 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Anisotropic gold nanostructures have gained increased attention for biomedical applications because of their remarkable optical properties. An emerging type of gold nanostructure—gold nanobipyramids (AuNBP)—has been shown to exhibit superior absorption properties compared to conventionally used gold nanoparticles, which makes them attractive for photothermal applications. We generated a high-shape-purity dispersion of AuNBP using a seed-mediated method and embedded them as photothermal conversion agents in a silk fibroin matrix to investigate their efficacy in photothermal sealing of incisional wounds in immunocompetent mice. These AuNBP-doped laser-activated sealants, or AuNBP-LASE were able to absorb near-infrared laser energy and convert it to heat, thereby inducing transient hyperthermia in the wound and the surrounding tissue. This photothermal conversion facilitated rapid sealing of the skin tissue by the AuNBP-LASE, which resulted in faster functional recovery of skin barrier function compared to nylon sutures at the early stages of repair. Further, the biomechanical properties of the healing skin closed with AuNBP-LASE those of intact skin more rapidly compared to incisions approximated with sutures. Histology studies indicated higher penetration of the LASE within the volume of the incision in skin tissue, lower scab formation, and a similar epidermal gap compared to conventional suturing. These results demonstrate that AuNBP-LASEs can be effective as wound approximation devices for photothermal sealing.
Introduction
Anisotropic metal nanostructures, including those made from gold, demonstrate unique optical properties, which include effective absorption of light energy in the near-infrared (NIR) region of the absorption spectrum [Citation1–4]. Given their relative ease of preparation, high yields, biocompatibility [Citation5], and high customizability [Citation6,Citation7], these particles have been evaluated for various biomedical applications including biosensing, diagnostics, and therapeutics [Citation3,Citation4, Citation8]. For example, the localized surface plasmon resonance (SPR) exhibited by gold nanorods (AuNRs) makes them attractive candidates for photothermal applications in cancer therapeutics and diagnostics [Citation4], and AuNR-mediated photothermal ablation has been studied in various cancers [Citation9–15].
Gold nanobipyramids (AuNBPs) are a relatively new class of anisotropic gold nanoparticles whose geometry is characterized by two pentagonal pyramids joined at their bases [Citation16,Citation17]. Although AuNBPs generally exhibit similar optical properties as those of AuNRs, they have been shown to demonstrate enhanced localized SPR activity because of field enhancement at the sharp tips [Citation18]. This enhanced SPR activity is characterized by smaller full-width half-maxima (FWHM) of the longitudinal SPR (LSPR) peak in the light absorption spectrum. These higher efficacies of optical absorption have led to investigations of AuNBPs in applications related to biomedical imaging [Citation19], diagnostics [Citation20], therapy [Citation16, Citation19,Citation20], theranostics [Citation21], biosensing [Citation22], and infection control [Citation23].
Photothermal approaches, facilitated by the conversion of laser energy to heat, are attractive for tissue sealing and repair [Citation24–30]. In this approach, laser irradiation of a biomaterial, embedded with photothermal converters such as dyes or nanoparticles, results in elevated localized temperatures, which are thought to induce changes in the biomaterial structure and/or in the tissue. This facilitates physical interdigitation of the biomaterial and tissue, leading to liquid-tight sealing [Citation31]. Conventional approximation methods including sutures and staples and some emerging approaches such as cyanoacrylate-based glues are commonly used in clinic. However, they can suffer from limitations including dehiscence, risk of surgical site infections, and poor cosmetic outcomes/scarring depending on the application [Citation32–34]. We previously investigated the use of gold nanorods as photothermal converters with different biomaterials including elastin-like polypeptides (ELPs), collagen, and silk for laser sealing of intestinal tissue and skin [Citation26–29]. In this study, we investigated the ability of AuNBPs as photothermal converters for application in the laser sealing of skin in live mice. We reasoned that the effective optical absorption properties of AuNBPs will make them attractive in tissue sealing and repair applications. We fabricated laser-activated sealants (LASE) in which, gold nanobipyramids were embedded within silk fibroin films, and investigated them for functional and biomechanical recovery of skin following laser sealing of full-thickness dorsal incisional wounds in live, immunocompetent mice.
Experimental
Materials
For all nanoparticle synthesis procedures, Nanopure™ water (NPW; resistivity: 18.2 MΩ cm−1) from ELGA PURELAB Classic machine was used. Chloroauric acid (HAuCl4), cetyltrimethylammonium chloride (CTAC), sodium citrate dihydrate, citric acid, silver nitrate (AgNO3), L-ascorbic acid, 8-hydroxyquinoline (8-HQL), sodium carbonate (Na2CO3), and lithium bromide (LiBr) were acquired from Sigma-Aldrich, St. Louis, MO, USA. Sodium borohydride (NaBH4) and cetyltrimethylammonium bromide (CTAB) were acquired from MP Biomedicals, Solon, OH, USA. Hydrochloric acid was procured from Fisher Scientific, Fair Lawn, NJ, USA.
Synthesis of gold nanobipyramids
Gold nanobipyramids were synthesized using a seed-mediated method reported previously with modifications [Citation35]. To prepare gold seeds, 200 µL HAuCl4 (0.01 M in NPW) and 18 µL hydrochloric acid (HCl; 1 N solution in NPW) were added to 4 mL of CTAC (25 wt% in water) solution. Next, 100 µL ice-cold NaBH4 (50 mM in NPW) was quickly injected under vigorous stirring (>800 rpm) to reduce Au3+ ions. The mixture was vigorously stirred for one minute to eliminate the hydrogen gas formed in the reaction. Next, 16 µL of citric acid (1 M in NPW) or sodium citrate (1 M in NPW) was added to the mixture and stirred for another minute. The vial was tightly capped and heated to 90–95 °C under slow stirring at 200 rpm for 90 min in an oil bath to introduce twinning defects in the seed crystal structure. The seeds were allowed to cool down to room temperature before using them in the generation of AuNBP.
Two different reducing agents—ascorbic acid [Citation36] and 8-HQL [Citation35]—were used in the preparation of AuNBP in order to identify effective methods for AuNBP formation. In the 8-HQL reduction method, 40 µL HAuCl4 (25 mM in NPW) and 18 µL AgNO3 (10 mM in NPW) were added to a 4 mL CTAB (47 mM in NPW) solution. Then, 40 µL of 8-HQL (0.4 M in ethanol) was added to the mixture. Reduction by 8-HQL changed the color of the solution from light yellow to almost colorless because of the reduction of Au3+ ions to Au+ ions. The desired volume of seed dispersion (10–40 µL; optical density at 520 nm or OD520 of stock dispersion: 0.32) was added, and the vial was stored in a 37 °C incubator. After 15 min, 25 µL of 8-HQL was added to the vial and gently mixed. The dispersion was allowed to incubate for another 1–2 h at 37 °C to allow the growth of AuNBPs before purification.
For AuNBP nanoparticles prepared by reduction with ascorbic acid, 200 µL HAuCl4 (10 mM in NPW), 40 µL AgNO3 (10 mM in NPW), and 80 µL HCl (1 N in NPW) were added to 4 mL CTAB (0.1 M in NPW). The vial was gently mixed, and 32 µL ascorbic acid (100 mM in NPW) was added to reduce Au3+ ions to Au+, turning the yellow solution colorless. Next, the desired volume of gold seed dispersion (10–40 µL; optical density of the stock seed dispersion at 520 nm or OD520: 0.32) was added, and the vial was incubated in a 37 °C incubator for at least 2 h before purification.
