Abstract
Purpose
This study aimed to investigate the efficacy and safety of ultrasound-guided percutaneous radiofrequency ablation (RFA) for the treatment of synovial hyperplasia in the knee joints of antigen-induced arthritis (AIA) model rabbits.
Methods
Forty Japanese large-eared white rabbits were divided into AIA and control groups. After successful induction of the AIA model, the knee joints were randomly assigned to RFA and non-RFA groups. The RFA group underwent ultrasound-guided RFA to treat synovial hyperplasia in the knee joint. Dynamic observation of various detection indices was conducted to evaluate the safety and effectiveness of the RFA procedure.
Results
Successful synovial ablation was achieved in the RFA group, with no intraoperative or perioperative mortality. Postoperative the circumference of the knee joint reached a peak before decreasing in the third week after surgery. The incidence and diameter of postoperative skin ulcers were not significantly different compared to the non-RFA group (p > .05). Anatomical examination revealed an intact intermuscular fascia around the ablated area in the RFA group. The ablated synovial tissue initially presented as a white mass, which subsequently liquefied into a milky white viscous fluid. Gross articular cartilage was observed, along with liquefied necrosis of the synovium on pathological histology and infiltration of inflammatory cells in the surrounding soft tissue.
Conclusion
The experimental results demonstrated that ultrasound-guided RFA of the knee in the treatment of synovial hyperplasia in AIA model animals was both effective and safe.
Introduction
Synovial hyperplasia is a prevalent pathological process observed in numerous osteoarthropathies, including rheumatoid arthritis, gouty arthritis, osteoarthritis, traumatic arthritis, and hemophilic arthropathy [Citation1–3]. It plays a pivotal role in disease progression. Rheumatoid arthritis (RA), a chronic systemic autoimmune disease, is primarily characterized by persistent synovial hyperplasia, synovitis, formation of synovial vascular opacities, and the release of various inflammatory mediators, protein hydrolases, and collagenases by immunoreactive cells [Citation4, Citation5]. These factors contribute to the erosion and lysis of joint cartilage, ligaments, tendons, and other tissues, leading to the destruction of joint cartilage, subchondral osteolysis, relaxation and destruction of the joint capsule, joint dislocation, fusion, and ossification. Ultimately, these processes result in ankylosis of the affected joint and complete loss of joint function, which is the primary cause of joint deformity.
Although disease-modifying antirheumatic drugs (DMARDs) [Citation6], particularly methotrexate (MTX) [Citation7], have been employed to enhance the prognosis of RA patients, some patients are compelled to discontinue treatment due to ineffective or unsatisfactory results and drug side effects [Citation8]. Current surgical open procedures used to treat synovial hyperplasia are highly invasive and have a slow recovery period [Citation9]. Arthroscopic synovectomy has a high recurrence rate post-surgery due to its limited incision and the difficulty in fully removing the synovium [Citation10,Citation11]. Some researchers have used radioisotopes to treat synovitis [Citation12,Citation13], which have shown some efficacy, but their use is restricted due to the presence of radioactivity. Consequently, there is an ongoing need to identify a safe and effective surgical treatment for synovial hyperplasia.
Ultrasound-guided percutaneous radiofrequency ablation has been extensively utilized to treat a variety of neoplastic lesions, such as liver cancer [Citation14,Citation15], kidney cancer [Citation16], thyroid nodules [Citation17], and breast nodules [Citation18]. It is considered an effective treatment method and a potential treatment modality for synovial hyperplasia. However, relevant clinical studies are still limited. In this study, we employed an animal model of immune-induced arthritis to simulate the pathological process of synovial hyperplasia in RA. We treated it with ultrasound-guided percutaneous radiofrequency ablation to investigate the safety and efficacy of this procedure.
Materials and methods
Animals
This study was undertaken at the Animal Experimentation Center of Wenzhou Medical University. All animals involved were treated in accordance with both Chinese laws and international animal welfare guidelines. The Japanese large-eared White rabbits utilized in the study were procured from the aforementioned Animal Experimentation Center.
The study included 40 male Japanese large-eared white rabbits of the clean-grade classification, aged six months, with weights ranging from 2.0 to 3.0 kg. The rabbits were divided into three groups: twenty were placed in the Antigen-Induced Arthritis (AIA) group, using the established AIA animal model methodology [Citation19]. Following the successful establishment of the animal model, one knee joint from each rabbit was selected for Radiofrequency Ablation (RFA) treatment, based on a computer-generated random number allocation. The treated side was designated as the Radiofrequency Ablation group (RFA group), while the untreated knee was regarded as the autologous control, referred to as the Non-Radiofrequency Ablation group (Non-RFA group). An additional 20 control animals received a local injection of saline and were thereafter raised in normal conditions without any further intervention.
Reagents and equipment
The reagents used included Ovalbumin (OVA, Sigma, USA) and Freund’s complete adjuvant (Sigma F5881, USA − 10 ml). SonoVue, a product of Braco, Italy, was employed as the ultrasound contrast agent. Prior to use, 5 ml of saline was added and the solution was agitated until it attained a uniform, milky consistency. It was then injected through the marginal ear vein, with each dose comprising 0.1 ml, followed by a 1 ml saline flush. The isolation strip injection used a 21 G PTC puncture needle, produced by Hachimitsu, Japan, and saline was used as the isolation solution.
