Abstract
Objective
Incomplete thermal ablation (ITA) fosters the malignancy of residual cells in Hepatocellular carcinoma (HCC) with unclear mechanisms now. This study aims to investigate the expression changes of NDST2 following ITA of HCC and its impact on residual cancer cells.
Methods
An in vitro model of heat stress-induced liver cancer was constructed to measure the expression of NDST2 using Quantitative Real-Time PCR and Western blotting experiments. The sequencing data from nude mice were used for validation. The clinical significance of NDST2 in HCC was evaluated by integrating datasets. Gene ontology and pathway analysis were conducted to explore the potential signaling pathways regulated by NDST2. Additionally, NDST2 was knocked down in heat stress-induced HCC cells, and the effects of NDST2 on these cells were verified using Cell Counting Kit-8 assays, scratch assays, and Transwell assays.
Results
NDST2 expression levels are elevated in HCC, leading to a decrease in overall survival rates of HCC patients. Upregulation of immune checkpoint levels in high NDST2-expressing HCC may contribute to immune evasion by liver cancer cells. Additionally, the low mutation rate of NDST2 in HCC suggests a relatively stable expression of NDST2 in this disease. Importantly, animal and cell models treated with ITA demonstrate upregulated expression of NDST2. Knockdown of NDST2 in heat stress-induced liver cancer cells results in growth inhibition associated with gene downregulation.
Conclusion
The upregulation of NDST2 can accelerate the progression of residual HCC after ITA, suggesting a potential role for NDST2 in the therapeutic efficacy and prognosis of residual HCC.
Introduction
Liver cancer is a prevalent and significant global health concern characterized by high incidence and mortality rates, imposing a substantial burden on the public health sector [Citation1–2]. Hepatocellular carcinoma (HCC), the primary histological subtype of liver cancer, accounts for 80%-90% of all primary liver cancer cases [Citation3–5]. It is now understood that timely diagnosis and treatment of HCC are pivotal for improving patient survival rates. Over the years, thermal ablation has emerged as a frontline treatment for early-stage HCC [Citation6–7], involving the destruction of tumor tissue through the application of high temperatures. However, due to tumor heterogeneity and limitations in the ablation technique, incomplete ablation may occur, leading to the persistence of residual cancer tissue [Citation8]. The rapid progression of residual liver cancer following thermal ablation poses a critical challenge. The heterogeneity of liver cancer cells results in distinct biological characteristics among subgroups, affecting sensitivity to thermal ablation, growth rates, invasion capacity, and more. The unchecked growth of residual cancer cells increases the risk of secondary recurrence and metastasis, significantly impacting patient prognosis. According to the literature [Citation9], the overall recurrence rate of HCC within 5 years after ablation is approximately 60%-70%, posing a substantial threat to patient survival rates. Therefore, further exploration of effective treatment methods to eliminate residual cancer tissue, as well as an in-depth investigation of the biological characteristics, gene regulatory mechanisms, and immune escape mechanisms of incompletely ablated liver cancer cells, hold great significance for the future management and prognosis of this patient population.
N-deacetylase/N-sulfotransferase 2 (NDST2) is a gene that codes for a protein functioning as an enzyme involved in sulfation processes. Specifically, NDST2 catalyzes the N-deacetylation of glucosamine glycosaminoglycans and the N-sulfation of N-acetylglucosamine (GlcNAc). By modifying the GlcNAc-GlcA disaccharide repeat sugar backbone, NDST2 contributes to the production of N-sulfated heparin, a crucial substrate for late-stage modification during heparin biosynthesis [Citation10]. Previous studies have highlighted the impact of heparin and heparin-like molecules on tumor occurrence and development [Citation11]. Primarily, these substances exert inhibitory effects on the growth and proliferation of tumor cells, significantly reducing the likelihood of tumor development. However, no research has hitherto investigated whether NDST2 plays a role in the occurrence and development of liver cancer or whether it can mitigate the rapid progression of residual cancer after incomplete thermal ablation.
This study sought to elucidate the role of NDST2 in the progression of residual hepatic cancer following incomplete thermal ablation. Overall, we aim to provide novel insights into the prognosis of liver cancer following thermal ablation therapy.
Materials and methods
Subcutaneous transplantation of incompletely ablated tumors in nude Mice model
6-week-old Balb/c nude mice obtained from the specific-pathogen-free laboratory at the Experimental Animal Center of Guangxi Medical University were selected for subcutaneous transplantation of HepG2 cells to establish a tumor model. The Cool-tip™ cold cycle radiofrequency ablation system was employed to simulate incomplete thermal ablation of liver cancer. After the experiment, the mice were euthanized following the ARRIVE guidelines and relevant regulations, and tumor tissues were collected for sequencing analysis. Pathological observation of the obtained tumor tissues confirmed the presence of tumor cells, necrotic cells, and degenerative cells, indicating the successful establishment of the subcutaneous transplantation model of incompletely ablated tumors, as previously described in our study [Citation12].