To remove unreacted reagents, the nanoparticle dispersion prepared with either 8-HQL or ascorbic acid reduction was centrifuged at 12000 rcf for 10 min at 40 °C. The supernatant was discarded, and the pellet was resuspended in fresh NPW. The nanoparticles were washed by centrifuging twice at 12000 rcf for 10 min. These purified nanoparticle dispersions were stored at room temperature before usage.
Preparation of gold nanorods
Gold nanorods were prepared by a seedless synthesis method based on literature methods, including from our previous work [Citation37–40]. In brief, 2 mL of HAuCl4 (10 mM in NPW), 1.08 mL of AgNO3 (0.004 M in NPW), 32 µL of concentrated HCl (13 M), and 280 µL of ascorbic acid (0.0788 M in NPW) was added to 40 mL of CTAB (0.1 M in NPW) under constant stirring at 800 rpm at 37 °C. Next, 60 µL of ice-cold NaBH4 (0.01 M in NPW) were added to the growth solution and the mixture was stirred at 800 rpm for about one minute. Next, the beaker was covered with aluminum foil, and the particles were allowed to grow overnight at 37 °C. To remove unreacted reagents and excess CTAB, CTAB-AuNR dispersion was distributed in microcentrifuge tubes and the tubes were centrifuged at 12000 rcf for 1 h. The supernatant was discarded and the pellets from two tubes were combined and resuspended in 1 mL of NPW. Next, the tubes were centrifuged at 12,000 rcf for 30 min, the supernatant was discarded, and the pellets were resuspended in 1 mL NPW. This step was repeated twice. All pellets were combined in one tube, and the final volume was adjusted to 2 mL with NPW. The gold nanorod dispersion was covered with aluminum foil at room temperature until further use.
Preparation of aqueous silk fibroin solution
Silk fibroin protein was extracted and enriched from Bombyx mori silkworm cocoons purchased from Mulberry Farms, CA, USA, using methods previously described by others [Citation41] and us [Citation42]. Each cocoon was cut into four pieces and the dead insect inside it was discarded. The cocoon pieces (10 g) were degummed in 4 L boiling water containing 0.02 M Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA). Excess Na2CO3 was washed three times by rinsing the degummed silk fibroin fibers in distilled water for 20 min each. The washed fibers were air-dried overnight at room temperature. Seven grams of dried fibers were weighed and then dissolved in 28 mL LiBr (Sigma-Aldrich, St. Louis, MO, USA) solution (9.3 M in NPW). The flask was incubated at 60 °C for 4 h to facilitate slow dissolution of the silk fibroin fibers. The silk fibroin-LiBr mixture was then dialyzed against 4 L NPW through a 3.5 kDa molecular weight cutoff (MWCO) dialysis membrane (Spectra-Por, Spectrum Labs, Rancho Dominguez, CA, USA) at 4 °C for 72 h. This aqueous silk fibroin solution was centrifuged at 12,400 rcf for 30 min to remove undissolved fibers. The centrifugation procedure was repeated until all undissolved fibers were removed. To measure the silk fibroin concentration, 500 µL of this solution was dried overnight at 60 °C in an oven and the dry weight was measured. The purified silk fibroin solution (called “silk” henceforth) was stored at 4 °C until further use.
Transmission electron microscopy (TEM) of AuNBP
An aqueous dispersion (8 µL) of AuNBP (ODLSPR: 2.4) was added to a copper grid coated with formvar/carbon and allowed to dry at room temperature overnight. Imaging was performed on a Philips CM200 or JEOL TEM/STEM 2010 F microscope. The physical dimensions of the particles were quantified using ImageJ and data from at least 15 AuNBP particles from different fields were used per group to determine the length, width, and aspect ratio of the nanoparticles.
Determination of AuNBP purity and full-width half-maxima (FWHM) of the absorbance spectrum
The shape purity of AuNBP was estimated either by TEM or spectroscopy. For TEM purity estimation, the number of AuNBP, nanospheres, and nanorods in TEM fields were manually counted using the Cell Counter plugin in ImageJ. Particles from 10 fields at different magnifications were used to calculate the purity that included at least 300 particles.
Spectroscopy can be employed to estimate the shape purity qualitatively. Higher intensity of the peaks other than the LSPR peak indicates the presence of impurities [Citation43]. Therefore, a higher ratio of absorbance at longitudinal SPR (LSPR) to transverse SPR (TSPR), or ALSPR/ATSPR, was considered indicative of higher shape-purity of AuNBPs in the mixture. FWHM was calculated as the wavelength range at half of the absorbance of the LSPR peak in Microsoft Excel.
Preparation of AuNBP-LASE films
AuNBP-LASE films were formed by incorporating gold nanobipyramids within the silk matrix by means of solvent evaporation methods. First, 662 µL of an aqueous dispersion of purified AuNBP (ODLSPR: ∼ 2.4) were added to 1.38 mL of an 8.7% (w/v) aqueous silk solution to prepare a 6% (w/v) silk—AuNBP mixture in NPW. To prepare AuNBP-LASE films, 500 µL of this mixture was spread evenly on a plastic coverslip (22 mm × 22 mm) for film formation by solvent evaporation. The loading of the particles in the film was approximately 5.7 µg per 30 mg of dry silk fibroin film. The loading was obtained by substituting the absorbance of AuNBPs at 400 nm in equation Au0 [mM] = 0.44 × A400nm [Citation44], where Au0 [mM] is the concentration of zerovalent gold ions, which form the nanoparticle. Substituting A400nm = 0.4, the volume of dispersion = 165.5 µL, and the atomic weight of Au = 196.97 Da, we approximate that 5.7 µg of Au0 is present in each film. After drying at room temperature in air overnight, the nanoparticle-dense edges of the films, formed because of the coffee-ring effect, were cut using scissors, and the homogenous central region was stored at room temperature for further usage. All AuNBP-LASE films were sterilized in ethylene oxide (Anprolene AN74, Anderson Sterilizers, Haw River, NC, USA) before in vivo studies.
AuNR-LASE films were similarly fabricated by adding aqueous AuNR dispersions to silk fibroin solution. Briefly, 150 µL of AuNR dispersion was added to 450 µL of 6% (w/v) silk fibroin solution. From this mixture, 500 µL silk-AuNR solution was added on a plastic coverslip (22 mm × 22 mm) and allowed to dry overnight at room temperature (approximately 25 °C). To ascertain similar loadings of the nanoparticle films before photothermal studies, the processing was optimized to ensure that the optical density of the dried AuNR-LASE films at 400 nm was similar to that of AuNBP-LASE films [Citation44].
Spectroscopic characterization of AuNBPs, AuNRs, AuNBP-LASEs, AuNR-LASEs
Ultraviolet-visible (UV-Vis) absorption spectroscopy was employed to characterize the light absorption behavior of the photothermal nanoparticles and LASE films. Purified AuNBP (200 µL) and AuNR (200 µL) dispersions were added to a well of a 96-well plate and a UV-Vis plate reader was used to determine the absorption spectra between 400 - 995 nm. NPW was used as a blank and its absorbance was subtracted from all the readings. For the spectroscopic characterization of AuNBP-LASE and AuNR-LASE, the entire film (after removing the external portion described above) was placed in the lid of a 12-well plate. To evaluate the effect of hydration on the LSPR of the AuNBP-LASE films, samples were hydrated with either 30, 60, or 100 µL of PBS and allowed to form a paste. Absorbance measurements were performed between the range of 400–995 nm for all films.
