Abstract
Purpose
The objective was to describe the technique and clinical outcome of microwave thermal ablation (MWA) and perfusion combined with synthetic bone substitutes in treating unicameral bone cysts (UBCs) in adolescents.
Materials and Methods
A total of 14 consecutive patients were enrolled by percutaneous MWA and saline irrigation combined with synthetic bone substitutes. Clinical follow-up included the assessment of pain, swelling, and functional mobility. Radiological parameters included tumor volume, physis-cyst distance, cortical thickness of the thinnest cortical bone, and the Modified Neer classification system.
Results
The mean follow-up was 28.9 months (26–52 months). All UBCs were primary, and all patients underwent the MWA, saline perfusion, and reconstruction combined with a synthetic bone substitute session, except for one patient (7.1%) who required a second session. All patients had good clinical results at the final follow-up. Satisfactory cyst healing was achieved in 13 cases according to radiological parameters. Tumor volume decreased from a mean of 49.7 cm3 before surgery treatment to 13.9 cm3 at the final follow-up (p < 0.01). The physis-cyst distance increased from a mean of 3.17–4.83 cm at the final follow-up (p < 0.01). Cortical thickness improved from a mean of 1.1 mm to 2.0 mm at the final follow-up (p < 0.01). According to the proposed radiological criteria, our results were considered successful (Grading I and II) in 13 patients (92.9%) at the final follow-up.
Conclusion
Percutaneous microwave ablation combined with a bone graft substitute is a minimally invasive, effective, safe, and cost-effective approach to treating primary bone cysts in the limbs of adolescents.
Introduction
Simple or unicameral bone cysts (UBCs) are commonly benign, fluid-filled bone lesions in adolescents or children, mainly located in long bones[Citation1, Citation2]. UBCs were reported to account for 3% of bone lesions and occur twice as often in boys as girls. The symptomatic cysts make the bone cortex thin, leading to pain or pathological fracture[Citation1, Citation2].
There is no current consensus around the treatment options for UBCs, but percutaneous treatments have been gaining popularity due to their success and minimally invasive, safe, and effective nature. Different modalities are available for the lesions, including mechanical curettage [Citation3], the injection of steroids [Citation3, Citation4], doxycycline sclerotherapy [Citation5], multiple drill holes [Citation6], and decompression with a cannulated screw [Citation7, Citation8], thermal ablation and cryoablation with or without bone-graft materials [Citation9]. Recurrence rates have been reported to range from 10–50% depending on the intervention [Citation1, Citation3–10], so a proportion of patients had to experience multiple surgeries to manage the lesions. The main goals of the treatments are to relieve or resolve pain, enhance cyst healing, and decrease the risk of pathological fracture.
The thorough disruption of the UBC’s wall lining is the critical point for the treatment of this lesion [Citation1, Citation8, Citation10]. Microwave ablation (MWA) is a thermal ablation technique that relies on an electromagnetic field to produce frictional energy that is converted into heat. With its relative insensitivity to impedance and perfusion, MWA can create a large ablation zone to radiate through all biological tissues, finally resulting in the inactivation of the target lesion within a few minutes [Citation11]. Only a few publications have reported the use of MWA for aneurysmal or unicameral bone cysts [Citation12, Citation13], with others often using this as a treatment for various bone metastases or osteoid osteomas [Citation14].
Although physical or chemical cauterization of the UBC wall lining can stop the progression of the disease, the posttreatment cortex remains fragile as the bone grows. Therefore, the introduction of bone graft substitutes has been used to enhance bone healing and strengthen fragile bone [Citation15]. Recently, an artificial biomimetic mineralized collagen (MC) bone repair material has been developed, which mimics the nanoscale fundamental unit of the natural bone [Citation16, Citation17]. Its microstructure accounts for its active osteo-induction and good osteogenic activity. This MC bone material has been effectively utilized for the treatment of different types of bone defects [Citation18, Citation19], including various benign bone tumors [Citation20].