Global open big Data analysis
The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and ArrayExpress databases were employed to obtain mRNA microarray data from specimens of both HCC and non-HCC (adjacent or normal liver tissue). To address batch effects, the “sva” package in R software was utilized after gene name matching, and gene profiles from the same platform were merged. Subsequently, the expression of NDST2 in the tumor and adjacent tissues was extracted from the dataset. Data analysis was carried out using GraphPad Prism 8, and statistical analysis employed the t-test for both groups. Standardized mean difference (SMD) forest plots and summary receiver operating characteristic (sROC) curves were generated using Stata 12, with a significance level set at p < 0.05. To identify protein molecules interacting with NDST2, a search was conducted using the STRING database. Subsequently, the SangerBox online platform was utilized for the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses.
Genetic mutation and immune cell infiltration analysis
RNA-seq data, mutation data, and corresponding clinical information for liver cancer were acquired from TCGA dataset. The “maftools” package in R software was employed to download and visualize the mutation data [Citation13]. The “immunedeconv” package was employed to assess the immune score of NDST2 in liver cancer, this package incorporates six advanced algorithms, namely Tumor Immune Estimation Resource (TIMER), xCell, MCP-counter, CIBERSORT, EPIC, and quanTIseq, due to the comprehensive categorization provided by the CIBERSORT algorithm, which offers nearly 22 different immune cell types, it was chosen for analysis and evaluation in this research. The “ggClusterNet” package was utilized for analysis and visualization in the form of a correlation network graph [Citation14]. The expression patterns of immune checkpoint-related genes were visualized using the “ggplot2” and “pheatmap” packages.
In vitro cell experiments
Cell culture
To verify the consistency of elevated NDST2 expression in response to heat stress across various liver cancer cells, an in vitro cell model was employed using MHCC97-H and MHCC97-L liver tumor cell lines. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium (Gibco, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. The cell incubator maintained a temperature of 37 °C with 5% CO2 concentration, providing optimal conditions for cell growth and maintenance.
siRNA design and Cell transfection
The siRNA sequence targeting NDST2 was custom-designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The synthesis report, including detailed information, is provided in . Cell transfection with siRNA transfection reagents was conducted according to the manufacturer’s instructions. Subsequently, the transfected cells were collected for further research purposes.
Table 1. The synthesis report sheet of siRNA sequences.
In vitro simulation of incomplete heat ablation in cells
Given the challenges associated with obtaining samples after pure ablation treatment in the clinical setting, this study simulated in vitro cellular ITA by subjecting liver cancer cells to heat in a water bath. The water bath temperature was set to 47 °C for 10 min each time. Liver cancer cells were inoculated in a six-well plate, and the water bath treatment was administered in the initial round when the cell growth reached 70%-80%. Subsequently, the cells were incubated in a cell culture incubator until the cell density reached 70%-80% again, and the next round of water bath treatment was applied. This process was repeated for a total of three rounds [Citation15].
Quantitative Real-Time PCR (RT-qPCR) experiment
Total RNA was extracted from cells using the FastPure Cell/Tissue Total RNA Isolation Kit, and the concentration was determined using a microvolume spectrophotometer. The PrimeScript™ RT Kit with gDNA Eraser was employed for reverse transcription to cDNA. The QuantStudio™ Real-Time PCR system was used to assess RNA expression. The PCR primer sequences used are provided in .
Table 2. Primer sequences of PCR.
Western blot experiment
Proteins were extracted from the cells using a prepared 1x protein extraction reagent. They were separated by 10% Sodium Dodecyl Sulfate (SDS)-Polyacrylamide gel electrophoresis and transferred onto a Polyvinylidene Fluoride (PVDF) membrane. Following blocking with 5% skim milk, overnight incubation with the primary antibody at 4 °C, and subsequent incubation with the secondary antibody at room temperature, chemiluminescent substrate was used to visualize the membrane, which was then imaged using the ChemiDoc™ imaging system. The primary antibodies used in this study were anti-NDST2 (NBP2-13645-25ul, Bio-Techne) and anti-GAPDH (10494-1-AP, Proteintech), while the secondary antibody was HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H + L) (SA00001-2, Proteintech).
Analysis of cellular biological behaviors
An in vitro heat-stressed liver cancer cell model was constructed to explore changes in cellular biological behaviors through Cell Counting Kit-8 (CCK-8) assays [Citation16], Transwell migration and invasion assays [Citation17], and scratch assays [Citation18].
Statistical analysis
Statistical analysis and graphing were performed using IBM SPSS Statistics 23, Stata, and GraphPad Prism 8 software. The t-test was employed to analyze the statistical differences between the two groups, and data were presented as mean ± standard deviation. p < 0.05 was considered statistically significant (Varying levels of significance were denoted with asterisks, specifically: * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001).