Viscoelastic characterization of AuNBP-LASE films
The viscoelastic properties of AuNBP-LASE films were characterized using dynamic mechanical analysis (DMA). The films were cut to 15 mm × 5 mm in dimension and their thickness was measured using a micrometer (Rexbeti, USA). The storage and loss moduli of the films were measured using a tension probe on a rheometer (TA Instruments, Discovery HR30, New Castle, DE, USA) by subjecting individual films to a frequency sweep between 1 and 10 Hz at a strain of 0.1%.
Photothermal characterization of AuNBP-LASE and AuNR-LASE films
Photothermal characterization of LASE films was carried out by irradiating them with an 808 nm NIR laser (LRD-0808, Laserglow Technologies, North York, Ontario, Canada) at different power densities; three “on” and three “off” cycles were used three times in succession to investigate the reversibility and reproducibility of the photothermal response. First, each film was cut into four pieces using scissors. A drop of 5 µL PBS was placed on top of a plastic coverslip and a piece of the film was placed on it. PBS was evenly distributed to hydrate the film. The handheld NIR laser (spot size: 1.6 mm) was set to power densities of 0.6, 1.2, or 2.4 W/cm2 with the help of a laser power meter (FieldMate, Coherent, Saxonburg, PA, USA) and fixed at 1 cm distance on top of the film with the help of clamps. The film was subjected to the individual on-and-off cycles of laser for 30 s each at different power densities. The temperature of the film was recorded using a thermal camera (A325sc NIR; FLIR, Nashua, NH, USA).
Skin sealing in live mice
Balb/c mice were purchased from Jackson Laboratories, ME, USA. At least one animal from the opposite sex was used in each group. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at ASU. Mice (age: 13 weeks) were anesthetized by an intraperitoneal (i.p.) injection (100 µL) of a cocktail containing ketamine (120 mg/kg) and xylazine (6 mg/kg). The dorsum was shaved using electric clippers and the surgical area was sterilized three times by cyclic application of 2% chlorhexidine gluconate and 70% ethanol.
For skin approximation or sealing studies, a full-thickness incision, 1 cm long, was made in the dorsal region using sterile scissors. The incision area was kept hydrated with 0.9% saline until the treatment. For the AuNBP-LASE group, the films were cut to the size of the incision. The incision was hydrated by the application of 0.9% saline using a sterile Q-tip. The film piece was carefully placed in the incision and allowed to form a paste. To approximate the incision, the two edges of the incision were pushed together using sterile forceps. After approximation, the film was irradiated with an NIR laser (808 nm) for 2 min. The power density of the laser was adjusted to maintain the temperature in the range of 60–65 °C. The temperature of the incision and the surrounding tissue was continuously monitored and recorded using a thermal camera. For approximation with sutures, the incision was closed with 4–0 black nylon sutures. Each incision was approximated with three simple-interrupted suture knots and each knot contained four throws. Post-operative anesthetic recovery for all animals was performed on heating pads set at the lowest heating setting.
Evaluation of barrier function recovery of skin
In all cases, barrier function recovery of skin was determined using daily measurements of transepidermal water loss (TEWL) with a vapometer (Delfin, FL, USA). Three values were recorded for every animal and the average of the three readings was considered as the mean TEWL value for the animal. TEWL readings for unwounded skin were collected similarly on an area of skin away from the incision.
Skin elasticity measurements
Skin elasticity measurements were performed using a Cutometer Dual MPA 580 (Courage Khazaka, Germany) with a suction probe (2 mm) on the skin within a 2–3 mm distance from the incision. This instrument measures the ability of the skin to return to its original state after being subjected to suction. The time vs. penetration depth curves obtained by the measurements were used to determine the various viscoelastic parameters of the skin. For our studies, the suction parameters were Mode: M1, pressure: 450 mBar, number of cycles: 5, on time: 3 s, and off time: 3 s. These parameters were adopted with modifications from published literature [Citation45–47]. Higher suction pressure compared to the published literature was used to reduce noise in the data. Mode M1 corresponds to a cycle that involves a linear increase in pressure followed by immediate removal of the suction pressure. Three measurements, including at least one on the opposite side of the incision, were recorded for each mouse. Measurements for unwounded (intact) skin were performed at a location of skin away from the incision.
Tissue harvesting and processing for histology
Mice were euthanized by CO2 asphyxiation on day 2 post wounding in order to investigate repair at early times after surgery. The skin containing the wound was excised using surgical scissors and placed between biopsy foam sponges in a plastic cassette. The tissues were stored in 10% neutral-buffered formalin (#HT501128, Sigma Aldrich, St. Louis, MO, USA) for at least 72 h before further processing. The tissues were then washed in two jars of phosphate buffer saline (PBS; NaCl: 137 mM, KCl: 2.7 mM, Na2HPO4: 10 mM, and K2HPO4: 1.8 mM; pH: 7.4) to remove excess formalin. They were then treated with increasing concentrations of ethanol and finally xylene before incubation in paraffin jars. Next, the tissues with incisions were cut into three pieces and embedded in Paraplast Plus paraffin (#19217, EMS Diasum, Hatfield, PA, USA) blocks. These blocks were cooled on ice and sectioned into 5 µm slices using an Accu-Cut® SRMTM 200 rotary microtome (Sakura Finetek USA, Torrance, CA, USA). The tissue sections were transferred onto positively charged glass slides (AHS90-WH, Hareta, Springside Scientific, Durham, NC, USA) in a water bath heated at 42 °C. Finally, the slide rack was placed in a 37 °C incubator overnight before staining.
Hematoxylin and eosin (H&E) staining
Glass slides with tissue sections were kept in a 60 °C oven for 30 min to melt the paraffin. After 30 min, the rack was immediately transferred to a xylene jar. Rehydration was performed using two jars containing xylene, then two jars containing decreasing concentrations of ethanol (90% and 70%), and finally tap water for 2 min each. After rehydrating the tissue sections, they were incubated in hematoxylin (Gill No. 2, #GHS232, Sigma Aldrich, St. Louis, MO, USA) for 3 min. After the hematoxylin staining step, the slides were washed in three tap water jars for 30 s, 1 min, and 2 min each. Next, the slides were treated with acid-alcohol solution (0.3% hydrochloric acid in 70% ethanol v/v) and immediately washed in tap water for 1 min. The slides were then treated with ammonia water (0.2% ammonium hydroxide in distilled water) for 30 s and washed in tap water for 1 min. Further, the slides were transferred to a jar containing eosin Y solution (0.5% in distilled water containing 0.2% glacial acetic acid) (#318906, Sigma Aldrich, St. Louis, MO, USA) for 4 min. Slides were submerged in two jars containing increasing concentrations of ethanol (90% and 100%) and two jars of xylene for 2 min each to facilitate dehydration. After drying the slides in the air for at least 1 h, they were mounted using Cytoseal-XYL (Electron Microscopy Sciences, Hatfield, PA, USA), and 1.5# glass coverslips (Globe Scientific Inc., Mahwah, NJ, USA). Slides were imaged using an Olympus BX43 microscope equipped with an Olympus DP74 CMOS camera. Image acquisition and epidermal gap quantification were carried out using cellSens software.