Therefore, the purpose of our study is to present a series of patients with UBCs treated with the technique of MWA and irrigation combined with MC bone repair materials under fluoroscopic guidance and investigate the clinical outcomes.
Materials and methods
Study population
This is a single-center prospective observational study, with the approval of the ethical committee of the investigators’ Hospital (No. XJS2022-101-01). Hospital medical records were reviewed from July 2018 until January 2020 to identify all patients with symptomatic UBCs who underwent the procedure of MWA with bone graft substitute filling. Symptoms are defined as painful, pathological fractures, or deformities.
Procedure technique
All procedures were performed under general anesthesia. The bone cyst was located under fluoroscopy and the upper and lower margins of the cyst were marked on the skin. Based on preoperative planning for an adequate approach, a 5 mm incision was made at the upper or lower center of the cyst. An 11-gauge or 14-gauge access cannula was first used to obtain biopsy samples for pathological examination. Microwave ablation was performed with an MWA system (2450 MHz, MTI-5A, Great Wall, Nanjing, China) under fluoroscopic guidance. The choice of needle gauge size depended on the size of the cyst and the expected amount of bone graft substitute. A second 5 mm incision was made along the remaining visible edge of the lesion and the other access cannula was placed. The surgeon fluoroscopically confirmed the placement of two access cannulas at the upper and lower central points of the lesion. A 14-gauge MWA antenna (1.8 mm in diameter and 180 mm in length), was then placed in the lesion center through an access cannula and the ablation procedure was initiated (). Typically, one ablation cycle lasted for 30 s and the total ablation time ranged from 2 to 5 min. During the ablation procedure, the surgeon should closely monitor the temperature to ensure that it remains between 60 and 70 °C. During the procedure, the skin was wrapped with gauze and continuously washed with ice saline solution to avoid skin burns. Generally, most patients are recommended to undergo 4–6 ablation cycles. However, when the ablation area is estimated to be close to peripheral nerves or blood vessels, repeated short ablation periods should be used. In particular, cyst fluid should not be allowed to drain out of the cavity because the fluid was regarded as an ablation medium.
Figure 1. Microwave ablation and saline perfusion of the tumor cavity. (a) An MWA antenna was then placed successively in the upper and lower midpoint of the lesion through the access cannulas. (b) After the ablation process, the membrane linings of the cyst wall were inactivated (Black). Through a syringe (Blue), the bone cyst was repeatedly perfused and flushed with normal saline, allowing the sanguineous fluid and necrotic tissue to be expelled through the other syringe (Red) until the fluid ran cool and clear.
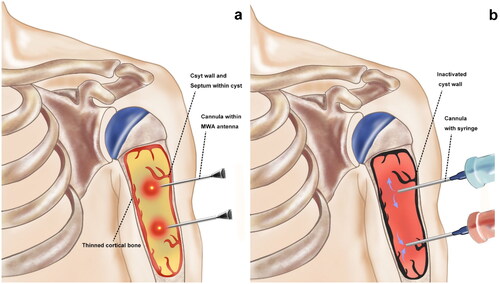
After the ablation was completed, both channels were connected to syringes. Through one syringe, normal saline was repeatedly perfused and flushed. During the procedure, it should be noted that a sanguineous fluid can be observed slowly flowing out from the other syringe (). If not, it may be due to misplaced puncture needles and it is necessary to evaluate whether there is any abnormal vascular flow. Finally, biomimetic mineralized collagen bone repair materials (Bone gold, Allgens Medical Technology Co., Ltd., China) were injected and pushed into the cavity with a clearing catheter through the channel. The volume of the synthetic bone substitute depended on the volume of the tumor cavity.