Results
ITA promotes the biological progression of residual liver cancer cells
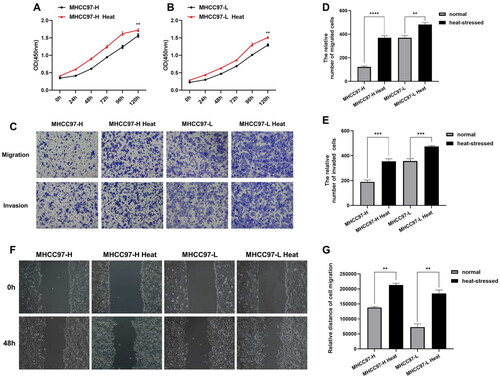
CCK-8 results indicated a significant increase in the proliferation capacity of liver cancer cells compared to normal liver cancer cells after exposure to heat stress (). The findings of Transwell assays demonstrated a notable increase in the number of migrated and invaded liver cancer cells compared to normal liver cancer cells (), indicating an enhanced migration and invasion capacity of liver cancer cells after heat stress. Migration ability was further examined using the scratch assay, where the comparison of wound healing areas at 0 and 48 h revealed enhanced locomotion of liver cancer cells after heat stress ().
Figure 1. ITA promotes the biological progression of residual liver cancer cells. (A–B) CCK-8 assays were performed to detect the proliferative capacity of heat-stressed liver cancer cells. (C–E) Transwell migration and invasion assays were conducted to evaluate the migratory and invasive abilities of heat-stressed liver cancer cells (magnification, ×100). (F–G) Scratch assays to measure changes in the migration capacity of liver cancer cells after heat stress (magnification, ×100).
Expression of NDST2 in liver cancer cells after heat stress
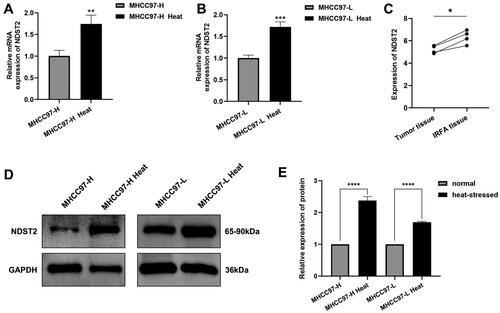
Through RT-qPCR experiments, it was revealed that NDST2 expression is upregulated in heat-stressed cells compared to normal liver cancer cells (). Western Blot results further confirmed the increase in NDST2 protein levels in cells after exposure to heat stress (). Additionally, mRNA expression analysis of NDST2 was performed through sequencing of residual tumor tissues in a nude mouse model of incomplete ablative subcutaneous grafting. These findings indicated elevated expression of NDST2 in the residual tumor tissues compared to normal liver cancer tissues after incomplete ablation ().
Figure 2. Upregulation of NDST2 expression in heat-stressed liver cancer cells. (A–B) RT-qPCR experiments were conducted to measure the expression changes of NDST2 in heat-stressed liver cancer cells. (C) Tissue sequencing results of residual tumor tissues in a nude mouse model showing increased NDST2 expression in incomplete ablation tumor tissues. (D–E) Western Blot assays to detect NDST2 protein levels in heat-stressed cells.
Global publicly available data analysis of NDST2 expression in tumors and liver cancer
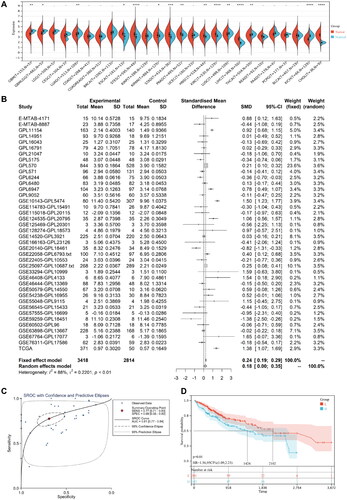
Based on TCGA data, NDST2 exhibited differential expression in 15 out of 26 cancer types included in TCGA database. Nine cancer types demonstrated high expression, particularly in LIHC, NDST2 showed elevated expression (). For further analysis, mRNA microarray data from 3418 cases of HCC and 2814 cases of non-HCC were collected from TCGA, ArrayExpress, and GEO databases. The results demonstrated significant heterogeneity (I2 = 88%, SMD = 0.18, p < 0.01), indicating higher expression of NDST2 in liver cancer than in non-liver cancer tissues (). Additionally, the area under the curve (AUC) of sROC was 0.81, with a sensitivity of 0.77 and specificity of 0.69, suggesting that NDST2 can distinguish between HCC and non-HCC (). To explore the impact of NDST2 on the overall survival of liver cancer patients, survival curves were generated. Patients were divided into low-expression and high-expression groups based on NDST2 levels, and the survival curves revealed a significant disparity. The low-expression group exhibited a considerably higher survival rate, indicating that patients with lower NDST2 expression experienced better overall survival rates than those with higher expression levels (). A search for 20 protein molecules interacting with NDST2 was conducted using the STRING database (Supplementary Figure S1). Further, the Sangerbox online platform was employed for KEGG and GO enrichment analysis. KEGG pathway enrichment analysis showed that NDST2 was significantly enriched in pathways such as glycosaminoglycan biosynthesis (heparan sulfate/heparin), metabolic pathways, and proteoglycans in cancer (Supplementary Figure S2A). GO annotation demonstrated that NDST2 was primarily involved in cellular components such as the endomembrane system, Golgi apparatus part, and Golgi apparatus (Supplementary Figure S2B). Its main biological processes in liver cancer include carbohydrate derivative metabolic process, glycosaminoglycan biosynthetic process, and aminoglycan biosynthetic process (Supplementary Figure S2C). The molecular functions of NDST2 were mainly related to transferase activity, sulfotransferase activity, and transferase activity, transferring sulfur-containing groups (Supplementary Figure S2D).