Masson’s trichrome staining
Similar to H&E staining, slides for Masson’s trichrome staining were deparaffinized in an oven and rehydrated using xylene and ethanol treatments. Next, the slides were transferred to a Coplin jar containing Bouin’s fixative (Electron Microscopy Sciences, Hatfield, PA, USA) and incubated in a 60 °C oven for 1 h. After fixation, the slides were thoroughly washed by submerging them in jars of distilled water until the yellow color of the wash solution disappeared. Next, the slides were treated with a solution containing 50% Wergert’s hematoxylin A and 50% hematoxylin B (Electron Microscopy Sciences, Hatfield, PA, USA) followed by repeated rinsing in distilled water until the wash solution became colorless. The slides were then transferred to Biebrich scarlet–acid Fuschin solution (Electron Microscopy Sciences, Hatfield, PA, USA) and incubated for 15 min followed by repeated rinsing in distilled water until the wash solution became colorless. Further, the slides were incubated in a solution containing phosphomolybdic acid–phosphotungstic acid (Electron Microscopy Sciences, Hatfield, PA, USA) for 15 min. The slides were then stained in aniline blue (Electron Microscopy Sciences, Hatfield, PA, USA) solution for 20 min and repeatedly rinsed in distilled water until the wash solution became colorless. Finally, the slides were differentiated in 1% acetic acid for 4 min to remove excess dye. The slides were then dehydrated by submerging them in 95% ethanol (2×), absolute ethanol (2×), and xylene (2×) for 2 min each. The slides were allowed to dry in the air for an hour before they were mounted using Cytoseal XYL.
Data and statistical analyses
Statistical analyses were performed using GraphPad Prism software (Boston, MA, USA). All three independent studies were carried out unless specifically indicated, and statistical significance was determined using analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test.
Results and discussion
Synthesis of AuNBPs
AuNBP particles were prepared using a seed-mediated method, which involves elevated temperatures (90–95 °C) intended to increase the shape purity of AuNBPs and decrease the content of spherical and rod-shaped nanoparticles in the mixture [Citation48]. We also investigated two reducing agents—ascorbic acid and 8-HQL—for generating AuNBPs. Our results indicated that the shape purity of AuNBPs (estimated from spectroscopy) generated using 8-HQL was higher than of those generated with ascorbic acid as determined by comparing the absorption peaks between 520–540 nm, which are associated with spherical impurities (). We observed that the color of the nanoparticle dispersion varied depending on the amounts of impurities and the type of reducing agent used. Typically, the formation of an orange color indicated a higher content of AuNBPs, and reddish tones indicated the presence of spherical impurities (not shown); ascorbic acid-facilitated reduction resulted in an orange dispersion, and 8-HQL-mediated reduction resulted in an orangish-brown nanoparticle dispersion. Treatment of seeds with sodium citrate, which was added to induce twinning defects in the growing crystal, resulted in reliable generation of AuNBPs, but the citric acid treatment did not yield characteristic AuNBP spectra (not shown). We therefore carried out all subsequent studies with sodium citrate.
Figure 1. Spectroscopic characterization of gold nanobipyramids (AuNBPs). (a) A representative absorption spectrum of the AuNBP seed solution. (b) Normalized AuNBP absorption spectra as a function of seed volume used in a 4 mL reaction (with 8-HQL as a reducing agent). (c) Normalized absorption spectra of AuNBPs for ascorbic acid or 8-HQL as reducing agents. (d) TEM image of high-purity AuNBPs indicates a uniform size distribution. A representative image from n = 3 independent TEM experiments is shown. Additional TEM images are included in Supplementary Figure S1.
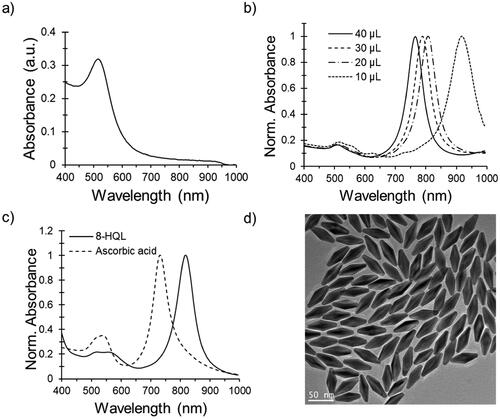
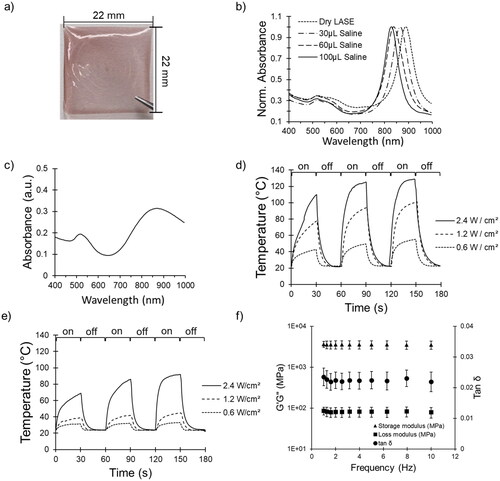
Figure 2. Characterization of AuNBP-LASE films. (a) Representative digital photograph of an AuNBP-LASE film prepared by solvent evaporation. (b) AuNBP absorption spectra normalized with maximum absorption in each spectrum as a function of hydration with saline (average of n = 3). (c) A representative absorption spectrum of dry AuNR-LASE. (d) photothermal (temperature) response of AuNBP-LASE and e) AuNR-LASE irradiated with an 808 nm laser at different power densities. The laser was irradiated (“on”) for 30 s and turned off (“off”) for 30 s for three consecutive cycles. Representative data from n = 3 independent experiments. (f) Dynamic mechanical analysis (DMA) of AuNBP-LASE (Average of n = 3).
Optical properties of AuNBPs
The orange-colored seed solution gradually turned reddish at elevated temperatures (90–95 °C for 90 min) indicating a gradual increase in seed size. The absorption spectrum of seeds showed a peak at about 520 nm (), which indicates the formation of largely spherical nanoparticles. AuNBPs exhibit two peaks—a smaller peak at ∼520 nm, which is a characteristic of TSPR, and a more intense peak at a higher wavelength, including in the NIR range (720–900 nm), which is a characteristic of LSPR (). The intensity of the peak depends on the concentration of the particles and the LSPR peak wavelength depends on their dimensions. The LSPR wavelength can be tuned by changing the volume of the seed solution (); increasing the volume causes a decrease in the number of gold ions per seed, leading to the formation of smaller AuNBP particles with shorter LSPR wavelengths (Supplementary Figure S1). In addition, the shape purity of AuNBPs increased from 80 to 90% when the seed volume was increased from 20 to 30 µL (Supplementary Figure S1). Spherical impurities exhibit a peak between 520 and 540 nm, which can also help in the estimation of AuNBP purity in the suspension using UV–Visible spectroscopy.
When the reducing agent was changed from ascorbic acid to 8-HQL, a significant decrease in shorter wavelength peak was observed, thus indicating a decreased proportion of impurities (). The ALSPR/ATSPR value increased from 2.9 to 6.7 when 8-HQL was used instead of ascorbic acid (Supplementary Figures S1 and S2). Transmission electron microscopy (TEM) images of AuNBP particles generated upon reduction with 8-HQL reduction confirmed the formation of uniform-sized particles with minimal presence of spherical or rod-shaped impurities (); the use of ascorbic acid resulted in higher amounts of impurities as visualized by TEM (Supplementary Figure S2).