The RF ablation instrument was a Cooltip-manufactured model, with the RF ablation needle model being ACT1510. The electrode length was 15 cm, with an exposed length of 1 cm. A consistent output power of 60 W was maintained during the RFA procedure.
The diagnostic ultrasound equipment used was a GE LOGIQ E9 model. For grayscale and energy Doppler ultrasound, an ML6-15 linear array probe was employed with a probe frequency of 7.0 MHZ, PWr – 15 dB, and mechanical index (MI) of 0.35. The instrument was fitted with a 9 L linear array probe, accompanied by ultrasonography software for ultrasonography. The frequency settings were GEN 7.0 MHZ, PWr – 15dB, and mechanical index (MI) 0.15, and an output energy of 10%. The gain was adjusted to an appropriate level prior to imaging.
Research methods
Establishment of animal model
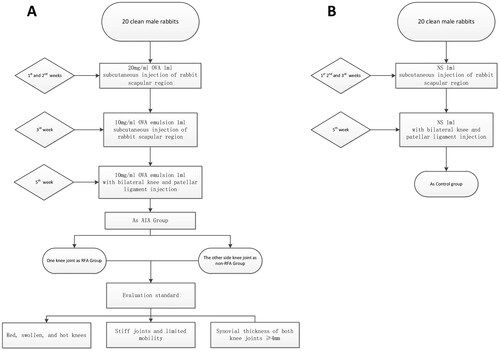
The model for arthritis was constructed employing the AIA technique (), comprising the following stages: (i) Primary Sensitization: 1 ml of Ovalbumin (OVA) saline solution (20 mg/ml) was administered subcutaneously at five distinct sites in the interscapular region of the rabbits for a period of 2 weeks. In the third week, an emulsion consisting of OVA and Freund’s complete adjuvant in a 1:1 ratio (10 mg/ml) was used at five different locations in the interscapular region of the rabbits. (ii) Knee Joint Injection: In the fifth week, both sides of the patellar ligament of the rabbits’ knee joint were injected with 1 ml of the 10 mg/ml OVA emulsion. (iii) Control Group Treatment: For the control group, 1 ml of saline solution was subcutaneously injected at five unique sites in the interscapular region of the rabbits during weeks 1 to 3. In the fifth week, 1 ml of saline solution was administered on both sides of the rabbits’ knee joint patellar ligament.
Model assessment
The following indicators were utilized to determine successful modeling: (i) General Condition Evaluation: Criteria include redness, swelling, heat, joint stiffness, and restricted movement of the knee joint. (ii) Ultrasound Evaluation: Successful modeling criteria include significant synovial proliferation in both knee joints.
Model monitoring
(i) Body weight and knee circumference of the rabbits were recorded on a weekly basis.(ii) Ultrasound Evaluation of Knee Osteoarthritis: Some research [Citation20] suggests that ultrasound offers superior efficacy compared to serology in assessing the AIA model. As such, ultrasound was adopted as an evaluation tool in this study. Measure the maximum thickness of the bursa effusion and synovial proliferation. The measurement of bursa effusion and synovial proliferation is based on measurements taken perpendicular to the bone surface of the knee joint, measuring the double-layer thickness of the synovium. Semi-quantitative scoring for synovitis was conducted using a 0-3 scale [Citation21].
Synovial radiofrequency ablation
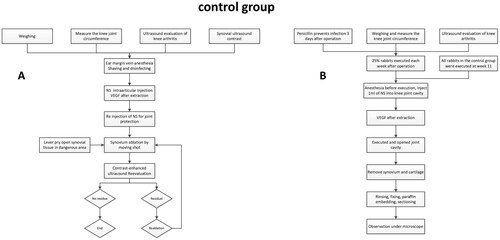
Preoperative measurements including the rabbit’s weight, knee circumference, and ultrasound evaluation of knee osteoarthritis were conducted (). Subsequently, the rabbits were intravenously anesthetized with 2% pentobarbital sodium (1.5 ml/kg) at the ear margin, shaved, and disinfected. Ultrasonography of the synovium was then conducted to observe synovial enhancement and to preserve the images. The ‘fluid isolation strip method’ [Citation22] was used as a strategy for joint cavity protection during ablation. Here, the joint cavity was filled with an injectable isolation fluid administered via a disposable 21 G PTC needle, aiming to protect the joint cavity, paracartilaginous tendons, and soft tissues. Ablation was performed by fixed personnel, including an ablation surgeon and an assistant, using the Moving-Shot technique for RFA under percutaneous real-time ultrasound guidance [Citation23]. Moving-Shot technique is performed in the synovial tissue by using the following methods: The electrode needle was percutaneously punctured to the distal end of the synovial tissue, then the ablation system was initiated and the needle was gradually withdrawn during the ablation process. This was paused when the needle reached the proximal end of the synovium, after which the direction was changed and the needle was reintroduced to the distal end of the synovium, before resuming ablation and needle withdrawal. In cases where synovial tissues adjacent to articular cartilage and significant tendons could not be fully separated by the isolation fluid, the "lever prying" method was employed as a response strategy [Citation24]. This involved prying up the synovial tissues in the risk area with the needle tip, separating them from the surrounding tissues and ablating all observable synovial tissues. Upon completion of synovial ablation, 0.1 ml of Sonovue was reinjected to perform contrast-enhanced ultrasound (CEUS), aiming to observe the presence of residual active synovial tissue and to determine the areas of surrounding soft tissue that may have been misablated. These images were saved and compared with preoperative CEUS. In instances of residual active synovial tissue, additional synovial ablation was performed until complete ablation was achieved (), and record the time(s) used for the ablation.