Figure 3. (A) Differential expression of NDST2 between tumor and non-tumor tissues among 26 types of TCGA cancers. (B) Data analysis from GEO, ArrayExpress, and TCGA databases comparing HCC tumors with non-tumor tissues, verifying the high expression of NDST2 in HCC. (C) sROC curve evaluating the potential of NDST2 differential expression as a discriminator in HCC (p = 0.01). (D) Cox regression analysis identifying the predictive value of NDST2 for overall survival in HCC patients.
NDST2 somatic mutations in liver cancer
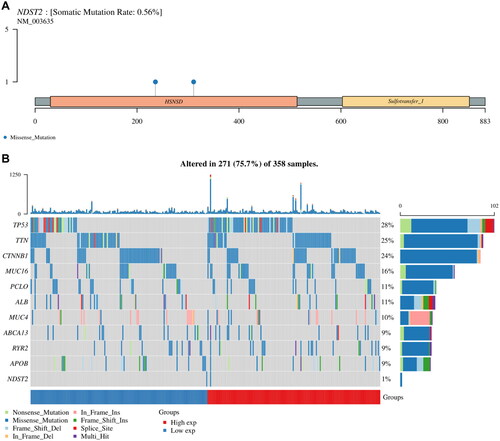
The mutation data of NDST2 was visualized by R software. A mutation waterfall plot was generated to display the gene mutation frequencies of TP53 (28%), TTN (25%), CTNNB1 (24%), MUC16 (16%), PCLO (11%), ALB (11%), MUC4 (10%), ABCA13 (9%), RYR2 (9%), APOB (9%), and NDST2 (1%) in liver cancer patients (). Notably, NDST2 exhibited a very low mutation frequency compared to other genes. The main mutation type in the NDST2 gene in liver cancer somatic cells was missense mutations, with a mutation frequency of 0.56% (). Single nucleotide polymorphisms (SNPs) predominated over insertions (INS) or deletions (DEL), as shown in Supplementary Figure S3. Among the detected SNV categories, C > T was the major SNP category. The number of mutations per sample is shown in Supplementary Figure S3, and each colored box represents a different mutation.
Figure 4. Genetic mutation landscape of NDST2 in liver cancer. (A) Lollipop plot displaying the distribution of NDST2 gene mutations, with missense mutations having a mutation frequency of 0.56%. (B) Overview of major mutations in liver cancer patients. The waterfall plot compares the mutation frequencies of NDST2 with the top 10 mutated genes in liver cancer patients, showing a very low mutation frequency for NDST2.
Tumor-infiltrating immune cells associated with NDST2
To assess the immune scores associated with NDST2 in liver cancer, the liver cancer specimens were categorized into a high-expression group (G1) and a low-expression group (G2) based on the expression levels of NDST2. Analysis using the CIBERSORT immune infiltration scoring revealed higher infiltration of T follicular helper cells, M0 Macrophages, and M1 Macrophages in G1. In G2, there was higher infiltration of M2 Macrophages (). The expression distribution of immune checkpoint genes in G1 and G2 was examined (), showing higher expression of immune checkpoint genes in G1 compared to G2. This suggests that high NDST2 expression may promote immune evasion in liver cancer cells. Furthermore, the association between NDST2 expression and immune cell scores, together with the correlation between immune scores themselves, was visualized using R software. The data indicated that NDST2 expression was positively correlated with M0 macrophages, M1 macrophages, T follicular helper cells, and CD8+ T cells, while a negative correlation was observed with M2 Macrophages, activated NK cells, gamma delta T cells, naive CD4+ T cells, and naive B cells (). Based on these findings, it can be inferred that high expression of NDST2 may promote immune evasion in liver cancer cells, leading to the activation of anti-tumor immune cells in liver cancer patients.