Different strategies are used for increasing the shape purity and yields of AuNBPs [Citation48–51] in dispersions. For example, the use of different capping agents (e.g. cetyltrimethylammonium chloride (CTAC), cetyltrimethylammonium bromide (CTAB), citrate [Citation35], reducing agents with different strengths (e.g. 8-HQL, ascorbic acid, 2-naphthol, 2-methyl-8-hydroxyquinoline) [Citation35], bipyramidal-shaped seeds [Citation52], and elevated temperatures for inducing crystal defects have all been explored. Seeds prepared with CTAC produce more reactive species because of lower affinity of CTAC to gold which can facilitate the deposition of Au0 on seeds [Citation35]. Furthermore, aging of seeds at high temperatures increases the proportion of polycrystalline seeds required to form bipyramidal structures and decrease spherical and nanorod impurities [Citation35]. Our results show that usage of 8-HQL instead of ascorbic acid significantly enhances the shape purity and optical properties of the synthesized AuNBPs [Citation35]. It is thought that the presence of the quinoline ring and the weaker reductive strength of 8-HQL above slows the deposition of Au0, which can limit the sphere formation, thus leading to higher shape-purities of gold nanobipyramid formation [Citation35].
Spectroscopic properties of AuNBP-LASE
Solvent-evaporation of silk-AuNBP aqueous dispersions resulted in the formation of light red/maroon-colored AuNBP-LASE films (), which show two characteristic light absorption peaks—one pertaining to LSPR at longer wavelength (near infrared region) and one about TSPR at shorter wavelength (visible region)—consistent with that seen for AuNBPs () in spectroscopy. Considering that the films were expected to be tested in a moist environment in vivo, we also studied the effect of hydration on the optical properties of the films. Hydration of films with saline resulted in a blue shift of the LSPR wavelength (). Compared to AuNBP-LASE, AuNR-LASE exhibited a broader LSPR peak at about 850 nm (, which likely indicates dimensional heterogeneity of AuNRs in the LASE film. In addition, the ratio of ALSPR to ATSPR is less than 2 for AuNR-LASE films (), indicating lower AuNR shape purity and LSPR effect compared to AuNBP-LASE films.
Photothermal characterization of AuNBP-LASE and comparison with AuNR-LASE films
Photothermal response of AuNBP-LASE and AuNR-LASE films was carried out by subjecting them to three consecutive on-and-off cycles of an 808 nm near-infrared laser irradiation with a 30 s duration for each cycle. As expected, the temperature immediately increases upon turning the laser on and drops rapidly when it is turned off (). The maximum temperature reached in an individual cycle increases in the second and third cycles as the film becomes drier and heat dissipation decreases. Increasing the power density resulted in a proportional increase in temperature () and tuning the power density made it possible to keep the temperature between 55°C and 65 °C, which is considered optimal for laser tissue sealing.
Upon laser irradiation, AuNR-LASE films, with similar amount of gold content, exhibited a lower increase in temperature compared to AuNBP-LASE, which is consistent with their lower LSPR absorbance (). At the highest power density tested (2.4 W/cm2), laser irradiation of AuNR-LASE immediately resulted in temperatures in the range of 60–70 °C in the first cycle of irradiation compared to 100–110 °C achieved by irradiating AuNBP-LASE films. The higher response of AuNBP-LASE films enables us to utilize lower power densities for laser-activated tissue sealing compared to those required with AuNR-LASE. This, in turn, can reduce potential nonspecific absorption by other biomolecules, which increases safety of the current approach. In addition, the fast kinetics of the photothermal response can further enhance safety because tissues need not be subjected to longer durations of elevated temperatures in photothermal sealing.
Viscoelastic characterization of AuNBP-LASE
Viscoelastic properties of AuNBP-LASE films were determined by subjecting them to frequency sweep on a rheometer. The films were found to be primarily elastic with a storage modulus of approximately 3.5 × 103 MPa, which remained relatively constant upon increasing the frequency from 1 to 10 Hz at 0.1% strain (). The loss modulus of the LASE films also remained nearly constant at ∼80 MPa (). The low values of loss angle, tan (δ), indicate a primarily elastic film. In vivo, the properties of the film may change upon hydration and lasering.
In vivo skin sealing using AuNBP-LASE
We investigated the efficacy of skin sealing in live immunocompetent Balb/c mice upon laser irradiation of AuNBP-LASE and compared it to that seen with conventional nylon sutures. AuNBP-LASE treatment successfully closed full-thickness incisions, and no visible signs of dehiscence, inflammation, edema, or infection were observed in any mouse for the duration of the study (day 2 post-surgery; ). We chose to investigate early time points—day 2 post surgery—because mouse skin heals rapidly by contraction and significant differences between sutures and laser sealing are not seen at later times following closure [Citation42]. In addition, efficient closure and early functional and biomechanical recovery are important for minimizing opportunistic infections and activating subsequent tissue repair processes, including the resolution of inflammation.
Figure 3. Skin sealing efficacy with AuNBP-LASE. (a) Digital photograph of an AuNBP-LASE used in laser skin sealing (left). Murine skin incisions sealed with sutures and AuNBP-LASE are shown on the right as indicated. (b) A cartoon showing vapometer and cutometer measurement sites in mice whose incisional wounds were laser-sealed with AuNBP-LASE. (c) Transepidermal water loss (TEWL) measurements of skin incisions sealed with sutures and AuNBP-LASE on day 2 post wounding d) Representative cutometer curves of the healing skin on day 2 post wounding using Mode 1 of the cutometer. (e) Comparison of dimensionless parameters obtained from cutometer measurements for unwounded skin, suture-approximated skin, and AuNBP-LASE-sealed skin. Descriptions of individual parameters are provided in . Representative images and data are shown from at least n = 3 independent experiments.
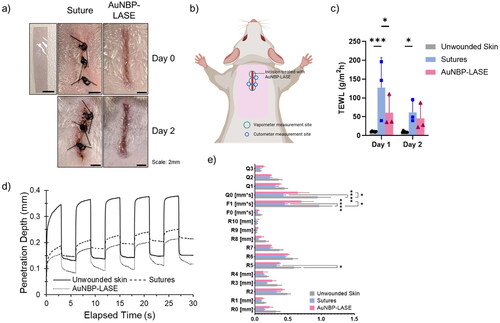
Restoration of skin barrier function was evaluated with transepidermal water loss or TEWL measurements at wound sites shown in . Skin sealing with AuNBP-LASE resulted in significantly lower values of TEWL compared to skin approximation with sutures on day 1, indicating better performance compared to sutures at early times (). However, TEWL values were similar for sutures and AuNBP-LASEs on day 2 (), likely because of the significant contraction of mouse skin. The ability of the LASE to act as a sealant and a filler in the incisional site can play a role in improving the barrier function, which can further augment functional tissue repair of skin.