Follow-up and evaluation
Post-operative rehabilitation guidance varies depending on the site, size, or other concrete conditions of the cysts. Appropriate limitations are necessary until follow-up radiographs suggest that healing has occurred, including partial weight-bearing and restrictions of mobility. The patients were followed up at 6-, 9- and 12-months and then annually in the clinic. Tumor volume was measured using anteroposterior (AP) and lateral radiographs or CT scans and by multiplying maximum length, width, and depth. Cortical thickness was defined as the thickness of the thinnest cortical bone surrounding the tumor whether on the AP, lateral or oblique radiograph. The treatment was considered successful (in terms of cyst involution and lack of compromise of the surrounding growth plate) when the following three parameters existed together: the cyst volume decreased by a minimum of 50%, the bone cortex became thicker and the distance to physis increased. In addition, this study evaluated the radiographic films using the modified Neer scale system16 reported in previous studies17 (). A cyst that healed well or healed with defects was not considered to require treatment, whereas a persistent cyst or recurrence indicated therapeutic failure, for which supplementary treatments were needed. Student’s paired t-test was used for statistical analysis; a difference between the pre- and post-operative situation was considered significant for p-values <0.05.
Table 1. Modified new classification of radiologic results.
Results
Patient characteristics
The results of the demographic characteristics are shown in . A total of 14 consecutive patients were included, with an average age of 12 years. The most common tumor location was the proximal humerus (seven patients, 50%), followed by the femur (five patients, 35.8%), mid-humerus (one patient, 7.1%), and ischial tubercle (one patient, 7.1%). The most common clinical presentation was pain in seven patients (50%), followed by pathological fracture in six patients (42.8%). Two patients (12.5%) with lower limb UBCs presented with a painful limp. Half of the patients had already been treated by immobilization before surgical treatment. The patients who underwent a previous treatment did not experience clinical improvement, with some persistence of clinical and radiological symptoms and signs in seven patients.
Table 2. Summarizes patients’ characteristics, number of sessions, and clinical results at the last follow-up.
Clinical results at final follow-up
The treatments achieved technical success in all cases. All patients gained complete pain relief within 1–2 months and achieved recovery of the affected limb by the final follow-up visit. The average follow-up period was 28.9 months. No instances of infection, poor wound healing, nonunion fracture, or growth restriction were observed. Besides MWA and bone grafting, two patients accepted osteosynthesis due to pathological fracture in the primary surgery (Patients 9 and 14). Only one patient (Patient 1) reported the complication of proximal femoral pathological fracture in the third postoperative month due to an accidental stumble. Two representative cases are shown in and .
Figure 2. A 9-year-old boy originally presented with right proximal humerus UBC with the symptoms of pain and pathological fracture. (a) Before treatment, an expansile lucent lesion was found in the proximal humerus in the frontal radiograph, with cortical thinning and interruption. (b) On the second day after the surgical procedure, scattered high-density shadows can be observed surrounding the lesion, indicative of the synthetic bone substitute utilized during the treatment. (c, b, and d): the radiographs were taken at 3-, 9- and 12-months postoperatively, respectively. Follow-up radiographs showed a gradual reduction in fracture lines and an increase in callus formation, indicating ongoing bone remodeling and consolidation, consistent with healing changes. The radiograph at 12 months (e) demonstrated near-complete remineralization of UBC and fracture healing and the cortical bone was thickened and remodeled.
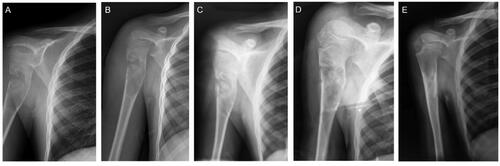
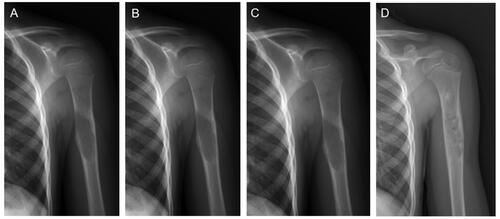
Figure 3. A representative MWA and bone grafting treatment to a mid-shaft humerus UBC.
(a) A 15-year-old boy was diagnosed with a bone cyst after experiencing pain. (b) Postprocedural radiograph on the second day. (c) Postprocedural radiograph after one and a half months. (d) The postoperative humerus AP radiograph in 6 months exhibited the vast majority of UBC remineralization.