Figure 5. Immune infiltration analysis of NDST2 in liver cancer. (A-B) Relative abundance of tumor-infiltrating immune cells in high-expressing NDST2 (G1) and low-expressing NDST2 (G2) liver cancer specimens. (C) Distribution of immune checkpoint gene expression in liver cancer samples with high expression of NDST2 (G1) and low expression of NDST2 (G2). (D) Network diagram depicting the correlation between NDST2 expression and tumor-infiltrating immune score, together with the correlation of the immunity scores themselves.
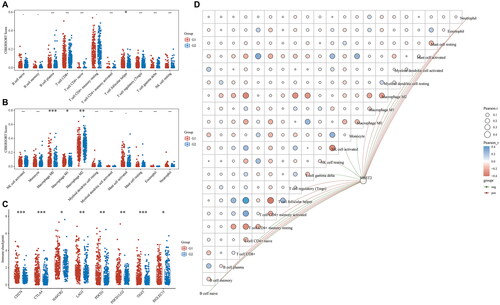
Downregulation of NDST2 inhibits proliferation, locomotion, and invasion of heat-stressed liver cancer cells
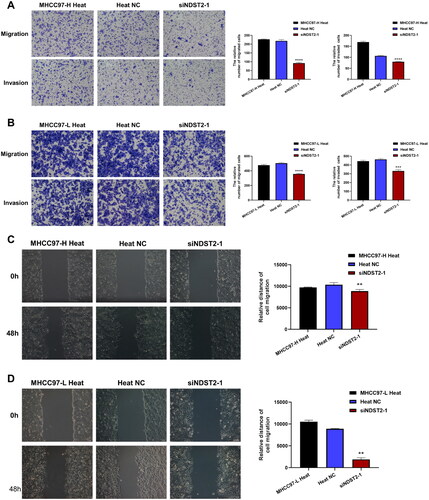
We used siRNA to knock down the expression of NDST2 in heat-stressed liver cancer cells, the cell group after silencing was defined as the siNDST2 group, and the cell group treated with Negative Control reagent was defined as the Heat NC group. Comparing the experimental data from RT-qPCR, the results showed a significant decrease in the expression level of NDST2 in the siNDST2 group compared to the Heat NC group and normal heat-stressed cells. Among the three transfection reagents, siNDST2-1 exhibited the best knockdown effect, with a transfection efficiency of over 50% (). The Western Blot results indicated a notable reduction in the protein level of the siNDST2-1 group compared to the Heat NC group and normal heat-stressed cells (). We chose siNDST2-1, which exhibited the most pronounced transfection effect for further experiments. The CCK-8 assays revealed that the downregulation of NDST2 effectively restrained the proliferation capacity of liver cancer cells subjected to heat stress (). Transwell migration and invasion assays showed that the number of migrated and invaded cells in the siNDST2 group was significantly lower than that in the Heat NC group and normal heat-stressed cells, indicating that downregulation of NDST2 expression could inhibit the locomotion and invasion ability of heat-stressed liver cancer cells (). The scratch assay revealed that downregulation of NDST2 expression inhibited the healing rate of scratch wounds in HCC cells (), demonstrating that downregulation of NDST2 expression could suppress the migration ability of heat-stressed liver cancer cells.
Figure 6. Biological progress of NDST2 downregulation in heat-stressed liver cancer cells. (A) Three transfection reagents were used to transfect the heat-stressed liver cancer cells, and the RT-qPCR results showed a decrease in NDST2 expression after transfection. Among them, siNDST2-1 exhibited the best transfection effect. (B) Western Blot experiments detected the knockdown effect of siNDST2-1, resulting in a decrease in NDST2 protein level after knockdown. (C) Western Blot results of MHCC97-H and MHCC97-L heat-stressed cells after knockdown. (D) The impact of NDST2 knockdown on the proliferation capacity of heat-stressed cells was determined using CCK-8 assays.
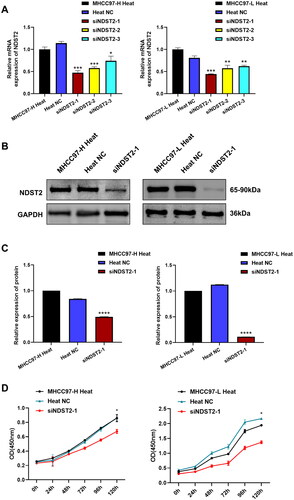
Figure 7. The locomotion and invasion abilities of heat-stressed liver cancer cells are altered after downregulation of NDST2. (A) Locomotion and invasive ability of heat-stressed liver cancer cells after MHCC97-H knockdown assessed by Transwell assays (magnification, ×100). (B) Locomotion and invasive ability of heat-stressed liver cancer cells after MHCC97-L knockdown assessed by Transwell assays (magnification, ×100). (C) Scratch assays measured the change in migration area of MHCC97-H heat-stressed cells after NDST2 knockdown (magnification, ×100). (D) Scratch assays measured the change in migration area of MHCC97-L heat-stressed cells after NDST2 knockdown (magnification, ×100).
