti-tumor immune response included activation of dendritic cells and T-cells, the release of proinflammatory cytokines and DAMPs, and downregulation of regulatory T-cells, IL-10, TGF-β, and VEGF-A [Citation53]. These immune effects potentially contributed to abscopal effects in untreated tumors and significantly increased survival [Citation53].
Pancreatic cancer
Recently, histotripsy ablation of normal pancreas and pancreatic tumors has been evaluated in small and large animal pancreatic tumor models. In a study by Hendricks-Wenger et al. evaluating the immune response, subcutaneous Pan02 tumors in C57BL/6 mice were partially ablated (60–75% tumor volume) with intrinsic threshold histotripsy [Citation54]. Results exhibited a reduction in tumor-associated immune cells, potentially contributing to improved tumor-progression-free and overall survival compared to controls [Citation54]. There was significant post-histotripsy upregulation of pathways associated with immune system activation, including HMGB1 signaling [Citation54].
Imran et al. successfully established human-derived orthotopic Panc1 tumors in RAG2/IL2RG double-knockout immunocompromised pigs [Citation55]. Tumors partially ablated with intrinsic threshold histotripsy (1000 pulses/point) demonstrated significantly reduced stromal compartment and collagen levels, similar to ex vivo ablation of human pancreatic tumors [Citation55].
Mouratidis et al. evaluated the combination of boiling histotripsy with immune checkpoint inhibition (anti-CTLA-4 and anti-PD-1 antibodies) in an orthotopic KPC (KrasG12D/+; Trp53R172H/+;Pdx-1-Cre) tumor model in immunocompetent C57BL/6J mice [Citation56]. Tumoral infiltration of CD8+IFNγ+ T cells increased, whereas CD11chigh cell population decreased in mice treated with combination therapy as compared to controls [Citation56]. Additionally, the ratio of CD8+IFNγ+ to CD3+CD4+FoxP3+ increased in the combination therapy cohort, suggestive of anti-tumor immune response [Citation56]. These findings were supported by improved survival outcomes in this group as compared to boiling histotripsy alone, ICI alone, and controls [Citation56].
Breast cancer
Histotripsy has been investigated for the treatment of highly aggressive breast cancer with encouraging results. Hendricks-Wenger et al. demonstrated the feasibility of intrinsic threshold histotripsy and demonstrated local and systemic immune responses and tumor suppression in a 4T1 breast cancer model [Citation57, Citation58].
In a clinical trial in dogs with spontaneously occurring mammary papillary adenocarcinoma, soft tissue sarcoma, lipoma, and low-grade mast cell tumor, Ashar et al. reported that partial intrinsic threshold histotripsy ablation (30–50% tumor volume targeted) resulted in partial remission to disease stabilization initially; however tumors eventually progressed [Citation59].
Nam et al. showed that boiling histotripsy augmented the efficacy of checkpoint inhibition (anti-PD-1 monoclonal antibody), resulting in tumor growth inhibition in orthotopic 4T1 triple-negative breast tumors as well as subcutaneous CT26 colorectal tumors in BALB/C mice [Citation60]. Standalone boiling histotripsy with 50 pulses significantly inhibited tumor growth, released pro-inflammatory cytokines, chemokines, DAMPs, and tumor antigens in the CT26 tumor model, and increased T cell infiltration in the 4T1 tumor model [Citation60]. Repeat treatments with boiling histotripsy increased tumor suppression as compared to single treatment and controls in both models [Citation60].
Sarcoma
Veterinary patients with spontaneously occurring sarcomas have been treated with histotripsy. Ruger et al. evaluated the feasibility and safety of intrinsic threshold histotripsy in three published studies in canine patients with spontaneously occurring soft tissue sarcomas and osteosarcomas, and the tumors were surgically resected within days post-treatment [Citation61–63]. Tumors were targeted with 500–1000 pulses/point, and the ablation zone demonstrated evidence of acute hemorrhage and coagulative and lytic necrosis on histology [Citation61–63]. Notably, the heterogeneity in tumor structural composition translated into differences in tumor and cavitation appearance on ultrasound imaging, histological appearance of ablation zones, and computed tomography (CT) enhancement patterns [Citation61, Citation62].
Recently, intrinsic threshold histotripsy feasibility was demonstrated in three cats with spontaneously arising soft tissue sarcoma, and tumors were surgically excised 3–6 days later in a study by Ruger et al. [Citation64] Tumors were targeted with 500 pulses/point, and results showed ablation zone necrosis with some intact tumor cells, suggesting that the histotripsy dose was likely underpowered [Citation64]. There was mild immune infiltration on histology and no significant changes in cytokine levels [Citation64].
Renal cancer
Renal cancer is a promising target for histotripsy with ongoing human clinical trials in patients with primary solid renal tumors. A shock-scattering histotripsy feasibility study was performed in the highly metastatic orthotopic VX2 tumor model using New Zealand rabbits by Styn et al. [Citation65] The number of treatment pulses delivered was based on real-time US feedback [Citation65]. Histology confirmed successful fractionation of the targeted tumor immediately post histotripsy, and at 24 h the ablation zone demonstrated a strong neutrophilic inflammatory response [Citation65]. In a follow-up study by Styn et al. in the same model to evaluate the risk of spontaneous metastases following shock-scattering histotripsy, it was shown that there was no statistically significant difference in the number or density of pulmonary metastases in treated versus control animals [Citation66].
Hendricks-Wenger et al. demonstrated the feasibility of intrinsic threshold histotripsy for treating RencaRCC renal cell carcinoma subcutaneous tumors in BALB/cCr mice [Citation57, Citation58].
A recent study by Schade et al. demonstrated the feasibility and immune response of partial boiling histotripsy ablation (50% tumor volume) using spontaneously generated orthotopic renal cell carcinoma tumors in genotyped Eker rats [Citation67]. Elevated intrarenal cytokine levels and circulating plasma levels of HMGB1 and TNF at 1 h post ablation suggested acute inflammatory response; this was followed by increased CD8+ T cell infiltration in both targeted and untargeted tumors at 48 h [Citation67]. A chronic study in the same tumor model by Brisbane et al. using boiling histotripsy pulses revealed that the ablation zone appeared fully acellularized by day 14 and only fibrous scar tissue remained by day 56 [Citation68].