At the final follow-up, all patients were pain-free, with a full range of movement in the affected limb and no swelling or redness. One patient presented with recurrent radiological changes without any clinical symptoms (Patient 14). One patient had a pathological fracture three months after surgery (Patient 1). The patient was treated by curettage, autograft combined with synthetic bone graft substitutes and external fixation, and was pain and symptom-free at the 13-month follow-up. Radiographic images of Patients 3 and 12 at the final follow-up showed residual radiolucent areas without any clinical or functional impact. Two patients (Patients 3 and 11) experienced mild limb shortening without any negative impact on their daily life activities.
Radiological results at the final follow-up
Tumor volume decreased from a mean of 49.7 cm3 before surgery treatment to 13.9 cm3 at the final follow-up (p < 0.01). The physis-cyst distance increased from a mean of 3.17 cm to 4.83 cm at the final follow-up (p < 0.01) (). Cortical thickness improved from a mean of 1.1 mm to 2.0 mm at the final follow-up (p < 0.01). According to the proposed radiological criteria, our results were considered successful (Grading I and II) in 13 patients (92.9%) at a minimum of 26 months follow-up.
Table 3. Comparison of X-ray parameters between pre-operation and post-operation.
Discussion
This study confirms the technical feasibility and clinical efficacy of microwave ablation, perfusion, and reconstruction with bone graft substitutes. The use of MWA can effectively disrupt cystic structures and relieve pain, while using MC for repairing bone defects caused by bone cysts is a promising option, offering long-term stable efficacy.
UBCs are still subject to debate regarding optimal management. Recently, Kim et al. [Citation10] compared the mainstream interventions in a total of 4973 patients with UBCs from 109 studies. The success rate of conservative treatment is 42.03%, and the cure rates of injection of methylprednisolone acetate, bone graft, bone substitute, or demineralized bone matrix range from 58.65 to 70.35%. Notably, curettage with or without bone autograft, substitute, demineralized bone matrix, or elastic stable intramedullary nail (ESIN) treatment success rate ranges from 69.73 to 88.61%, an average of 79.97%. Among them, the success rate of curettage with ESIN + bone graft/bone substitute is the highest, reaching 88.61%. Interestingly, decompression with cannulated screws is only 50%, close to conservative treatment. Compared to traditional techniques, MWA integrates their benefit and offers unique advantages, including the inactivation of lesions, pain relief, less invasiveness, and simplicity. Previous studies have reported that MWA is effective in achieving pain relief for patients suffering from bone lesions [Citation14, Citation21]. In our study, all patients reported complete pain relief at the final visit. The high temperature can thoroughly cauterize the cyst’s wall in a short time. Since the cysts are filled with fluid, the range and effectiveness of inactivation can be further magnified by heating the cystic fluid. In most cases, the cyst fluid fills the whole cavity, which allows the ablation heat to radiate through the entire space of the tumor cavity. In other words, the cyst fluid acts as a medium during the ablation heating procedure. This could potentially explain the lower recurrence rate observed in our center. For comparison, it is difficult for the tumor curettage to ensure that all of the three-dimensional and irregular cavity of the tumor is removed as planned, especially when the surgeons would like to save as much of the remaining cortical bone as possible. In addition, its simplicity and short duration are among the advantages. In our study, the entire ablation and inactivation process only lasted for 2–5 min in each patient. For example, cryoablation commonly took more than half an hour for the protocol, which consists of several freezing cycles and thawing cycles [Citation22]. The technique described in the present study saves surgical time, shortens the duration of physical damage, and reduces the impact on the surrounding tissues. Furthermore, 5-mm stab incisions for cannulation with a needle make it less invasive than traditional curettage options.