Discussion
Over the past decade, there has been a notable rise in the incidence of liver cancer, prompting continuous optimization of treatment methods. Early screening, diagnosis, and treatment have been established as effective strategies for improving patient survival rates in liver cancer [Citation19–20]. Surgical resection and interventional ablation are currently the most widely employed treatments for early-stage liver cancer, with thermal ablation emerging as the preferred choice due to its minimal impact on the body [Citation21–22]. However, the potential for accelerated proliferation and invasion of residual tumor tissue following thermal ablation poses a risk of recurrence or metastasis [Citation15]. Goldberg SN, Ahmed M, and others delved into the impact of non-lethal thermal ablation on tumor cells in their research. They found that during the process of thermal ablation, tumor cells damaged by heat could secrete a series of pro-tumorigenic factors such as IL-6, STAT 3, HGF, VEGF, which could stimulate the proliferation of tumor cells [Citation23]. In recent years, Goldberg SN, Ahmed M, and their peers further revealed that fibroblast growth factors (FGFs) secreted by thermally injured liver cells may act as initiators of a series of crucial signaling pathways, making the inhibition of FGF production essential in suppressing the occurrence and progression of HCC [Citation24]. In this study, we observed an upregulation of NDST2 expression in residual liver cancer tissues after thermal ablation, prompting further exploration of the role and significance of NDST2 in non-lethal thermal ablation of liver cancer.
NDST2, a protein-coding gene, encodes a crucial bifunctional enzyme involved in the N-deacetylation and N-sulfation of glucosamine in heparan sulfate synthesis [Citation25]. It plays a pivotal role in determining the sulfation pattern of heparan sulfate by modifying the GlcNAc-GlcA disaccharide repeat backbone, a prerequisite substrate for late-stage modification of heparin biosynthesis—N-sulfated heparin [Citation26–27]. Previous research has underscored the significant impact of heparin and heparin-like molecules on tumor initiation and progression, with the ability to impede tumor cell proliferation, inhibit growth, curb migration, and suppress invasion [Citation28]. This study explored the significance and impact of NDST2 in incomplete residual tumors after liver cancer ablation.
Herein, an analysis of TCGA data revealed that NDST2 exhibited elevated expression specifically in liver cancer, among the 26 types of cancer studied. Examination of mRNA microarray data from 3418 cases of HCC and 2814 cases of non-HCC sourced from TCGA, ArrayExpress, and GEO databases validated that NDST2 expression in tumor tissues was higher than in non-cancerous tissues. Our findings suggest that the differential expression of NDST2 in HCC holds the potential for discrimination and serves as a predictive marker for overall survival in HCC patients. Exploring protein interactions, we employed the String database to identify 20 molecules that interact with the NDST2 protein. Subsequent KEGG and GO enrichment analysis revealed a significant enrichment of NDST2 in the endomembrane system of cells. Its involvement in biological processes such as Glycosaminoglycan biosynthesis of heparan sulfate/heparin and carbohydrate derivative metabolic processes in liver cancer was emphasized. We further investigated genetic mutations and immune cell infiltration of NDST2 in liver cancer. NDST2 mutations were predominantly missense mutations, with a significantly low mutation rate (0.56%). These findings underscore the relatively stable expression of NDST2 in liver cancer, highlighting its consistent role in the disease. Employing the CIBERSORT algorithm to scrutinize immune cell infiltration of NDST2 in liver cancer, we observed heightened infiltration of T follicular helper cells, M0 Macrophages, and M1 Macrophages in the NDST2 high-expression group. Conversely, the NDST2 low-expression group exhibited elevated infiltration of M2 Macrophages. Previous literature reports supported the pro-inflammatory role of M1 Macrophages, which secretes abundant pro-inflammatory cytokines, suppressing tumor progression through diverse mechanisms. In contrast, M2 Macrophages typically promote tumor cell growth by releasing various immune inhibitory factors, cytokines, and growth factors, participating in tumor angiogenesis [Citation29]. Another study revealed that T-cell follicular helper cells play a pivotal role in promoting the formation of tertiary lymphoid structures, which was associated with an increase in tumor immune infiltration, contributing to the inhibition of tumor growth [Citation30]. However, these results indicated that the NDST2 high-expression group may possess an enhanced capability to trigger anti-tumor immune cell activation in liver cancer patients, contradicting our initial research findings. This discrepancy highlights the intricacies surrounding the role of NDST2 in modulating the immune response in liver cancer and underscores the need for further in-depth investigation to unravel the underlying mechanisms. Additionally, a concurrent assessment of the distribution of immune checkpoint gene expression in liver cancer was carried out. The results revealed that the NDST2 high-expression group exhibited upregulated levels of immune checkpoints compared to the low-expression group. This observation suggests that the elevated NDST2 expression may potentially contribute to immune evasion by liver cancer cells.