Postoperative treatment
Post-surgery, the ablation site was subjected to cooling via an ice pack for a period of 2 h (). To avert potential infections, penicillin (160,000 units/ml, 2.5 ml) was intramuscularly administered for the first three consecutive days post-surgery. Body weight and knee joint circumference measurements were conducted weekly until the rabbits were euthanized. Knee arthritis was evaluated using ultrasound on a weekly basis. A quarter of the experimental animals were euthanized each week for the four weeks following the surgery, whereas all of the control animals were euthanized in the 11th week. Following euthanasia, the patella and the soft tissue flap were lifted, and the knee joint was flexed to its maximum to fully expose the joint cavity. Observations were made regarding the distribution and residual status of the synovial membrane in both knees, any adhesions on the knee joint surface of the experimental side, and any damage to cartilage and bone. It was also verified that the knee tendons were free of injuries. These findings were duly recorded. The synovial tissue was excised, rinsed with chilled saline, and subsequently fixed in 10% neutral formaldehyde for 48 h. The skin, subcutaneous tissue, ligaments, tendons, synovium, cartilage, and subchondral bone of the knee joint’s tibial plateau were excised, rinsed with distilled water, and then placed in 10% neutral formaldehyde for fixation for 48 h. This was followed by decalcification with ethylenediaminetetraacetic acid, embedding in paraffin, and then sectioning continuously at 5 μm for microscopic analysis.
Statistical analysis
The statistical analysis was conducted using the SPSS 19.0 software. Measurement data conforming to a normal distribution were compared between two groups and presented as Mean ± SD. Two-way ANOVA was utilized to compare data with equal variance across groups, while the Sidak test was employed for pairwise comparison between two groups, and the Tukey test was used for pairwise comparison amongst three groups. A p-value of less than .05 was considered statistically significant. Graphpad Prism 8.0 software was used for generating and analyzing curves and bar graphs.
Results
Establishment and evaluation of the AIA model
During the establishment of the AIA model, three out of 20 Japanese large-eared white rabbits unfortunately died. The remaining 17 rabbits were successfully molded in one attempt, demonstrating a success rate of 85% (17/20). An escalation in the knee joint circumference emerged after the intra-articular injection in the fifth week of modeling (). This phenomenon was associated with joint erythema and edema, augmented skin temperature, and knee skin ulceration (58.8%, 10/17), with ulcers measuring between 5 and 10 mm in diameter (mean 7.50 ± 2.64 mm). By the seventh week, ultrasound assessment confirmed synovial hyperplasia in the knee joint, satisfying the criteria for a successful model (). The synovial membrane thickness in the RFA group varied between 4 to 9 mm, and 4 to 15 mm in the non-RFA group (; ). Synovitis scores in the RFA group ranged from 0 to 3 points (mean 1.47 ± 1.07 points) and the non-RFA group showed similar scoring (mean 1.29 ± 0.92 points). No statistically significant disparities were observed between the groups (all p > .05, ). The control group, consisting of 20 rabbits, had no fatalities during the feeding process. The rabbits in the AIA group experienced marginally lower weight gain than the control group; the weight difference did not reach statistical significance until the seventh week (all p < .01, ).
Figure 3. Weight and knee circumference change over time; A: weight-time change curve; B: knee circumference-time change curve; C: two-way ANOVA of weight-time change residual plots; D: two-way ANOVA of residual plots of circumference time change. In statistical comparisons, the RFA group differed significantly from the Control group (aP < .05), the RFA group from the non-RFA group (bP < .05), and the non-RFA group from the Control group (cP < .05). *p < .05, **p < .01, ***p < .001.
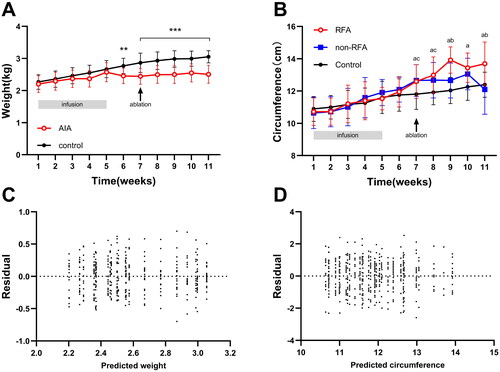
Figure 4. Observations after synovial ablation; A: ultrasound assessment of knee effusion depth; B: ultrasound assessment of synovial hyperplasia thickness; C: ultrasound semi-quantitative assessment of synovitis score; D: skin ulcer diameter before and after ablation (*p < .05, **p < .01, ***p < .001).