Prostate cancer
Histotripsy has been used for targeting prostate cancer in large and small animal tumor models. In a study by Schade et al., transabdominal shock-scattering histotripsy in an orthotopic ACE-1 prostate canine tumor model demonstrated successful necrotic homogenization of tumor with focal regions of hemorrhage on acute histology as well as at 3 weeks post-treatment [Citation69].
Chevillet et al. demonstrated the release of tumor-derived microRNA using boiling histotripsy in a subcutaneous MatLyLu prostate cancer model in Copenhagen rats [Citation70]. Subcellular debris was observed in the ablation zone; however, some connective tissue structures such as larger blood vessels were intact on histology [Citation70].
Thymoma and lymphoma
Boiling histotripsy has been investigated in thymoma and lymphoma murine models. Hoogenboom et al. showed the safety and feasibility of using MR-guided boiling histotripsy, using MR imaging and histology for treatment evaluation in a subcutaneous EL4 thymoma tumor model in C57BL/6 mice [Citation71]. No perfusion was observed within the ablation zone on contrast MR, however, some micro-hemorrhage was noted in the transition zone [Citation71]. Histology revealed mostly disintegrated cellular structure cells within the ablation zone as seen on histology [Citation71]. In a follow-up study, MR-guided boiling histotripsy (100 pulses/point and 200 pulses/point) was compared in EL4 thymoma, B16OVA melanoma, and 9464D neuroblastoma tumor models, with MR and histological characterization [Citation72]. With 100 pulses/point, the EL4, and 9464D tumor ablation zones were completely disintegrated, but the B16OVA tumor ablation zone showed partially viable cells and remnants of micro-vessels within the cell debris. Increasing the dose to 200 pulses/point revealed more homogenous ablation in B16OVA tumors, suggesting that treatment parameters need to be optimized based on the mechanical characteristics of the target tumor [Citation72].
Bijgaart et al. investigated the immunomodulatory effects and mechanism of boiling histotripsy in a subcutaneous EG7 lymphoma model in C57BL/6NCrl and B6.SJL-PtprcaPepcb/BoyJ mice [Citation73]. Results showed immune activation of dendritic cells and T-cells [Citation73]. Additionally, fragmented RNA, DNA, and protein profiles and a reduction in immunosuppressive factors TGF-β and IL-10 were observed in the post-ablation tumor debris [Citation73].
Summary
Overall, in vivo tumor studies have established the feasibility of using intrinsic threshold histotripsy, shock-scattering histotripsy, and boiling histotripsy for cancer treatment. Notably, the histotripsy treatment dose (typically defined as the number of pulses/point or the number of pulses per volume) and the parameters investigated have varied widely based on the type and location of the tumor and the animal model. In general, lower dose and pulse repetition frequency (PRF) have been used in small animal tumor models as compared to larger animal tumor models, ranging from delivering a few pulses at 1 Hz PRF in orthotopic murine brain tumors to 1000 pulses/point at 500 Hz PRF in naturally occurring canine tumors. Boiling histotripsy studies have utilized lower PRF (1–5 Hz) since millisecond pulses are used, compared to higher PRF (1 Hz-1000 Hz) needed in shock-scattering histotripsy and intrinsic threshold histotripsy studies since they use microsecond pulses. Across studies, the acute appearance of the ablation zone is strikingly similar, showing acellularized debris with regions of focal hemorrhage. While higher treatment doses have shown more complete homogenization of the target region, in some sensitive organs such as the brain, the excessive dose has caused undesirable injury and inflammation [Citation51]. Conversely, it was observed that stiffer tumors (e.g., cholangiocarcinoma, B16OVA melanoma) require higher doses to achieve similar levels of homogenization compared to relatively softer tumors (e.g., 4T1 breast cancer, EL4 thymoma, 9464D neuroblastoma) [Citation58, Citation72]. Fortunately, histotripsy has been associated with minimal bleeding side-effects, even in animals that have received therapeutic doses of anticoagulants, as evidenced by studies involving histotripsy of the normal prostate in canine patients, and histotripsy of the normal liver and kidneys in porcine patients [Citation74, Citation75]. However further studies evaluating bleeding risk after histotripsy are needed.
Most studies agree that while histotripsy can inhibit tumor growth and exert a measurable local and systemic anti-tumor immune response, the strength of the response can depend on the immunogenicity of the tumor, the type of histotripsy approach, and the treatment parameters used. Interestingly, the use of immune checkpoint inhibitors combined with histotripsy has shown promise even in immunologically cold tumors [Citation43]. A limited number of longitudinal survival studies in different rodent tumor models have reported improved overall survival outcomes as compared to controls, however, only a few studies have demonstrated tumor-free survival with complete tumor regression at the study endpoint [Citation7, Citation44]. Further investigations are necessary to fully understand and optimize the histotripsy mechanisms and immune responses in vivo.
In addition to in vivo cancer research, histotripsy is also being investigated in humans. In the first-in-human trial in Spain, eleven tumors were targeted in eight patients with unresectable end-stage multifocal primary liver cancer (HCC), colorectal cancer liver metastases, breast cancer liver metastases, and cholangiocarcinoma (CCA) [Citation37]. Technical success was achieved in all cases, as evidenced by MR or computerized tomography (CT) imaging of the planned ablation volume 1 day post-ablation [Citation37]. Notably, one patient with colorectal liver metastases exhibited abscopal effects as evidenced by tumor burden reduction of nontargeted tumor lesions, as well as prolonged reduction of carcinoembryonic antigen tumor marker several weeks post ablation [Citation38]. A recent multi-center human clinical trial for liver cancer (Hope4Liver) in Europe (NCT04573881) and the United States (NCT04572633) evaluated the safety and efficacy of the investigational shock-scattering histotripsy system manufactured by Histosonics, Inc. (Ann Arbor, MI). The data from the Hope4Liver trial has not yet been published, although the trial is complete. In October 2023, the FDA approved Histosonics Inc.’s shock-scattering histotripsy system (EdisonTM) for the treatment of liver tissue in humans. At this time, there are no other approved anatomic locations for histotripsy treatment in humans, although early human clinical trials are ongoing to evaluate histotripsy for primary solid renal tumors in the US (NCT05820087) and UK (NCT05432232). Despite recent major advancements, there is scope for additional technical, preclinical, and clinical investigations before histotripsy can be adopted clinically to treat cancer in different locations.