In addition, this study explored the alterations in expression and potential biological functions of NDST2 in residual liver cancer tumors after thermal ablation through both in vivo and in vitro experiments. Utilizing cellular and animal models of incomplete thermal ablation, we observed changes in NDST2 expression. Our investigation revealed that, following incomplete thermal ablation, both gene and protein expression of NDST2 exhibited upregulation compared to normal liver cancer samples. Further validation was carried out through in vitro cell experiments involving the knockdown of NDST2 expression in heat-stressed liver cancer cells. Our results demonstrated a significant reduction in the proliferation, locomotion, and invasion abilities of heat-stressed liver cancer cells after knockdown, suggesting that downregulation of NDST2 can impede the rapid progression of heat-stressed liver cancer cells. This finding offers a promising avenue for enhancing the prognosis of liver cancer thermal ablation therapy.
Nevertheless, it is essential to highlight the limitations of this study. Firstly, the specific regulatory mechanism through which NDST2 affects the rapid progression of heat-stressed liver cancer cells remains unclear. Indeed, the use of ChIP-seq can extensively explore the target genes of NDST2 in liver cancer and elucidate its potential mechanisms [Citation31]. Secondly, sequencing results from in vitro cell models of incomplete ablation can provide robust evidence for studying the expression changes of NDST2 in residual liver cancer tumors. Furthermore, clinical sample PCR and immunohistochemical analysis can be employed to validate the expression of NDST2 in human tissue samples, representing a potential avenue for future validation [Citation32].
Conclusion
In summary, our research findings indicate a significant correlation between elevated NDST2 expression in heat-stressed liver cancer cells and prognosis. Furthermore, growth inhibition of heat-stressed liver cancer cells upon NDST2 downregulation revealed a tumor-suppressive role for NDST2 in this scenario. These findings not only suggest a potential therapeutic target for improving the prognosis of liver cancer thermal ablation therapy by focusing on NDST2 but also open up new avenues for future research in this field.
Ethics approval and consent to participate
Animal experiment in this study was approved by the ethics committee of The First Affiliated Hospital of Guangxi Medical University (NO.2019-KY-(084)). All experiments were performed in accordance with ARRIVE guidelines and relevant regulations.
Supplemental Material
Download MS Word (1.6 MB)Acknowledgements
Thanks to Key Laboratory of Ultrasonic Molecular Imaging and Artificial Intelligence, Guangxi Zhuang Autonomous Region Engineering Research Center for Artificial Intelligence Analysis of Multimodal Tumor Images, Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, and Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education for their support of this study. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
TCGA dataset (https://portal.gdc.com), ArrayExpress dataset (https://www.ebi.ac.uk/arrayexpress/), GEO dataset (https://www.ncbi.nlm.nih.gov/geo/) and STRING database (http://www.string-db.org/) were used in the study. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. And the Nude Mice sequencing datasets generated and analyzed during the current study are available in the GEO repository (record GSE234283). The following secure token has been created to allow review of record GSE234283 while it remains in private status: cvebmegybtgdvqr.
Additional information
Funding
References
- Anwanwan D, Singh SK, Singh S, et al. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):1. doi: 10.1016/j.bbcan.2019.188314.
- Ghafouri-Fard S, Honarmand Tamizkar K, Hussen BM, et al. MicroRNA signature in liver cancer. Pathol Res Pract. 2021;219:153369. doi: 10.1016/j.prp.2021.153369.
- Salazar J, Le A. The heterogeneity of liver cancer metabolism. Adv Exp Med Biol. 2021;1311:127–13. doi: 10.1007/978-3-030-65768-0_9.
- Feng M, Pan Y, Kong R, et al. Therapy of primary liver cancer. Innovation . 2020;1(2):100032. doi: 10.1016/j.xinn.2020.100032.
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3.
- Bo XW, Sun LP, Yu SY, et al. Thermal ablation and immunotherapy for hepatocellular carcinoma: recent advances and future directions. World J Gastrointest Oncol. 2021;13(10):1397–1411. doi: 10.4251/wjgo.v13.i10.1397.
- Langenbach MC. RFA vs resection of HCC: exploring the past to improve the future. Eur Radiol. 2019;29(5):2677–2678. doi: 10.1007/s00330-019-6000-y.