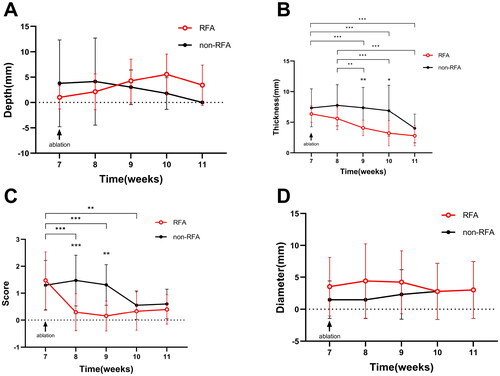
Table 1. Comparison of the knees in the RFA and non-RFA groups after modeling.
Radiofrequency ablation and intraoperative monitoring
A total of 17 rabbits in the AIA group underwent a seamless intravenous anesthesia process at the ear margins. Post-anesthesia, a preoperative contrast-enhanced ultrasound (CEUS) was performed on the RFA group (), revealing a high enhancement (76.47%, 13/17 cases) and uniform enhancement (64.71%, 11/17 cases) of the synovium during the arterial phase. The remaining displayed moderate to slightly low enhancement with heterogeneous contrast and slow fading in the parenchymal phase. The peripheral fluid accumulation area consistently showed a filling defect region with no enhancement.
In each case of synovial ablation, ultrasound was used for guidance, a liquid isolation band method for injection, a lever prying technique in areas close to the joint surface, and the Moving-shot technique for synovial ablation (). CEUS evaluation showed that after the first attempt, 8 out of 17 cases (47.06%) achieved complete ablation, and after re-ablation, 15 out of 17 cases (88.24%) achieved complete ablation, with the remaining two cases completely ablated after the third attempt (). The differences in complete ablation of the rabbit synovial joints after one, two, and three attempts were statistically significant [(5.17 ± 1.17) mm vs (6.75 ± 0.89) mm vs 7.00 mm, F = 5.325, p = .020], and the differences between one-time ablation vs two or three times were statistically significant (p values of .010 and .038, respectively).
Figure 5. Bilateral knee ultrasound evaluation and monitoring during synovial ablation; A, B: non-RFA side of knee synovial (↑) thickness 6 mm, CDFI synovitis score 1 (↑); C: ultrasound-guided synovial ablation showing RFA pin (↑); D, E: same rabbit RFA side of knee synovial thickness (↑) 7 mm, CDFI synovitis score 1 (↑); F: synovial ablation with CDFI showed annular shape (↑).
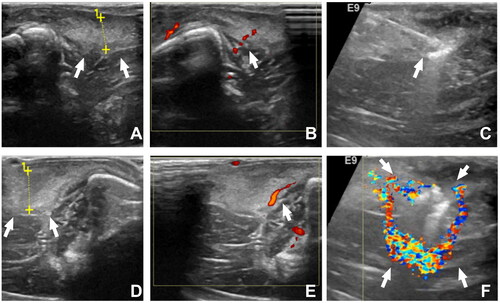
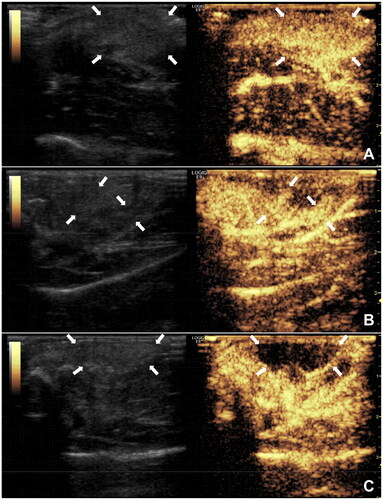
Figure 6. CEUS for monitoring and evaluating the effectiveness of synovial ablation; A: preoperative CEUS showing highly hyperplastic synovium (↑); B: CEUS monitoring during ablation showing residual synovium (↑); C: CEUS after re-ablation showing no residual synovium (↑).
In the 17 cases of RFA, there were no reports of deaths related to anesthesia or surgical procedures. Additionally, intraoperative CEUS revealed that in the RFA group, there were no areas of non-enhancement in the peripheral tissues, indicating no inadvertent ablation in all rabbits.
Postoperative efficacy evaluation of RFA
Post-synovial RFA, the 17 rabbits in the AIA group received standard anti-infection treatment for three days. No mortalities were observed during the postoperative observation period until the point of sacrifice. The weekly postoperative sacrifice numbers were four rabbits per week during weeks 8–10 and five rabbits in week 11.