Challenges and considerations in planning histotripsy tumor model studies
This section highlights the main considerations and challenges for planning and execution of in vivo tumor model studies. The selection and implications of the histotripsy approach, instrumentation, study design, histotripsy treatment dose, and post-treatment tumor monitoring are discussed.
Histotripsy instrumentation, approach, and setup
The choice of the histotripsy therapy transducer varies based on factors such as acoustic access, depth of the intended target location, desired ultrasound waveform, power requirements, and the underlying histotripsy mechanism. For example, transducers with a sharp focus (low f-number <0.8) are preferred in the intrinsic threshold approach, whereas shock-scattering histotripsy transducers have higher f-numbers (0.8–1.2) [Citation1, Citation2]. A variety of custom-built and commercially available ultrasound therapy transducers have been used in vivo in small and large animal models. Although it would be ideal to evaluate the same histotripsy transducer in all preclinical and human clinical investigations, the size and depths of intended targets make this impractical in small animal studies. To address this, the frequency and size of the transducers are scaled; small animal studies in mice and rats have typically used transducers with smaller apertures, fewer elements, and shorter focal lengths as compared to transducers used in large animal canine or porcine studies [Citation41, Citation58, Citation61]. While this strategy has been successful in ablating tumors and observing the therapeutic response, it should be noted that certain parameters such as the histotripsy dose evaluated in small animal studies may not directly translate to large animal studies and human trials.
Intrinsic threshold histotripsy and shock-scattering histotripsy produce similar bioeffects. Yet, intrinsic threshold histotripsy can generate a more compact cavitation cloud with high accuracy compared to shock-scattering histotripsy when using a transducer with the same center frequency and f-number. Thus, intrinsic threshold histotripsy has been used more frequently in rodent studies requiring a sharper focal zone, and shock-scattering histotripsy has been used more frequently in large animal studies and human trials. Boiling histotripsy-generated bubbles are larger compared to both intrinsic threshold histotripsy and shock-scattering histotripsy. The acute histology of the liquefied tissue appears similar across all three types of histotripsy, but the long-term effects and immunological response seem to differ. A fair comparison study using the three histotripsy approaches in the same animal tumor model with the same transducer frequency needs to be conducted to evaluate the differences in long-term effects and immunological response. To compare the biological effects, f-number, and acoustic parameters may be varied due to the requirements of different histotripsy modalities, e.g., boiling histotripsy requires highly non-linear shockwave, while intrinsic threshold histotripsy requires high peak negative pressure. To compare treatment efficacy, treatment speed (complete ablation volume/total treatment time) can be used as a metric instead of the dose (the number of pulses per volume).
Imaging guidance is critical for histotripsy treatment, which is typically guided by either real-time ultrasound or MR imaging, although stereotactic systems have been developed and validated [Citation48]. The choice of imaging is most often dictated by equipment availability. However, it should be noted that some tumors can be difficult to delineate on ultrasound imaging, especially orthotopic tumor targets such as in the liver, with the image clarity further compromised due to the standoff introduced by the coupling medium. Contrast bubbles may be used to enhance images, but care must be taken to completely destroy them prior to initiating histotripsy, as they may serve as excitation nuclei for subsequent cavitation and can also lower the cavitation threshold [Citation76–80].
Treatment time is an important factor that can directly influence clinical adoption. To optimize time, volumetric ablation can be achieved with either mechanical steering (using a robotic arm or mechanical positioning system) or electronic focal steering approaches with different scan patterns. Researchers are investigating techniques to rapidly and uniformly ablate the intended volume while preventing tissue heating, off-target tissue damage, and cavitation memory effects [Citation14, Citation18, Citation81, Citation82]. Signal attenuation and aberration are other major challenges, especially for deeper targets, and algorithms are being implemented for aberration correction, especially in large animal model targets such as the liver which has extensive rib blockage, and the brain through the skull [Citation26, Citation83–86]. Respiratory motion during treatment can interfere with imaging quality making it difficult to discern tumor boundaries, impact targeting and delivery of therapy pulses to intended locations, and can cause undesirable off-target damage, particularly in small animal models. Different strategies are being used to minimize issues due to respiratory motion [Citation26, Citation83–85, Citation87–89].
Histotripsy study design
While initial studies mainly focused on establishing safety and feasibility, recent studies have been exploring the cellular-level mechanisms and immune responses to histotripsy. Another area of interest has been the evaluation of combination therapies, such as the use of histotripsy and immunotherapy, or histotripsy and chemotherapy. A major consideration in designing such studies is choosing the tumor model and determining the study cohorts and experimental timeline. For a cancer type, multiple tumor models may be available; some factors for consideration include whether the tumor model can support study objectives, difficulty of establishing the tumor model, tumor growth kinetics, immune competence of host animal, and availability of assays and other tools for tumor response evaluation. Establishing study cohorts and timelines can be tricky, especially in cases of combination and immune assessment studies. For example, to design a study evaluating the combination of histotripsy with immunotherapy, considerations include the timings, dose, and interval of immunotherapy, and whether to initiate immunotherapy before or post histotripsy ablation. As the complexity of the research goal increases, the study design needs to be carefully and ethically formulated, while taking into consideration all available resources.