In general, the major complications of MWA stem from thermal-mediated cauterization, including neurocutaneous damage, local infections, or secondary fractures. Historically, MWA has been utilized to safely treat bony lesions [Citation14, Citation23]. Khan et al. [Citation21] reported a cohort of 69 patients treated with MWA for spinal metastases, with an overall complication rate of 2.9% (nerve thermal injury and skin burn). In a retrospective study, two cases of aneurysmal bone cysts (ABCs) treated with percutaneous MWA were reported, with none of them showing complications or recurrence [Citation12]. In a systematic review, it was indicated that MWA had a clinical complication rate of 4.0% (10/249) in the treatment of bone tumors, with only one case of secondary fracture reported [Citation14]. In our study, two cases reported transient complications: minor skin burns and tingling. Both have been gradually recovering postoperatively, with no lingering sequelae. Concerning the major complication, only one secondary patient underwent a second surgery due to the complication of a femoral fracture (Patient 9) in our series. To a certain extent, the elevated temperatures caused by MWA could contribute to an increase in bone fragility. However, according to the Mirels scoring system [Citation24], this patient has a score of 9, categorizing them as being at high risk for fractures. Building upon this, the force from a fall may be the direct cause of the fracture. This technique does not involve the use of bone cement filling or internal fixation to achieve immediate stabilization of the lesions, which is also one of its limitations. In addition, percutaneous MWA in the present study does not need to achieve large bone necrosis (commonly used in the treatment of malignant tumors).
Our study shows that all patients achieved good radiological results based on the modified Neer classification. Tumor volume, physis-cyst distance, and cortical thickness at final follow-up show significant changes compared with those before surgery. Compared to curettage alone, management of the residual cavity can effectively reduce the recurrence in treating unicameral bone cysts. In the present study, normal saline is repeatedly perfused and flushed through the channel after MWA treatment. The purpose of this step is to drain the apoptotic tumor from the residual cavity. Afterward, in terms of cavity filling and osteogenic induction, biomimetic mineralized collagen bone repair materials were injected and pushed into the cavity with a clearing catheter through the channel. Previous studies have reported that synthetic bone substitute materials should have good biocompatibility and biodegradability, and simultaneously promote the formation of new bone tissues [Citation25, Citation26]. The results of the present study show that radiolucent area(s) are significantly different before and after the treatment, which indicates that osteogenic induction is successful via the cavity-filling methods.
In conclusion, our study demonstrated that percutaneous MWA, perfusion, and reconstruction with a synthetic bone substitute is an effective and safe imaging-guided technique for the treatment of UBCs in pediatric patients. The procedure was technical and radiologically successful with favorable clinical outcomes, no major complications, or technical failure. We believe that the procedure is a suitable treatment of choice for the management of UBCs, but additional studies with larger cohorts and follow-ups are needed to determine the long-term efficacy and safety.
The study has inherent limitations. Chief among them is the modest sample size, and the single-arm and retrospective design poses challenges for initiating more rigorous clinical outcomes. Additionally, variations in the size and location of the cysts result in differences in the power, depth, duration of MWA, and volume of bone substitute materials, leading to discrepancies potentially. Moreover, the technique does not involve stabilization devices. Additional internal fixation may be required in cases of weight-bearing bone fractures.
Acknowledgments
We sincerely thank the patients and researchers for their invaluable contributions to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data utilized and analyzed in the present study can be obtained from the corresponding author upon a reasonable request.
Additional information
Funding
References
- Zhao J-G, Wang J, Huang W-J, et al. Interventions for treating simple bone cysts in the long bones of children. Cochrane Database Syst Rev. 2017;2(2):CD010847. doi: 10.1002/14651858.CD010847.; PubMed Central PMCID: PMCQ1. eng.
- Neer CS, Francis KC, Johnston AD, et al. Current concepts on the treatment of solitary unicameral bone cyst. Clin Orthop Relat Res. 1973;97(97):40–51.
- Canavese F, Wright JG, Cole WG, et al. Unicameral bone cysts: comparison of percutaneous curettage, steroid, and autologous bone marrow injections. J Pediatr Orthop. 2011;31(1):50–55.