The AIA group of rabbits continued to show reduced weight gain up to four weeks postoperatively, with a significant statistical difference compared to the control group (both p < .001) (). Knee circumference in the RFA group started to increase immediately postoperatively, peaked at week 9 (postoperative week 2), then subsequently decreased, showing a statistically significant difference (p < .05) at weeks 9 and 11 in comparison to the non-RFA group. After reaching its apex at week 10 (postoperative week 3), the knee circumference in the non-RFA group diminished by week 11.
The 17 rabbits in the AIA group underwent RFA for a duration of 100 to 190 s (156.5 ± 24.0s). presents the statistical correlation between the ablation time and synovial thickness, joint effusion, and skin breakdown before RFA and at 1, 2, and 4 weeks post-operation. The results showed a significant positive correlation between the duration of RFA and the thickness of synovial proliferation before the operation and at 1-week post-operation (p < .001).
Table 2. Correlation analysis between time (s) of RFA and synovial thickness, knee effusion, and skin rupture in AIA group.
A comparison of preoperative and postoperative ultrasonographic evaluation indices is presented (; ). In the RFA group, compared to pre-operation (week 7), a significant reduction in synovial thickness was observed between weeks 9 and 11 post-operation, and the synovitis score significantly decreased between weeks 8 and 10, with the differences being statistically significant (all p < .05). The synovitis score reached its lowest point at week 9 (the 3rd week post-operation). An increase in bursal fluid was observed during weeks 8 to 10 (the initial three weeks post-operation), followed by a decrease in week 11. Upon examining the ultrasound images and dissecting the animals post-euthanasia, it was observed that the synovial tissue gradually liquefied over time following RFA.
Table 3. Comparison of evaluation indexes before and after ablation between RFA group and non-RFA group.
Post-surgery, within the first two weeks, knee joint erythema and swelling, along with heightened surface skin temperature, were observed in the RFA group. Skin ulceration or enlargement of preexisting skin ulcers was detected in 4 cases (23.53%), but there was no statistically significant difference in overall ulcer area compared to the preoperative period (all p > .05). The differences were also non-significant when compared with the non-RFA group (all p > .05).
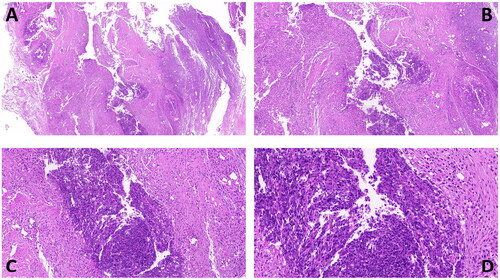
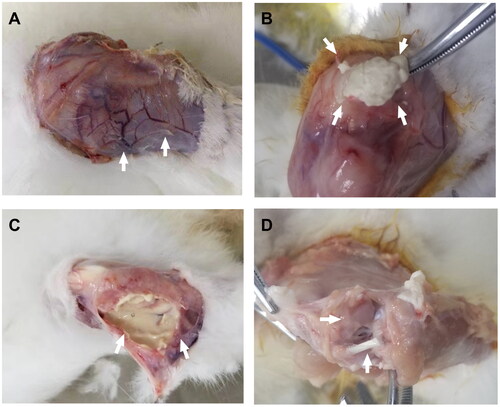
Joint anatomical evaluation of the AIA group rabbits demonstrated (): intact muscle fascia surrounding the ablation area in the RFA group with no significant muscle, tendon, or ligament adhesions; synovial tissue appearing as a white mass at weeks 8–9 (), with most of the synovial membrane liquefying into a milky white viscous fluid by weeks 10–11 (); the gross articular cartilage was preserved, and the morphology remained intact, with no significant differences compared to the non-RFA group (). During dissection, we observed the relationship between skin ulcers and deeper tissues and found no evidence of penetrating injuries inside the joint. The non-RFA group showed synovial hyperplasia with accompanying synovitis (). The histopathology of the ablation area () revealed that in the AIA group, there were no necrotic areas in the subcutaneous tissues, ligaments, tendons, synovium, or cartilage. In the RFA group, liquefactive necrosis was observed in the synovial membrane (13/17), and inflammatory cell infiltration was present in the surrounding soft tissues (15/17), whereas the non-RFA group exhibited synovial proliferation accompanied by synovitis.
Figure 7. Anatomy of rabbit knee joint in AIA group, A: increased synovial vascular thickening 1 week after surgery in non-RFA group (↑); B–C: after synovial ablation in RFA group, B is 2 weeks after surgery, synovial membrane is lumpy or cheese-like (↑), C is 4 weeks after surgery, synovial membrane is milky white viscous fluid (↑); D: joint surface has intact ligaments and rough cartilage (↑).
Discussion
Synovial tissue (ST) is a soft, innermost layer of the joint capsule, lining the joint space. This light-red, smooth, thin, and pliable tissue comprises loose connective tissue, providing a connective tissue membrane covering the inner surface of the joint capsule [Citation1]. Synovial tissue presents a non-adhesive, deformable interface between tissues, offering lubrication for the cartilage and nourishment to chondrocytes [Citation25]. Since rheumatoid arthritis (RA) was first described, synovial proliferation has been highlighted in clinical trials as a critical component of the RA pathophysiological process [Citation26,Citation27].