Tumor model characteristics and clinical relevance
Most of the in vivo histotripsy cancer studies have been performed in subcutaneous murine models. While this provides a relatively straightforward approach due to the ease of execution and the availability of options for histological analysis and immune profiling, subcutaneous tumors cannot fully mimic the natural orthotopic tumor microenvironment [Citation90]. Tumors generated in animal models may also be nodular and confined with limited ability to form spontaneous metastases [Citation91]. Another compounding factor is that subcutaneous tumors cannot be ablated fully with margins due to the risk of injury to the overlying skin [Citation40, Citation42]. The metabolism of mice is also faster compared to humans; typically, mouse tumors grow to treatable sizes within days to weeks post-inoculation, and human tumors have a comparatively slower growth trajectory [Citation92, Citation93]. In many investigations, the rodents are only a few weeks old with relatively healthy immune systems; most human cancer patients are at an advanced age with compromised immune systems. In most in vivo histotripsy studies, tumors have been ablated at a relatively ‘early stage’ of the disease, additional research is needed into whether similar treatment outcomes can be achieved when tumors are targeted at a ‘late stage’.
Orthotopic tumor models in small animals are available; however, the tumor size and proximity to surrounding critical structures, the ability to accurately identify tumors on imaging, and acoustic access for histotripsy can be challenging. Spontaneously occurring orthotopic tumors in large animal models are preferred for histotripsy evaluation as they are closest to human size and structure. However, the limited availability of such models is a major hindrance. Where such tumor models are available, the cost and resources may limit the sample size in studies. Recently, the ONCOPIG, a genetically modified immunocompromised pig model has been developed, and can be used to engraft human-derived tumors [Citation94]. However, the immunocompromised status does not allow in-depth immune analysis. To overcome these limitations, researchers have been evaluating histotripsy in veterinary patients with naturally occurring tumors [Citation59, Citation62, Citation64].
Histotripsy dose standardization and optimization
In most studies, histotripsy treatment doses and tumor ablation percentages have been arbitrarily chosen based on smaller pilot studies or previous work. This approach makes it difficult to directly compare therapeutic outcomes between different studies. Additionally, owing to the differences in histotripsy approach, acoustic parameters, and transducer geometry used in small and large animal experiments, the optimal histotripsy dose needed for effective tumor ablation may differ. Most importantly, the differences in animal models, tumor types, and protocols are the main roadblocks preventing comparison across studies. Using the same animal tumor model, treatment protocol, and transducer frequency could be a good starting point to compare the degree of damage (by acute histology), ablation volume, and ablation speed. As the focal volume can vary across different transducer geometries and focal spacings, the number of pulses per volume may be a better histotripsy dose metric than the number of pulses per point. Researchers are currently working on techniques to accurately localize, quantify, and standardize the histotripsy dose in terms of the cavitation dynamics and amplitude by monitoring the acoustic cavitation emission signals [Citation95–97].
Additionally, it should be noted that the susceptibility of tissues to histotripsy-induced damage is dependent on their mechanical properties [Citation98–100]. Hence, the optimal treatment parameters may differ based on the tumor type and structurally stiffer tumors may require higher doses compared to softer tumors. The number of histotripsy pulses to completely break down the target tumor also varies with the histotripsy type and parameters used. For example, even when similar transducer frequencies are used, it typically takes a lower number of pulses for boiling histotripsy (that uses millisecond-length pulses), to completely ablate the target tumor compared to shock-scattering histotripsy or intrinsic threshold histotripsy (that uses microsecond-length pulses). Therefore, to compare boiling histotripsy with the other two approaches, ablation speed (cc/min) may be a better metric than the number of pulses.
In addition to the number of pulses per volume, the induced damage can vary with other acoustic parameters (such as pressure and PRF). Within the same histotripsy modality, it may take fewer histotripsy pulses to completely ablate the target tumor when higher peak negative focal pressure is used. However, using a higher-than-required dose may result in unintended damage and inflammation, which may negatively impact treatment outcomes, such as in the case of brain cancer applications [Citation51]. A higher PRF and cavitation memory effect (where the cavitation bubbles do not have sufficient time to fully collapse, resulting in residual gas nuclei that persist and act as seeding for subsequent cavitation events) may be desirable in histotripsy-resistant tissues where the generated focal pressure is inadequate to sustain cavitation cloud with every pulse [Citation101]. It is possible to factor the pressure and PRF into the histotripsy dose, e.g., by multiplying a factor correlated with the pressure level and the number of pulses per volume. The PRF is already factored into the ablation speed.
Characterization of treatment response
One of the advantages of histotripsy is the ability to distinguish ablated versus non-ablated tumors in real-time using imaging guidance [Citation7, Citation40]. Imaging modalities such as CT and MR have also been used to assess and quantify histotripsy ablation by comparing pre and post-treatment imaging, without the need to wait weeks after treatment [Citation16, Citation62, Citation102]. Researchers have also characterized the temporal evolution of tumors in response to histotripsy in many tumor models [Citation49]. For example, in the case of T2-weighted MR imaging of orthotopic liver tumors, tumor appearance changes to hypointense within a few hours post-ablation, then becomes hyperintense 2–3 days post-ablation, and when the ablation zone involutes to form scar tissue in a few weeks, it appears as a mm-sized hypointense area [Citation7, Citation44]. However, this evolution sequence cannot be generalized to all tumor models. In the Hep3B human-derived subcutaneous murine model, the untreated tumor has a heterogenous appearance on T2-weighted MR imaging, which becomes hyperintense as the tumor liquefies immediately post-treatment [Citation40].
Conclusions
Histotripsy has shown promising results for treating cancers in different anatomic locations in vivo. Preclinical studies have reported on the safety, feasibility, radiological and histological characterization of tumors, survival outcomes, immunomodulatory effects, and treatment outcomes with combination approaches in a spectrum of tumor models as well as in veterinary patients with naturally occurring tumors. Ongoing human clinical trials have shown promising early results. Histotripsy has recently been approved by the FDA to noninvasively treat liver tumors. In addition to being used as a standalone therapy, the combination of histotripsy with other treatment modalities such as immunotherapy and chemotherapy is currently under investigation in various tumor models. We hope that the insights and challenges discussed in this review will help future in vivo preclinical development of histotripsy, with the goal of clinical adoption.