Restoration of skin biomechanical properties was investigated using a Cutometer MPA580 (Courage Khazaka, Germany) instrument, which is commonly used for determining different skin parameters [Citation45–47] as summarized in , and variables that are used to measure these parameters are shown schematically in Supplementary Figure S3. The elastic properties of the skin were determined at three points next to the incision as shown in and representative cutometer curves are shown in . Even though skin closed with AuNBP-LASE exhibited average values that were closer to the unwounded skin compared to sutures for almost all cutometer parameters, the difference was statistically significant only for R5 (net elasticity), F1 (elasticity), and Q0 (maximum recovery) between the AuNBP-LASE and suture treatments (). F0 and F1 are areas associated with elastic deformation and elastic recovery, respectively. For a completely elastic material, the values of F1 will be close to 0, indicating an immediate return to its original state. Of particular significance, the net elasticity (R5) of the skin returned to that of intact skin for AuNBP-LASE, but not in the case of those wounds approximated with sutures (). We posit that this is a result of the homogenous distribution of mechanical stress around incisions sealed with LASE [Citation53] enabling a quick elastic recovery. Q-parameters are used to evaluate the viscoelastic recovery of the skin. Q0, which indicates the total or maximum recovery area, is the area under the curve during the suction phase. Higher Q0 values indicate lower firmness of the skin. In our studies, statistically significant lower values of Q0 in suture-treated incisions compared to intact skin indicate increased firmness of the healing skin. Even though Q0 was observed to be lower in suture-treated mice, elastic (Q1), viscous (Q2), and viscoelastic (Q3 = Q1 + Q2) recoveries were comparable among all groups.
Table 1. Description of cutometer parameters.
Elasticity measurements during wound healing can provide insights into the health of the wound and may be prognostic in determining abnormally healing wounds or even hypertrophic scar formation [Citation54–56]. During healing, restoration of the mechanical properties of the skin takes place primarily in the proliferation and remodeling phase. The proliferation phase begins around four days post-injury and involves the rapid proliferation of cells involved in the deposition of collagen and other extracellular matrix (ECM) components [Citation57]. The remodeling phase, comprising of collagen deposition by myofibroblast and ECM remodeling, which enhances skin elasticity and tensile strength [Citation57], begins after around three weeks post injury and lasts several months. In human subjects, Cutometer parameters are correlated with elastin and collagen content in skin; for example, the R7 parameter has been found to depend on the elastin fiber network [Citation58]. In clinical research, skin elasticity measurements using a Cutometer have been studied in burn wounds and scars [Citation54–56, Citation59,Citation60]. Significant correlation has been found in objective scar measurements by Cutometer, subjective scar assessment tools including the Vancouver Scar Scale (VSS) and Patient and Observer Scar Assessment Scale (POSAS), and a patient’s quality of life [Citation59], underscoring the importance of noninvasive elasticity measurements. Studies in non-human subjects are somewhat limited. In one such study, enhanced gross elasticity, measured by the R2 parameter in Cutometer was observed in hairless mice upon collagen tripeptide treatment following UVB irradiation [Citation61], indicating utility of these measurements in preclinical research. However, further studies such as the current one will be needed to further standardize and employ Cutometer parameters in animal wound healing.
Histology
Histological analyses of the closed incisional wounds on day 2 post closure were performed using H&E and Masson’s staining. Upon laser sealing, AuNBP-LASE was seen to penetrate within the full thickness of the skin cavity, evenly occupied the volume of the incision, and integrated well with the tissue ( top; blue box). This is consistent with the cutometer observation that the net elasticity approached that of intact skin with AuNBP-LASE likely because of complete space-filling and homogeneous strain in the incisional wound. These phenomena were not seen with sutures likely because of the discrete and not continuous mode of skin approximation with these closure devices. Scab formation was significantly reduced in the case of AuNBP-LASE compared to sutures. However, there were signs of dermal collagen denaturation, potentially because of localized thermal effects, in AuNBP-LASE-treated wounds ( top; indicated by green box). Such structural changes in tissue proteins and/or in the LASE biomaterial and their subsequent interdigitation are thought to be the underlying mechanism of wound closure in laser tissue sealing [Citation62]. Although the epidermal gap was statistically comparable between the groups, local hyperthermia in the LASE group appeared to compromise the epidermis ( right). This epidermal gap is expected to significantly decrease by day 7 based on previous observations [Citation42].
Figure 4. Histological analyses of mice incisions closed with sutures and AuNBP-LASE on day 2 post wounding. Representatives of n = 3 independent images are shown here; other images are shown in Supplementary Figure S4. (Top) Hematoxylin and eosin (H&E) staining (red lines indicate the dermal gap, black arrows indicate the epidermal edge, blue box shows the area of glue integration with the tissue, and green box illustrates the effect of laser-induced hyperthermia on dermal collagen) and (bottom) Masson’s trichrome staining. (Right) Quantification of epidermal gap for incisions closed using sutures and AuNBP-LASE.
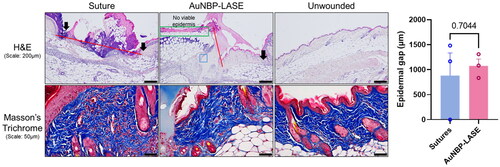
Investigation of tissues stained with Masson’s trichrome (collagen stain) indicated denser collagen bundles and loss of typical “basket-weave” pattern observed in the skin adjacent to wound sealed with AuNBP-LASE ( bottom) compared to those closed with sutures or unwounded skin; additional histology images of other subjects are shown in Supplementary Figure S4. This collagen rearrangement is a likely consequence of the photothermal sealing mechanism [Citation60, Citation63–65]. Dermal collagen denaturation can begin at temperatures of 45–50 °C at which, the process of breaking down the collagen triple helix begins which is followed by the divergence of the fibers and their interlacing [Citation60]. As the temperature increases to 65 °C—the temperature required for laser-assisted sealing—the bundles split into fibers and collagen amorphization occurs at and around the periphery of the laser spot [Citation60]. Our observations on the visible loss of the “basket-weave” pattern 1-1.5 mm from the wound ( top; indicated by green box) are consistent with literature findings on collagen amorphization. Collagen amorphization can also explain the enhanced restoration of net elasticity in wounds closed with AuNBP-LASE compared to sutures. For example, salt denatured collagen was found to have higher elasticity than rigid crystalline collagen [Citation66]. In general, it is believed that collagen fibers provide mechanical strength to the skin and elastin fibers provide elasticity [Citation67]. Our study indicates that changes in collagen structure because of hyperthermia caused by laser irradiation can influence mechanical properties of the recovered skin.
Conclusions
Anisotropic gold nanostructures have interesting optical properties that make them attractive in several biomedical applications including imaging, therapeutics, diagnostics, and theranostics. Here, we investigated gold nanobipyramids as photothermal convertors for tissue sealing and repair in live mice. Synthesis of AuNBP was carried out using a seed-mediated method in which the shape purity and optical properties of AuNBP were easily tuned by optimizing the seed volume. Use of 8-HQL as a reducing agent improved the shape-purity of AuNBPs. Solvent-evaporated AuNBP-silk fibroin LASE biomaterials resulted in rapid and effective laser sealing of full thickness incisions in Balb/c mice with higher barrier function recovery at early times post-surgery and reduced scab formation compared to that seen with sutures. A particular advantage of AuNBP-LASE was the observation that the sealant can occupy the entire volume of the incision evenly, which allows for more homogeneous coverage of the wound volume and better restoration of mechanical properties. Laser sealing with AuNBP-LASE can serve as an alternative to staples, sutures, or cyanoacrylate glues that are currently used as approximation devices for incisional wound closure. In addition, the ability to locally deliver bioactives that promote tissue repair and antibiotic drugs that combat surgical site infections will further enhance the efficacy of the LASE sealing approach. Long term effects of LASE, laser application and hyperthermia in the wound, performance in wounds under tension, efficacy in infected wounds, and detailed studies on fibrosis and scarring will determine the translational outlook for laser sealing in skin sealing and tissue repair applications.