The primary cell populations impacted by RA are synovial cells and chondrocytes. Synovial cells can be categorized into fibroblast-like and macrophage-like synoviocytes. The overproduction of pro-inflammatory cytokines is primarily attributed to macrophage-like synoviocytes, whereas fibroblast-like synoviocytes exhibit abnormal behavior in RA. In an experimental model, some authors have concurrently implanted fibroblast-like synoviocytes with cartilage, leading to fibroblast invasion of the cartilage [Citation28]. Extensive evidence suggests that joint destruction and osteoclast activation are key mechanisms leading to bone erosion [Citation29,Citation30]
As mainstream surgical treatments for synovial hyperplasia, open surgery and arthroscopic synovectomy [Citation9,Citation10] possess certain limitations in clinical application [Citation11]. Even though Disease-Modifying Antirheumatic Drugs (DMARDs) mitigate disease progression, complete elimination of synovial hyperplasia remains a formidable challenge. The accumulated experience from the application of percutaneous Radiofrequency Ablation (RFA) in the treatment of liver, thyroid, breast nodules, and other organs demonstrates percutaneous RFA as an effective treatment modality for a variety of tumors, thereby making percutaneous RFA a worthwhile option for the treatment of synovial hyperplasia.
The mature application of ultrasound in the evaluation of musculoskeletal diseases enables gray-scale ultrasound and color Doppler to accurately ascertain synovial thickness and semi-quantitatively assess the degree of synovial inflammation [Citation15]. Furthermore, ultrasonography can distinguish synovial tissue from other necrotic tissues in the joint cavity and determine the residual synovial tissue post-thermal ablation. This study employed ultrasound as a means of guidance, monitoring, and evaluation of synovial ablation.
Establishment and evaluation of AIA animal models
Ovalbumin (OVA) is widely employed as an exogenous antigen to establish various immune-induced animal models [Citation31], among which the Antigen-Induced Arthritis (AIA) model effectively simulates the pathophysiological process of RA. The advantage of the AIA model is that it does not naturally recover from arthritis with time following successful modeling, providing a more effective means to observe the therapeutic effect of RFA. In this study, the AIA group rabbits had a high modeling success rate (17/20, 85%) and could be consistently used for ultrasound-guided synovial ablation studies. Post-modeling, the AIA group rabbits exhibited reduced food intake, slow or non-existent weight gain compared to the control group, knee joint erythema and swelling post-joint cavity injection, reduced hind limb movement, and increased knee joint diameter.
Safety of RFA procedure
Complications associated with synovial Radiofrequency Ablation (RFA) align with those of other superficial sites, predominantly encompassing risks of anesthesia, bleeding, infection, thermal injury to skin and subcutaneous tissues at the treatment site, adhesions of surrounding tissues (particularly muscles, tendons, and ligaments), synovial and joint cavity effusion, and loss of joint function [Citation32]. No anesthesia- or surgery-related fatalities were observed amongst the 17 RFA cases.
The percutaneous puncture approach employed in this study, under real-time ultrasound guidance, effectively circumvented large blood vessels and important anatomical structures, resulting in minimal intraoperative bleeding. Moreover, with postoperative anti-infection treatment support, no significant systemic or soft tissue infections were observed following surgery.
In this study, complete ablation of synovial tissue was achieved with one, two, and three ablation attempts in 8, 7, and 2 cases, respectively. We observed that with increasing synovial tissue thickness, more ablation attempts and real-time CEUS assessments during surgery were needed to avoid accidental ablation of surrounding joint tissues and achieve complete synovial tissue ablation.
The lever prying method, currently mainly used for radiofrequency and microwave ablation of thyroid nodules in clinical practice, aims to displace the ablation area from its original location to prevent heat damage to important structures like nerves. This study’s lever prying technique is based on this principle. Despite using measures like liquid isolation bands, the Moving-shot technique, lever prying, and postoperative ice application, the RFA group still showed common knee joint redness and swelling, increased skin temperature, and fluid accumulation within two weeks post-operation. In four instances, skin ulcers were observed or existing ulcers worsened, although the difference was not statistically significant compared to pre-operation and the contralateral side. While the difference was not significant (p > .05), this issue still warrants attention. However, during dissection, no penetrating injuries were found within the joint related to skin ulcers, suggesting these ulcers were more associated with repeated joint injections during modeling rather than thermal deposition effects of RFA [Citation24, Citation33].
Skin and subcutaneous tissue injury is a common complication of superficial site thermal ablation. Apart from synovial ablation, RFA application has been reported in numerous clinical studies involving sites like the thyroid, breast, lymph nodes, and superficial tumors. In analyzing the four instances of skin ulcers or enlarged existing ulcers, we observed that these rabbits’ knee synovial membrane were more extensive and thicker. The same output power used for RFA in this study resulted in longer total ablation times and greater local tissue thermal deposition in these instances. Therefore, the resolution lies in controlling total RFA time, possibly achieved via a second-stage procedure.