Disclosure statement
Drs. Timothy Hall, Man Zhang, Mishal Mendiratta-Lala, Clifford S. Cho, and Zhen Xu have financial and/or other relationships. The relationships are described below: Dr. Timothy Hall: Histosonics, Inc: co-founder, stockholder, consultant; Dr. Man Zhang: Histosonics, Inc: consultant; Dr. Mishal Mendiratta-Lala: University of Michigan Site-PI of the Hope4Liver national multi-center clinical trial (NCT04573881) sponsored by HistoSonics, Inc; Dr. Clifford S Cho: Histosonics, Inc: research support. Co-national PI of the Hope4Liver national multi-center clinical trial (NCT04573881) sponsored by HistoSonics, Inc; Dr. Zhen Xu: Histosonics, Inc: co-founder, stockholder, consultant. All other authors declare no conflict of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(6):726–736. doi: 10.1109/TUFFC.2004.1304271.
- Lin KW, Kim Y, Maxwell AD, et al. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):251–265. doi: 10.1109/tuffc.2014.6722611.
- Maxwell AD, Wang TY, Cain CA, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am. 2011;130(4):1888–1898. doi: 10.1121/1.3625239.
- Vlaisavljevich E, Maxwell A, Mancia L, et al. Visualizing the histotripsy process: bubble cloud-cancer cell interactions in a tissue-mimicking environment. Ultrasound Med Biol. 2016;42(10):2466–2477. doi: 10.1016/j.ultrasmedbio.2016.05.018.
- Parsons JE, Cain CA, Abrams GD, et al. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32(1):115–129. doi: 10.1016/j.ultrasmedbio.2005.09.005.
- Maxwell AD, Cain CA, Hall TL, et al. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med Biol. 2013;39(3):449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004.
- Worlikar T, Mendiratta-Lala M, Vlaisavljevich E, et al. Effects of histotripsy on local tumor progression in an in vivo orthotopic rodent liver tumor model. BME Front. 2020;2020:9830304. doi: 10.34133/2020/9830304.
- Vlaisavljevich E, Greve J, Cheng X, et al. Non-invasive ultrasound liver ablation using histotripsy: chronic study in an in vivo rodent model. Ultrasound Med Biol. 2016;42(8):1890–1902. doi: 10.1016/j.ultrasmedbio.2016.03.018.
- Hall TL, Kieran K, Ives K, et al. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol. 2007;21(10):1159–1166. doi: 10.1089/end.2007.9915.
- Khokhlova TD, Canney MS, Khokhlova VA, et al. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130(5):3498–3510. doi: 10.1121/1.3626152.
- Canney MS, Khokhlova VA, Bessonova OV, et al. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol. 2010;36(2):250–267. doi: 10.1016/j.ultrasmedbio.2009.09.010.
- Pahk KJ, Gélat P, Sinden D, et al. Numerical and experimental study of mechanisms involved in boiling histotripsy. Ultrasound Med Biol. 2017;43(12):2848–2861. doi: 10.1016/j.ultrasmedbio.2017.08.938.
- Simon JC, Sapozhnikov OA, Khokhlova VA, et al. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57(23):8061–8078. doi: 10.1088/0031-9155/57/23/8061.
- Wang TY, Xu Z, Hall TL, et al. An efficient treatment strategy for histotripsy by removing cavitation memory. Ultrasound Med Biol. 2012;38(5):753–766. doi: 10.1016/j.ultrasmedbio.2012.01.013.
- Kim Y, Vlaisavljevich E, Owens GE, et al. In vivo transcostal histotripsy therapy without aberration correction. Phys Med Biol. 2014;59(11):2553–2568. doi: 10.1088/0031-9155/59/11/2553.
- Longo KC, Knott EA, Watson RF, et al. Robotically assisted sonic therapy (RAST) for noninvasive hepatic ablation in a porcine model: mitigation of body wall damage with a modified pulse sequence. Cardiovasc Intervent Radiol. 2019;42(7):1016–1023. doi: 10.1007/s00270-019-02215-8.
- Vlaisavljevich E, Kim Y, Allen S, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39(8):1398–1409. doi: 10.1016/j.ultrasmedbio.2013.02.005.
- Shi A, Xu Z, Lundt J, et al. Integrated histotripsy and bubble coalescence transducer for rapid tissue ablation. IEEE Trans Ultrason Ferroelectr Freq Control. 2018;65(10):1822–1831. doi: 10.1109/TUFFC.2018.2858546.
- Vlaisavljevich E, Aydin O, Lin KW, et al. The role of positive and negative pressure on cavitation nucleation in nanodroplet-mediated histotripsy. Phys Med Biol. 2016;61(2):663–682. doi: 10.1088/0031-9155/61/2/663.
- Li Y, Hall TL, Xu Z, et al. Enhanced shock scattering histotripsy with pseudomonopolar ultrasound pulses. IEEE Trans Ultrason Ferroelectr Freq Control. 2019;66(7):1185–1197. doi: 10.1109/TUFFC.2019.2911289.
- Pahk KJ. Control of the dynamics of a boiling vapour bubble using pressure-modulated high intensity focused ultrasound without the shock scattering effect: a first proof-of-concept study. Ultrason Sonochem. 2021;77:105699. doi: 10.1016/j.ultsonch.2021.105699.
- Thomas GPL, Khokhlova TD, Sapozhnikov OA, et al. Enhancement of boiling histotripsy by steering the focus axially during the pulse delivery. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70(8):865–875. doi: 10.1109/TUFFC.2023.3286759.
- Eranki A, Farr N, Partanen A, et al. Mechanical fractionation of tissues using microsecond-long HIFU pulses on a clinical MR-HIFU system. Int J Hyperthermia. 2018;34(8):1213–1224. doi: 10.1080/02656736.2018.1438672.
- Li Y, Wang R, Lu M, et al. Histotripsy using fundamental and second harmonic superposition combined with hundred-microsecond ultrasound pulses. Ultrasound Med Biol. 2018;44(10):2089–2104. doi: 10.1016/j.ultrasmedbio.2018.05.024.
- Guan Y, Lu M, Li Y, et al. Histotripsy produced by hundred-microsecond-long focused ultrasonic pulses: a preliminary study. Ultrasound Med Biol. 2016;42(9):2232–2244. doi: 10.1016/j.ultrasmedbio.2016.01.022.