Supplemental Material
Download MS Word (2.8 MB)Acknowledgements
We acknowledge the use of facilities within the Eyring Materials Center at Arizona State University supported in part by NNCI-ECCS-2025490. We also acknowledge the service of Department of Animal Care and Technologies (DACT) staff at ASU for their invaluable help with the animal studies described in this manuscript and for providing access to the ethylene oxide sterilization facility.
Disclosure statement
KR is affiliated with Synergyan, LLC and Endotat Biotechnologies, LLC.
Additional information
Funding
References
- Xia K, Zhang L, Huang Y, et al. Preparation of gold nanorods and their applications in photothermal therapy. J Nanosci Nanotechnol. 2015;15(1):63–73. doi: 10.1166/jnn.2015.9586.
- Vines JB, Yoon J-H, Ryu N-E, et al. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167. doi: 10.3389/fchem.2019.00167.
- Huang H-C, Barua S, Sharma G, et al. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155(3):344–357. doi: 10.1016/j.jconrel.2011.06.004.
- Huang H-C, Ramos J, Grandhi TSP, et al. Gold nanoparticles in cancer imaging and therapeutics. Nano LIFE. 2010;01(03n04):289–307. doi: 10.1142/S1793984410000274.
- Shukla R, Bansal V, Chaudhary M, et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21(23):10644–10654. doi: 10.1021/la0513712.
- MacLeod MJ, Goodman AJ, Ye H-Z, et al. Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene-thiolate ligands. Nat Chem. 2019;11(1):57–63. doi: 10.1038/s41557-018-0159-8.
- Li J, Zhu B, Zhu Z, et al. Simple and rapid functionalization of gold nanorods with oligonucleotides using an mPEG-SH/tween 20-Assisted approach. Langmuir. 2015;31(28):7869–7876. doi: 10.1021/acs.langmuir.5b01680.
- Amin MU, Li L, Zhang R, et al. Rapid and ultrasensitive solution-based SERS detection of drug additives in aquaculture by using polystyrene sulfonate modified gold nanobipyramids. Talanta. 2023;251:123800. doi: 10.1016/j.talanta.2022.123800.
- Ali MR, Rahman MA, Wu Y, et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc Natl Acad Sci U S A. 2017;114(15):E3110–E3118.
- Ali MRK, Wu Y, Tang Y, et al. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc Natl Acad Sci U S A. 2017;114(28):E5655–E5663.
- Choi WI, Kim J-Y, Kang C, et al. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers. ACS Nano. 2011;5(3):1995–2003. doi: 10.1021/nn103047r.
- Dickerson EB, Dreaden EC, Huang X, et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269(1):57–66. doi: 10.1016/j.canlet.2008.04.026.
- von Maltzahn G, Park J-H, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69(9):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242.
- Huang HC, Rege K, Heys JJ. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano. 2010;4(5):2892–2900. doi: 10.1021/nn901884d.
- Huang H-C, Yang Y, Nanda A, et al. Synergistic administration of photothermal therapy and chemotherapy to cancer cells using polypeptide-based degradable plasmonic matrices. Nanomedicine (Lond). 2011;6(3):459–473. doi: 10.2217/nnm.10.133.
- Liu X, Zhou W, Wang T, et al. Highly localized, efficient, and rapid photothermal therapy using gold nanobipyramids for liver cancer cells triggered by femtosecond laser. Sci Rep. 2023;13(1):3372. doi: 10.1038/s41598-023-30526-x.
- Wu X, Mu L, Chen M, et al. Bifunctional gold nanobipyramids for photothermal therapy and temperature monitoring. ACS Appl Bio Mater. 2019;2(6):2668–2675. doi: 10.1021/acsabm.9b00344.
- Chow TH, Li N, Bai X, et al. Gold nanobipyramids: an emerging and versatile type of plasmonic nanoparticles. Acc Chem Res. 2019;52(8):2136–2146. doi: 10.1021/acs.accounts.9b00230.
- Li C, Mei E, Chen C, et al. Gold-Nanobipyramid-based nanotheranostics for Dual-Modality imaging-Guided phototherapy. ACS Appl Mater Interfaces. 2020;12(11):12541–12548. doi: 10.1021/acsami.0c00112.
- Feng J, Chen L, Xia Y, et al. Bioconjugation of gold nanobipyramids for SERS detection and targeted photothermal therapy in breast cancer. ACS Biomater Sci Eng. 2017;3(4):608–618. doi: 10.1021/acsbiomaterials.7b00021.
- Tabish TA, Dey P, Moska S, et al. Smart gold nanostructures for light mediated cancer theranostics: combining optical diagnostics with photothermal therapy. Adv Sci (Weinheim). 2020;7(15):1903441.
- Bhardwaj H, Sumana G, Marquette CA. Gold nanobipyramids integrated ultrasensitive optical and electrochemical biosensor for aflatoxin B(1) detection. Talanta. 2021;222:121578. doi: 10.1016/j.talanta.2020.121578.
- Mobed A, Hasanzadeh M, Seidi F. Anti-bacterial activity of gold nanocomposites as a new nanomaterial weapon to combat photogenic agents: recent advances and challenges. RSC Adv. 2021;11(55):34688–34698. doi: 10.1039/d1ra06030a.
- Urie R, Flake T, Rege K. Laser tissue welding in wound healing and surgical repair. In: Bioengineering in wound healing. World Scientific; 2017. p. 303–324.
- Urie R, Ghosh D, Ridha I, et al. Inorganic nanomaterials for soft tissue repair and regeneration. Annu Rev Biomed Eng. 2018;20(1):353–374. doi: 10.1146/annurev-bioeng-071516-044457.
- Huang HC, Walker CR, Nanda A, et al. Laser welding of ruptured intestinal tissue using plasmonic polypeptide nanocomposite solders. ACS Nano. 2013;7(4):2988–2998. doi: 10.1021/nn303202k.
- Urie R, Quraishi S, Jaffe M, et al. Gold nanorod-collagen nanocomposites as photothermal nanosolders for laser welding of ruptured porcine intestines. ACS Biomater Sci Eng. 2015;1(9):805–815. doi: 10.1021/acsbiomaterials.5b00174.
- Mushaben M, Urie R, Flake T, et al. Spatiotemporal modeling of laser tissue soldering using photothermal nanocomposites. Lasers Surg Med. 2018;50(2):143–152. doi: 10.1002/lsm.22746.
- Urie R, Guo C, Ghosh D, et al. Rapid soft tissue approximation and repair using laser-activated silk nanosealants. Adv Funct Mater. 2018;28(42):1802874. doi: 10.1002/adfm.201802874.
- Urie R, McBride M, Ghosh D, et al. Antimicrobial laser-activated sealants for combating surgical site infections. Biomater Sci. 2021;9(10):3791–3803. doi: 10.1039/d0bm01438a.
- Matteini P, Rossi F, Menabuoni L, et al. Microscopic characterization of collagen modifications induced by low-temperature diode-laser welding of corneal tissue. Lasers Surg Med. 2007;39(7):597–604. doi: 10.1002/lsm.20532.
- Sandy-Hodgetts K, Carville K, Leslie GD. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J. 2015;12(3):265–275. doi: 10.1111/iwj.12088.
- Liu X, Sprengers M, Nelemans PJ, et al. Risk factors for surgical site infections in dermatological surgery. Acta Derm Venereol. 2018;98(2):246–250. doi: 10.2340/00015555-2844.
- Shupak RP, Blackmore S, Kim RY. Skin hypersensitivity following application of tissue adhesive (2-octyl cyanoacrylate). Baylor University Medical Center Proceedings. Vol. 34; 2021. p. 736–738. doi: 10.1080/08998280.2021.1935140.