Fortunately, no significant misablation of muscles or ligaments was detected at necropsy in this animal group. There were also no significant postoperative muscle, tendon, or ligament adhesions observed, nor was there any difference in thermal damage to articular cartilage in the RFA group compared with the non-RFA group at gross autopsy. Hence, it can be inferred that damage to other surrounding tissues during RFA treatment of synovial hyperplasia was limited and controlled. Regarding joint function loss, the animals in the Antigen-Induced Arthritis (AIA) group exhibited reduced knee motion in the RFA group within two weeks postoperatively, but knee motion function gradually returned after 2 weeks. Therefore, percutaneous RFA’s performance in the treatment of synovial hyperplasia is deemed overall safe.
Efficacy of RFA procedure
In the RFA group, the knee joint’s circumferential diameter peaked in the second week post-operation and then decreased, corresponding to an increase in joint effusion, soft tissue swelling, and slow absorption of the synovium within the first two weeks post-operation. All these processes showed significant improvement in the third-week post-operation. Specifically, the fluid volume in the bursa initially increased in the first three weeks post-operation and then started to decrease by week 11. The synovium gradually liquefied after the surgery, and the synovitis score rapidly declined. These observations indicate that RFA directly facilitated the clearance of synovial proliferation in rabbit joints. However, the AIA model has a persistent arthritic effect, and in some cases of the RFA group, synovial tissue reappeared by week 9 post-operation, as detected by ultrasound, when the underlying cause of the condition had not been eliminated.
Of note was the gradual decrease in synovial thickness, synovitis score, and bursal effusion in the non-RFA group following surgery, with a significant downward trend apparent at postoperative weeks 3–4. The possible mechanism behind this is speculated to be the amelioration of secondary infection through the administration of antibiotics, or the activation of the body’s immune response by the RFA procedure, leading to improvements in inflammatory joint lesions in the non-RFA group.
The AIA model is similar to the RA, in the treatment of RA, effective management is typically gauged by a significant reduction in synovial inflammation and the prevention of joint destruction. While there isn’t a universally accepted specific level of synovial hyperplasia and synovitis reduction that is considered as definitive evidence of treatment effectiveness, clinical decisions are often based on the overall reduction of these symptoms and the prevention of joint damage. Therefore, overall, the results of this study indicate that RFA is effective for treating localized synovial proliferation in joints, in terms of reducing synovial proliferation, synovitis, joint effusion, and other soft tissue aspects.
Limitations
This study presents several limitations. Primarily, the relatively small sample size, which was further diminished when experimental animals were euthanized 4 weeks postoperatively, suggests that the results of the study can only indicate trends. Furthermore, the mechanism behind the reduction in knee diameter and synovium in the non-RFA group due to RFA’s action on synovial tissue remains unclear, necessitating further research for elucidation.
Another important point to note is that the follow-up period for this study was only 4 weeks after RFA procedure. During RFA treatment for synovial ablation, the temperature inside the joint significantly increases. Although no issues with cartilage damage were observed during the short-term follow-up, there is a possibility that thermal injury could exacerbate cartilage damage in the long term. Therefore, future studies involving long-term controlled observations are needed to confirm the possibility of such complications.
Conclusion
Ultrasound-guided radiofrequency ablation of the rabbit knee synovium provides an effective surgical treatment for immune-mediated synovial hyperplasia removal. With its high surgical safety, minimal invasiveness, few postoperative complications, and repeatability, this method merits further investigation to confirm whether it has the effect of activating the immune response to the pathophysiological process of the disease.
Author contributions
Xu Q and Yan ZH contributed to the study conception and design; Xu XH, Liu ZZ, Zhu JB, Ding HH participated in the material preparation and data collection; Jin CC performed the analyses. Xu Q drafted the initial manuscript and revised the article; all authors read and approved the final manuscript.
Acknowledgments
We are grateful to Weiyi Wang for his constructive suggestions during the revision of article and for planning the complementary experimental study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American college of rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1–13. doi: 10.1002/acr.21772.
- Barg A, Knupp M, Kapron AL, et al. Total ankle replacement in patients with gouty arthritis. J Bone Joint Surg Am. 2011;93(4):357–366. doi: 10.2106/JBJS.J.00957.
- Smith MM, Cake MA, Ghosh P, et al. Significant synovial pathology in a meniscectomy model of osteoarthritis: modification by intra-articular hyaluronan therapy. Rheumatology (Oxford). 2008;47(8):1172–1178. doi: 10.1093/rheumatology/ken219.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8.
- Asif Amin M, Fox DA, Ruth JH. Synovial cellular and molecular markers in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):385–393. doi: 10.1007/s00281-017-0631-3.
- Pincus T, Yazici Y, Sokka T, et al. Methotrexate as the "anchor drug" for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol. 2003;21(5 Suppl 31):S179–S85.
- Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: still an "anchor" drug? Autoimmun Rev. 2014;13(11):1102–1108. doi: 10.1016/j.autrev.2014.08.026.
- van der Heijden JW, Dijkmans BA, Scheper RJ, et al. Drug insight: resistance to methotrexate and other disease-modifying antirheumatic drugs–from bench to bedside. Nat Clin Pract Rheumatol. 2007;3(1):26–34. doi: 10.1038/ncprheum0380.