- Wright JG, Yandow S, Donaldson S, et al. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90(4):722–730.
- Rajeswaran S, Khan A, Samet JD, et al. Minimally invasive treatment for unicameral bone cysts with chemical sclerosis and bone graft substitute: a preliminary report. Cardiovasc Intervent Radiol. 2022;45(2):190–196.
- Shinozaki T, Arita S, Watanabe H, et al. Simple bone cysts treated by multiple drill-holes. 23 cysts followed 2-10 years. Acta Orthop Scand. 1996;67(3):288–290.
- Brecelj J, Suhodolcan L. Continuous decompression of unicameral bone cyst with cannulated screws: a comparative study. J Pediatr Orthop B. 2007;16(5):367–372.
- Hou H-Y, Wu K, Wang C-T, et al. Treatment of unicameral bone cyst: surgical technique. J Bone Joint Surg Am. 2011;93 Suppl 1:92–99.
- Alkuhaimi TS, Alduraywish I, Alghamdi T, et al. Feasibility of percutaneous Image-Guided combined treatment of symptomatic bone cyst using cryoablation and bone graft substitute. Cardiovasc Intervent Radiol. 2023;46(4):512–518.
- Ruiz-Arellanos K, Larios F, Inchaustegui ML, et al. Treatment and outcomes of 4,973 unicameral bone cysts: a systematic review and Meta-Analysis. JBJS Rev. 2024;12(1):.
- Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38(2):61–67. doi: 10.1067/j.cpradiol.2007.08.011.
- Arleo TL, Hawkins CM, Fabregas JA, et al. Percutaneous image-guided treatment of aneurysmal bone cysts: is there a superior treatment option? Pediatr Radiol. 2022;52(8):1539–1549.
- Filippiadis D, Charalampopoulos G, Zampakidis X, et al. Percutaneous microwave ablation and osteoplasty of an aneurysmal bone cyst. J Vasc Interv Radiol. 2021;32(6):923–925.
- Cazzato RL, de Rubeis G, de Marini P, et al. Percutaneous microwave ablation of bone tumors: a systematic review. Eur Radiol. 2021;31(5):3530–3541.
- Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016;98-B(1 Suppl A):6–9.
- Zhu W, Li W, Yao M, et al. Mineralized collagen/polylactic acid composite scaffolds for load-bearing bone regeneration in a developmental model. Polymers (Basel). 2023;15(20):4194.
- Zhu X, Wang C, Bai H, et al. Functionalization of biomimetic mineralized collagen for bone tissue engineering. Mater Today Bio. 2023;20:100660.
- Wang X, Kou J-M, et al. Clinical outcome comparison of polymethylmethacrylate bone cement with and without mineralized collagen modification for osteoporotic vertebral compression fractures. 2018.
- Jiang H-J, Xu J, Qiu Z-Y, et al. Mechanical properties and cytocompatibility improvement of vertebroplasty PMMA bone cements by incorporating mineralized collagen. Materials. 2015;8(5):2616–2634. doi: 10.3390/ma8052616.
- Gao C, Qiu Z-Y, Hou J-W, et al. Clinical observation of mineralized collagen bone grafting after curettage of benign bone tumors. Regen Biomater. 2020;7(6):567–575.
- Khan MA, Deib G, Deldar B, et al. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. AJNR Am J Neuroradiol. 2018;39(7):1376–1383.
- Baust JG, Snyder KK, Santucci KL, et al. Cryoablation: physical and molecular basis with putative immunological consequences. Int J Hyperthermia. 2019;36(sup1):10–16.
- Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6 Suppl 1(Suppl 1):S65–S69.
- Jawad MU, Scully SP. In brief: classifications in brief: mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468(10):2825–2827.
- Walsh WR, Vizesi F, Michael D, et al. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29(3):266–271.
- Teramoto H, Kawai A, Sugihara S, et al. Resorption of apatite-wollastonite containing glass-ceramic and beta-tricalcium phosphate in vivo. Acta Med Okayama. 2005;59(5):201–207.