- Chateau D, Liotta A, Vadcard F, et al. From gold nanobipyramids to nanojavelins for a precise tuning of the plasmon resonance to the infrared wavelengths: experimental and theoretical aspects. Nanoscale. 2015;7(5):1934–1943. doi: 10.1039/c4nr06323f.
- Zhao S, Tian Y, Liu W, et al. High and low molecular weight hyaluronic acid-coated gold nanobipyramids for photothermal therapy. RSC Adv. 2018;8(16):9023–9030. doi: 10.1039/c7ra11667e.
- Huang H-C, Koria P, Parker SM, et al. Optically responsive gold nanorod-polypeptide assemblies. Langmuir. 2008;24(24):14139–14144. doi: 10.1021/la802842k.
- Huang HC, Barua S, Kay DB, et al. Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes. ACS Nano. 2009;3(10):2941–2952. doi: 10.1021/nn900947a.
- Walker CR, Pushpavanam K, Nair DG, et al. Generation of polypeptide-templated gold nanoparticles using ionizing radiation. Langmuir. 2013;29(32):10166–10173. doi: 10.1021/la400567d.
- Ramos J, Potta T, Scheideler O, et al. Parallel synthesis of poly(amino ether)-templated plasmonic nanoparticles for transgene delivery. ACS Appl Mater Interfaces. 2014;6(17):14861–14873. doi: 10.1021/am5017073.
- Rockwood DN, Preda RC, Yücel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379.
- Ghosh D, Salinas CM, Pallod S, et al. Temporal evaluation of efficacy and quality of tissue repair upon laser-activated sealing. Bioeng Transl Med. 2023;8(2):e10412.
- Scarabelli L, Sánchez-Iglesias A, Pérez-Juste J, et al. A tips and tricks practical guide to the synthesis of gold nanorods. J Phys Chem Lett. 2015;6(21):4270–4279. doi: 10.1021/acs.jpclett.5b02123.
- Khlebtsov NG, Khlebtsov BN, Kryuchkova EV, et al. Universal determination of gold concentration in colloids with UV–vis spectroscopy. J Phys Chem C. 2022;126(45):19268–19276. doi: 10.1021/acs.jpcc.2c05843.
- Abbas DB, Lavin CV, Fahy EJ, et al. Standardizing dimensionless cutometer parameters to determine in vivo elasticity of human skin. Adv Wound Care (New Rochelle). 2022;11(6):297–310. doi: 10.1089/wound.2021.0082.
- Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002;277(48):46616–46621. doi: 10.1074/jbc.M209003200.
- Wang Y, Marshall KL, Baba Y, et al. Hyperelastic material properties of mouse skin under compression. PLoS One. 2013;8(6):e67439. doi: 10.1371/journal.pone.0067439.
- Sánchez-Iglesias A, Winckelmans N, Altantzis T, et al. High-Yield seeded growth of monodisperse pentatwinned gold nanoparticles through thermally induced seed twinning. J Am Chem Soc. 2017;139(1):107–110. doi: 10.1021/jacs.6b12143.
- Chateau D, Desert A, Lerouge F, et al. Beyond the concentration limitation in the synthesis of nanobipyramids and other pentatwinned gold nanostructures. ACS Appl Mater Interfaces. 2019;11(42):39068–39076. doi: 10.1021/acsami.9b12973.
- Nafisah S, Morsin M, Sanudin R, et al. Effect of additive acid on seeded growth of gold nanobipyramids. J Phys Chem Solids. 2021;148:109764. doi: 10.1016/j.jpcs.2020.109764.
- Fang C, Zhao G, Xiao Y, et al. Facile growth of high-Yield gold nanobipyramids induced by chloroplatinic acid for high refractive index sensing properties. Sci Rep. 2016;6(1):36706. doi: 10.1038/srep36706.
- Lee JH, Gibson KJ, Chen G, et al. Bipyramid-templated synthesis of monodisperse anisotropic gold nanocrystals. Nat Commun. 2015;6(1):7571. doi: 10.1038/ncomms8571.
- Ridha I, Basiri A, Godeshala S, et al. Chromophore-free sealing and repair of soft tissues using mid-infrared light-activated biosealants. Adv Funct Mater. 2021;31(6):2007811. doi: 10.1002/adfm.202007811.
- Muller B, Mazza E, Schiestl C, et al. Longitudinal monitoring and prediction of long-term outcome of scar stiffness on pediatric patients. Burns Trauma. 2021;9:tkab028.
- Fong SS, Hung LK, Cheng JC. The cutometer and ultrasonography in the assessment of postburn hypertrophic scar–a preliminary study. Burns. 1997;23 Suppl 1(Suppl 1):S12–S18. doi: 10.1016/S0305-4179(96)00095-2.
- Nedelec B, Correa JA, Rachelska G, et al. Quantitative measurement of hypertrophic scar: interrater reliability and concurrent validity. J Burn Care Res. 2008;29(3):501–511. doi: 10.1097/BCR.0b013e3181710881.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi: 10.1177/147323000903700531.
- Kim SH, Lee SJ, Kim HJ, et al. Aging-related changes in the mid-face skin elasticity in east Asian women. Arch Craniofac Surg. 2019;20(3):158–163. doi: 10.7181/acfs.2019.00213.
- Busche MN, Thraen A-CJ, Gohritz A, et al. Burn scar evaluation using the cutometer(R) MPA 580 in comparison to “patient and observer scar assessment scale” and Vancouver scar scale. J Burn Care Res. 2018;39(4):516–526. doi: 10.1093/jbcr/irx009.
- Ignatieva N, Zakharkina O, Dadasheva A, et al. Transformation of the dermal collagen framework under laser heating. J Biophotonics. 2019;12(12):e201960024.
- Pyun H-B, Kim M, Park J, et al. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev Nutr Food Sci. 2012;17(4):245–253. doi: 10.3746/pnf.2012.17.4.245.
- Kramer EA, Rentschler ME. Energy-based tissue fusion for sutureless closure: applications, mechanisms, and potential for functional recovery. Annu Rev Biomed Eng. 2018;20(1):1–20. doi: 10.1146/annurev-bioeng-071516-044702.
- Huang J, Xia S, Jia M, et al. Experimental study of the effect of temperature on collagen conformational changes in skin tissue welded by femtosecond laser. Optik. 2023;288:171184. doi: 10.1016/j.ijleo.2023.171184.
- Derman İD, Şenel EC, Ferhanoğlu O, et al. Effect of heat level and expose time on denaturation of collagen tissues. Cell Mol Bioeng. 2021;14(1):113–119. doi: 10.1007/s12195-020-00653-w.
- Kirsch KM, Zelickson BD, Zachary CB, et al. Ultrastructure of collagen thermally denatured by microsecond domain pulsed carbon dioxide laser. Arch Dermatol. 1998;134(10):1255–1259. doi: 10.1001/archderm.134.10.1255.
- Reich S, Katchalsky A, Oplatka A. Dynamic-elastic investigation of the chemical denaturation of collagen fibers. Biopolymers. 1968;6(8):1159–1168. doi: 10.1002/bip.1968.360060810.
- Mehta-Ambalal SR. Neocollagenesis and neoelastinogenesis: from the laboratory to the clinic. J Cutan Aesthet Surg. 2016;9(3):145–151. doi: 10.4103/0974-2077.191645.