- Biehl C, Braun T, Thormann U, et al. Radiocarpal fusion and midcarpal resection interposition arthroplasty: long-term results in severely destroyed rheumatoid wrists. BMC Musculoskelet Disord. 2018;19(1):286. doi: 10.1186/s12891-018-2172-x.
- Lipina M, Makarov M, Mukhanov V, et al. Arthroscopic synovectomy of the knee joint for rheumatoid arthritis. Int Orthop. 2019;43(8):1859–1863. doi: 10.1007/s00264-018-4160-z.
- Yang JH, Kwon HH, Lee JK, et al. Successful arthroscopic treatment of refractory and complicated popliteal cyst associated with rheumatoid arthritis in combination with osteoarthritis: case series and literature review. Rheumatol Int. 2019;39(12):2177–2183. doi: 10.1007/s00296-019-04278-9.
- Miszczyk M, Jochymek B, Miszczyk L, et al. The results of 394 consecutive cases of knee joint radiation synovectomy (radiosynoviorthesis) using 90Y. Ann Nucl Med. 2020;34(2):94–101. doi: 10.1007/s12149-019-01418-w.
- Amini A, Yahyanezhad S, Velez E, et al. Prospective evaluation of phosphorus-32 radiation synovectomy in patients with severe and chronic rheumatoid arthritis unresponsive to conventional medical treatment. Nucl Med Commun. 2020;41(1):65–72. doi: 10.1097/MNM.0000000000001116.
- Hasegawa K, Aoki T, Ishizawa T, et al. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol. 2014;21 Suppl 3(S3):S348–S55. doi: 10.1245/s10434-014-3585-x.
- Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in asia: consensus statement from the asian oncology summit 2009. Lancet Oncol. 2009;10(11):1111–1118. doi: 10.1016/S1470-2045(09)70241-4.
- Yu J, Zhang X, Liu H, et al. Percutaneous microwave ablation versus laparoscopic partial nephrectomy for cT1a renal cell carcinoma: a propensity-matched cohort study of 1955 patients. Radiology. 2020;294(3):698–706. doi: 10.1148/radiol.2020190919.
- Aldea Martínez J, Aldea Viana L, López Martínez JL, et al. Radiofrequency ablation of thyroid nodules: a long-term prospective study of 24 patients. J Vasc Interv Radiol. 2019;30(10):1567–1573. doi: 10.1016/j.jvir.2019.04.022.
- Li P, Xiao-Yin T, Cui D, et al. Evaluation of the safety and efficacy of percutaneous radiofrequency ablation for treating multiple breast fibroadenoma. J Cancer Res Ther. 2016;12(Supplement):C138–C142. doi: 10.4103/jcrt.JCRT_966_16.
- Rafayelyan S, Meyer P, Radlanski RJ, et al. Effect of methotrexate upon antigen-induced arthritis of the rabbit temporomandibular joint. J Oral Pathol Med. 2015;44(8):614–621. doi: 10.1111/jop.12265.
- Chen S, Zheng Q, Liu H, et al. Sonography is superior to Serum-Based biomarkers for measuring disease status in experimental rheumatoid arthritis. J Ultrasound Med. 2016;35(10):2223–2230. doi: 10.7863/ultra.15.10044.
- Carotti M, Galeazzi V, Catucci F, et al. Clinical utility of eco-color-power doppler ultrasonography and contrast enhanced magnetic resonance imaging for interpretation and quantification of joint synovitis: a review. Acta Biomed. 2018;89(1-S):48–77. doi: 10.23750/abm.v89i1-S.7010.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623. doi: 10.3348/kjr.2017.18.4.615.
- Baek JH, Jeong HJ, Kim YS, et al. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid. 2008;18(6):675–676. doi: 10.1089/thy.2007.0274.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655. doi: 10.3348/kjr.2018.19.4.632.
- Kurowska-Stolarska M, Alivernini S. Synovial tissue macrophages: friend or foe? RMD Open. 2017;3(2):e000527. doi: 10.1136/rmdopen-2017-000527.
- Humby FC. Synovial tissue sampling in rheumatological practice-past developments and future perspectives. Front Med (Lausanne). 2019;6:4. doi: 10.3389/fmed.2019.00004.
- Veale DJ. Synovial tissue biopsy research. Front Med (Lausanne). 2019;6:72. doi: 10.3389/fmed.2019.00072.
- Müller-Ladner U, Kriegsmann J, Franklin BN, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149(5):1607–1615.
- Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, et al. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52(7):1999–2002. doi: 10.1002/art.21118.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4.
- Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40(3):193–200. doi: 10.1080/08923973.2018.1434793.
- Zhang W, Jin ZQ, Baikpour M, et al. Clinical application of ultrasound-guided percutaneous microwave ablation for benign breast lesions: a prospective study. BMC Cancer. 2019;19(1):345. doi: 10.1186/s12885-019-5523-6.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20. doi: 10.1080/02656736.2019.1622795.