- Sukovich J, Xu Z, Kim Y, et al. Targeted lesion generation through the skull without aberration correction using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63(5):671–682. doi: 10.1109/tuffc.2016.2531504.
- Bader KB, Hendley SA, Bollen V. Assessment of collaborative robot (cobot)-assisted histotripsy for venous clot ablation. IEEE Trans Biomed Eng. 2021;68(4):1220–1228. doi: 10.1109/TBME.2020.3023630.
- Khokhlova TD, Monsky WL, Haider YA, et al. Histotripsy liquefaction of large hematomas. Ultrasound Med Biol. 2016;42(7):1491–1498. doi: 10.1016/j.ultrasmedbio.2016.01.020.
- Gerhardson T, Sukovich JR, Chaudhary N, et al. Histotripsy clot liquefaction in a porcine intracerebral hemorrhage model. Neurosurgery. 2020;86(3):429–436. doi: 10.1093/neuros/nyz089.
- Khokhlova TD, Kucewicz JC, Ponomarchuk EM, et al. Effect of stiffness of large extravascular hematomas on their susceptibility to boiling histotripsy liquefaction in vitro. Ultrasound Med Biol. 2020;46(8):2007–2016. doi: 10.1016/j.ultrasmedbio.2020.04.023.
- Smallcomb M, Simon JC. Histotripsy in collagenous tendons. Proc Meet Acoust. 2019;146(4):2992. doi: 10.1121/1.5137356.
- Owens GE, Miller RM, Ensing G, et al. Therapeutic ultrasound to noninvasively create intracardiac communications in an intact animal model. Catheter Cardiovasc Interv. 2011;77(4):580–588. doi: 10.1002/ccd.22787.
- Xu Z, Owens G, Gordon D, et al. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742–749. doi: 10.1161/CIRCULATIONAHA.109.889071.
- Villemain O, Robin J, Bel A, et al. Pulsed cavitational ultrasound softening: a new non-invasive therapeutic approach of calcified bioprosthetic valve stenosis. JACC Basic Transl Sci. 2017;2(4):372–383. doi: 10.1016/j.jacbts.2017.03.012.
- Maxwell AD, Owens G, Gurm HS, et al. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011;22(3):369–377. doi: 10.1016/j.jvir.2010.10.007.
- Schuster TG, Wei JT, Hendlin K, et al. Histotripsy treatment of benign prostatic enlargement using the vortx Rx system: Initial human safety and efficacy outcomes. Urology. 2018;114:184–187. doi: 10.1016/j.urology.2017.12.033.
- Vidal-Jove J, Serres X, Vlaisavljevich E, et al. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. Int J Hyperthermia. 2022;39(1):1115–1123. doi: 10.1080/02656736.2022.2112309.
- Vidal-Jove J, Serres-Creixams X, Ziemlewicz TJ, et al. Liver histotripsy mediated abscopal effect-case report. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):3001–3005. doi: 10.1109/TUFFC.2021.3100267.
- Messas E, IJsselmuiden A, Goudot G, et al. Feasibility and performance of noninvasive ultrasound therapy in patients with severe symptomatic aortic valve stenosis: a first-in-human study. Circulation. 2021;143(9):968–970. doi: 10.1161/CIRCULATIONAHA.120.050672.
- Worlikar T, Vlaisavljevich E, Gerhardson T, et al. Histotripsy for non-invasive ablation of hepatocellular carcinoma (HCC) tumor in a subcutaneous xenograft murine model. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:6064–6067. doi: 10.1109/EMBC.2018.8513650.
- Hendricks-Wenger A, Weber P, Simon A, et al. Histotripsy for the treatment of cholangiocarcinoma liver tumors: in vivo feasibility and ex vivo dosimetry study. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2953–2964. doi: 10.1109/TUFFC.2021.3073563.
- Hendricks-Wenger A, Saunier S, Simon A, et al. Histotripsy for the treatment of cholangiocarcinoma in a patient-derived xenograft mouse model. Ultrasound Med Biol. 2022;48(2):293–303. doi: 10.1016/j.ultrasmedbio.2021.10.002.
- Qu S, Worlikar T, Felsted AE, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer. 2020;8(1):e000200. doi: 10.1136/jitc-2019-000200.
- Worlikar T, Zhang M, Ganguly A, et al. Impact of histotripsy on development of intrahepatic metastases in a rodent liver tumor model. Cancers (Basel). 2022;14(7):1612. doi: 10.3390/cancers14071612.
- Pepple AL, Guy JL, McGinnis R, et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front Immunol. 2023;14:1012799. doi: 10.3389/fimmu.2023.1012799.
- Singh MP, Sethuraman SN, Miller C, et al. Boiling histotripsy and in-situ CD40 stimulation improve the checkpoint blockade therapy of poorly immunogenic tumors. Theranostics. 2021;11(2):540–554. doi: 10.7150/thno.49517.
- Sukovich JR, Macoskey JJ, Lundt JE, et al. Real-time transcranial histotripsy treatment localization and mapping using acoustic cavitation emission feedback. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(6):1178–1191. doi: 10.1109/TUFFC.2020.2967586.
- Choi SW, Gerhardson TI, Duclos SE, et al. Stereotactic transcranial focused ultrasound targeting system for murine brain models. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(1):154–163. doi: 10.1109/TUFFC.2020.3012303.
- Choi SW, Duclos S, Camelo-Piragua S, et al. Histotripsy treatment of murine brain and glioma: temporal profile of magnetic resonance imaging and histological characteristics post-treatment. Ultrasound Med Biol. 2023;49(8):1882–1891. doi: 10.1016/j.ultrasmedbio.2023.05.002.
- Gerhardson T, Pal A, Sheetz L, et al. Histotripsy mediated immunomodulation in a mouse GL261 intracranial glioma model. Int Symp Therapeutic Ultrasound. 2018. doi: 10.13140/RG.2.2.19788.51845.
- Duclos S, Golin A, Fox A, et al. Transcranial histotripsy parameter study in primary and metastatic murine brain tumor models. Int J Hyperthermia. 2023;40(1):2237218. doi: 10.1080/02656736.2023.2237218.
- Iwanicki I, Wu LL, Flores-Guzman F, et al. Histotripsy induces apoptosis and reduces hypoxia in a neuroblastoma xenograft model. Int J Hyperthermia. 2023;40(1):2222941. doi: 10.1080/02656736.2023.2222941.
- Eranki A, Srinivasan P, Ries M, et al. High-intensity focused ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin Cancer Res. 2020;26(5):1152–1161. doi: 10.1158/1078-0432.CCR-19-1604.
- Hendricks-Wenger A, Sereno J, Gannon J, et al. Histotripsy ablation alters the tumor microenvironment and promotes immune system activation in a subcutaneous model of pancreatic cancer. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2987–3000. doi: 10.1109/TUFFC.2021.3078094.
- Imran KM, Gannon J, Morrison HA, et al. Successful in situ targeting of pancreatic tumors in a novel orthotopic porcine model using histotripsy. Ultrasound Med Biol. 2023;49(11):2361–2370. doi: 10.1016/j.ultrasmedbio.2023.07.013.
- Mouratidis PXE, Costa M, Rivens I, et al. Pulsed focused ultrasound can improve the anti-cancer effects of immune checkpoint inhibitors in murine pancreatic cancer. J R Soc Interf. 2021;18(180):20210266. doi: 10.1098/rsif.2021.0266.
- Hendricks AD, Howell J, Schmieley R, et al. Histotripsy initiates local and systemic immunological response and reduces tumor burden in breast cancer. J Immunol. 2019;202(1_Supplement):194.30–194.30. doi: 10.4049/jimmunol.202.Supp.194.30.
- Hendricks-Wenger A, Arnold L, Gannon J, et al. Histotripsy ablation in preclinical animal models of cancer and spontaneous tumors in veterinary patients: a review. IEEE Trans Ultrason Ferroelectr Freq Control. 2022;69(1):5–26. doi: 10.1109/TUFFC.2021.3110083.
- Ashar H, Singh A, Kishore D, et al. Enabling chemo-immunotherapy with HIFU in canine cancer patients. Ann Biomed Eng. 2023. doi: 10.1007/s10439-023-03194-1.
- Nam GH, Pahk KJ, Jeon S, et al. Investigation of the potential immunological effects of boiling histotripsy for cancer treatment. Adv Therapeutics. 2020;3(8):1900214. doi: 10.1002/adtp.201900214.
- Ruger LN, Hay AN, Vickers ER, et al. Characterizing the ablative effects of histotripsy for osteosarcoma: in vivo study in dogs. Cancers (Basel). 2023;15(3):741. doi: 10.3390/cancers15030741.
- Ruger L, Yang E, Gannon J, et al. Mechanical high-intensity focused ultrasound (histotripsy) in dogs with spontaneously occurring soft tissue sarcomas. IEEE Trans Biomed Eng. 2023;70(3):768–779. doi: 10.1109/TBME.2022.3201709.
- Ruger LN, Hay AN, Gannon JM, et al. Histotripsy ablation of spontaneously occurring canine bone tumors in vivo. IEEE Trans Biomed Eng. 2023;70(1):331–342. doi: 10.1109/TBME.2022.3191069.
- Ruger L, Yang E, Coutermarsh-Ott S, et al. Histotripsy ablation for the treatment of feline injection site sarcomas: a first-in-cat. Int J Hyperthermia. 2023;40(1):2210272. doi: 10.1080/02656736.2023.2210272.
- Styn NR, Wheat JC, Hall TL, et al. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J Endourol. 2010;24(7):1145–1150. doi: 10.1089/end.2010.0123.
- Styn NR, Hall TL, Fowlkes JB, et al. Histotripsy of renal implanted VX-2 tumor in a rabbit model: investigation of metastases. Urology. 2012;80(3):724–729. doi: 10.1016/j.urology.2012.06.020.
- Schade GR, Wang YN, D'Andrea S, et al. Boiling histotripsy ablation of renal cell carcinoma in the eker rat promotes a systemic inflammatory response. Ultrasound Med Biol. 2019;45(1):137–147. doi: 10.1016/j.ultrasmedbio.2018.09.006.
- Brisbane W, Khokhlova T, Whang S, et al. MP100-02 boiling histotripsy ablation of renal carcinoma in a chronic rat model. J Urol. 2017;197(4S):e1329–e1330. doi: 10.1016/j.juro.2017.02.3109.
- Schade GR, Keller J, Ives K, et al. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J Urol. 2012;188(5):1957–1964. doi: 10.1016/j.juro.2012.07.006.
- Chevillet JR, Khokhlova TD, Giraldez MD, et al. Release of cell-free microRNA tumor biomarkers into the blood circulation with pulsed focused ultrasound: a noninvasive, anatomically localized, molecular liquid biopsy. Radiology. 2017;283(1):158–167. doi: 10.1148/radiol.2016160024.
- Hoogenboom M, Eikelenboom D, den Brok MH, et al. In vivo MR guided boiling histotripsy in a mouse tumor model evaluated by MRI and histopathology. NMR Biomed. 2016;29(6):721–731. doi: 10.1002/nbm.3520.
- Hoogenboom M, Eikelenboom DC, van den Bijgaart RJE, et al. Impact of MR-guided boiling histotripsy in distinct murine tumor models. Ultrason Sonochem. 2017;38:1–8. doi: 10.1016/j.ultsonch.2017.02.035.
- van den Bijgaart RJE, Mekers VE, Schuurmans F, et al. Mechanical high-intensity focused ultrasound creates unique tumor debris enhancing dendritic cell-induced T cell activation. Front Immunol. 2022;13:1038347. doi: 10.3389/fimmu.2022.1038347.
- Wheat JC, Hall TL, Hempel CR, et al. Prostate histotripsy in an anticoagulated model. Urology. 2010;75(1):207–211. doi: 10.1016/j.urology.2009.09.021.
- Mauch SC, Zlevor AM, Knott EA, et al. Hepatic and renal histotripsy in an anticoagulated porcine model. J Vasc Interv Radiol. 2023;34(3):386–394.e2. doi: 10.1016/j.jvir.2022.11.034.
- Bader KB, Haworth KJ, Shekhar H, et al. Efficacy of histotripsy combined with rt-PA in vitro. Phys Med Biol. 2016;61(14):5253–5274. doi: 10.1088/0031-9155/61/14/5253.
- Edsall C, Khan ZM, Mancia L, et al. Bubble cloud behavior and ablation capacity for histotripsy generated from intrinsic or artificial cavitation nuclei. Ultrasound Med Biol. 2021;47(3):620–639. doi: 10.1016/j.ultrasmedbio.2020.10.020.
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241(1):95–106. doi: 10.1148/radiol.2411051170.
- Vlaisavljevich E, Durmaz YY, Maxwell A, et al. Nanodroplet-mediated histotripsy for image-guided targeted ultrasound cell ablation. Theranostics. 2013;3(11):851–864. doi: 10.7150/thno.6717.
- Tran BC, Seo J, Hall TL, et al. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50(10):1296–1304. doi: 10.1109/tuffc.2003.1244746.
- Lundt JE, Allen SP, Shi J, et al. Non-invasive, rapid ablation of tissue volume using histotripsy. Ultrasound Med Biol. 2017;43(12):2834–2847. doi: 10.1016/j.ultrasmedbio.2017.08.006.
- Duryea AP, Cain CA, Roberts WW, et al. Removal of residual cavitation nuclei to enhance histotripsy fractionation of soft tissue. IEEE Trans Ultrason Ferroelectr Freq Control. 2015;62(12):2068–2078. doi: 10.1109/TUFFC.2015.007202.
- Yeats E, Lu N, Sukovich JR, et al. Soft tissue aberration correction for histotripsy using acoustic emissions from cavitation cloud nucleation and collapse. Ultrasound Med Biol. 2023;49(5):1182–1193. doi: 10.1016/j.ultrasmedbio.2023.01.004.
- Lu N, Hall TL, Sukovich JR, et al. Two-step aberration correction: application to transcranial histotripsy. Phys Med Biol. 2022;67(12):125009. doi: 10.1088/1361-6560/ac72ed.
- Lu N, Gupta D, Daou BJ, et al. Transcranial magnetic resonance-guided histotripsy for brain surgery: pre-clinical investigation. Ultrasound Med Biol. 2022;48(1):98–110. doi: 10.1016/j.ultrasmedbio.2021.09.008.
- Sukovich JR, Hall TL, Choi S-W, et al. Neuronavigation-guided transcranial histotripsy, results in a cadaveric model. J Acous Soc Am. 2022;152(4_Supplement):A154–A154. doi: 10.1121/10.0015864.
- Thomas GPL, Khokhlova TD, Khokhlova VA. Partial respiratory motion compensation for abdominal extracorporeal boiling histotripsy treatments with a robotic arm. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2861–2870. doi: 10.1109/TUFFC.2021.3075938.
- Longo KC, Zlevor AM, Laeseke PF, et al. Histotripsy ablations in a porcine liver model: feasibility of respiratory motion compensation by alteration of the ablation zone prescription shape. Cardiovasc Intervent Radiol. 2020;43(11):1695–1701. doi: 10.1007/s00270-020-02582-7.
- Khokhlova TD, Schade GR, Wang YN, et al. Pilot in vivo studies on transcutaneous boiling histotripsy in porcine liver and kidney. Sci Rep. 2019;9(1):20176. doi: 10.1038/s41598-019-56658-7.
- Chulpanova DS, Kitaeva KV, Rutland CS, et al. Mouse tumor models for advanced cancer immunotherapy. Int J Mol Sci. 2020;21(11):4118. doi: 10.3390/ijms21114118.
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451–452. doi: 10.1038/nrc3972.
- Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6 Spec No(Suppl 1):S39–S44. doi: 10.1038/sj.embor.7400422.
- Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65(4):284–297. doi: 10.1002/(SICI)1096-9098(199708)65:4<284::AID-JSO11>3.0.CO;2-2.
- Schachtschneider KM, Schwind RM, Newson J, et al. The oncopig cancer model: an innovative large animal translational oncology platform. Front Oncol. 2017;7:190. doi: 10.3389/fonc.2017.00190.
- Macoskey JJ, Choi SW, Hall TL, et al. Using the cavitation collapse time to indicate the extent of histotripsy-induced tissue fractionation. Phys Med Biol. 2018;63(5):055013. doi: 10.1088/1361-6560/aaae3b.
- Jensen CR, Ritchie RW, Gyöngy M, et al. Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology. 2012;262(1):252–261. doi: 10.1148/radiol.11110670.
- Sukovich JR, Hall TL, Komaiha M, et al. Acoustic cavitation localization during histotripsy using transmit-receive capable arrays. J Acous Soc Am. 2023;153(3_supplement):A316–A316. doi: 10.1121/10.0018981.
- Vlaisavljevich E, Kim Y, Owens G, et al. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol. 2014;59(2):253–270. doi: 10.1088/0031-9155/59/2/253.
- Vlaisavljevich E, Maxwell A, Warnez M, et al. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):341–352. doi: 10.1109/TUFFC.2014.6722618.
- Mancia L, Vlaisavljevich E, Xu Z, et al. Predicting tissue susceptibility to mechanical cavitation damage in therapeutic ultrasound. Ultrasound Med Biol. 2017;43(7):1421–1440. doi: 10.1016/j.ultrasmedbio.2017.02.020.
- Xu Z, Fowlkes JB, Cain CA. A new strategy to enhance cavitational tissue erosion using a high-intensity, initiating sequence. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(8):1412–1424. doi: 10.1109/tuffc.2006.1665098.
- Knott EA, Swietlik JF, Longo KC, et al. Robotically-assisted sonic therapy for renal ablation in a live porcine model: initial preclinical results. J Vasc Interv Radiol. 2019;30(8):1293–1302. doi: 10.1016/j.jvir.2019.01.023.