- Zeng X, Liao G, Li S, et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology. 2023;77(4):1122–1138. doi: 10.1002/hep.32585.
- Zhu S, Wu Y, Zhang X, et al. Targeting N7-methylguanosine tRNA modification blocks hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Mol Ther. 2023;31(6):1596–1614. doi: 10.1016/j.ymthe.2022.08.004.
- Herrera-Heredia SA, Hsu HP, Kao CY, et al. Heparin is required for the formation of granules in connective tissue mast cells. Front Immunol. 2022;13:1000405. doi: 10.3389/fimmu.2022.1000405.
- Atallah J, Khachfe HH, Berro J, et al. The use of heparin and heparin-like molecules in cancer treatment: a review. Cancer Treat Res Commun. 2020;24:100192. doi: 10.1016/j.ctarc.2020.100192.
- Wang XD, Peng JB, Zhou CY, et al. Potential therapies for residual hepatoblastoma following incomplete ablation treatment in a nude mouse subcutaneous xenograft model based on lncRNA and mRNA expression profiles. Oncol Rep. 2020;43(6):1915–1927. doi: 10.3892/or.2020.7545.
- Bi F, Chen Y, Yang Q. Significance of tumor mutation burden combined with immune infiltrates in the progression and prognosis of ovarian cancer. Cancer Cell Int. 2020;20(1):373. doi: 10.1186/s12935-020-01472-9.
- Wen T, Xie P, Yang S, et al. ggClusterNet: an R package for microbiome network analysis and modularity-based multiple network layouts. iMeta. 2022;1(3):e32. doi: 10.1002/imt2.32.
- Guo Y, Ren Y, Dong X, et al. An overview of hepatocellular carcinoma after insufficient radiofrequency ablation. J Hepatocell Carcinoma. 2022;9:343–355. doi: 10.2147/JHC.S358539.
- Wang L, Li S, Luo H, et al. PCSK9 promotes the progression and metastasis of Colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res. 2022;41(1):303. doi: 10.1186/s13046-022-02477-0.
- Justus CR, Marie MA, Sanderlin EJ, et al. Transwell in vitro cell migration and invasion assays. Methods Mol Biol. 2023;2644:349–359. doi: 10.1007/978-1-0716-3052-5_22.
- Martinotti S, Ranzato E. Scratch wound healing assay. Methods Mol Biol. 2020;2109:225–229. doi: 10.1007/7651_2019_259.
- Xing M, Wang X, Kiken RA, et al. Immunodiagnostic biomarkers for hepatocellular carcinoma (HCC): the first step in detection and treatment. Int J Mol Sci. 2021;22(11):6139. doi: 10.3390/ijms22116139.
- Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7(3):308–319. doi: 10.1016/j.gendis.2020.01.014.
- Jin Q, Chen X, Zheng S. The security rating on local ablation and interventional therapy for hepatocellular carcinoma (HCC) and the comparison among multiple anesthesia methods. Anal Cell Pathol . 2019;2019:2965173. doi: 10.1155/2019/2965173.
- Granata V, Grassi R, Fusco R, et al. Diagnostic evaluation and ablation treatments assessment in hepatocellular carcinoma. Infect Agent Cancer. 2021;16(1):53.
- Markezana A, Ahmed M, Kumar G, et al. Moderate hyperthermic heating encountered during thermal ablation increases tumor cell activity. Int J Hyperthermia. 2020;37(1):119–129. doi: 10.1080/02656736.2020.1714084.
- Markezana A, Paldor M, Liao H, et al. Fibroblast growth factors induce hepatic tumorigenesis post radiofrequency ablation. Sci Rep. 2023;13(1):16341. doi: 10.1038/s41598-023-42819-2.
- Carlsson P, Presto J, Spillmann D, et al. Heparin/heparan sulfate biosynthesis: processive formation of N-sulfated domains. J Biol Chem. 2008;283(29):20008–20014. doi: 10.1074/jbc.M801652200.
- Pikas DS, Eriksson I, Kjellén L. Overexpression of different isoforms of glucosaminyl N-deacetylase/N-sulfotransferase results in distinct heparan sulfate N-sulfation patterns. Biochemistry. 2000;39(15):4552–4558. doi: 10.1021/bi992524l.
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502.
- Korhan P, Yılmaz Y, Bağırsakçı E, et al. Pleiotropic effects of heparins: from clinical applications to molecular mechanisms in hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2018;2018:7568742–7568748. doi: 10.1155/2018/7568742.
- Cheng K, Cai N, Zhu J, et al. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun. 2022; 42(11):1112–1140. doi: 10.1002/cac2.12345.
- Yu D, Walker LSK, Liu Z, et al. Targeting TFH cells in human diseases and vaccination: rationale and practice. Nat Immunol. 2022;23(8):1157–1168. doi: 10.1038/s41590-022-01253-8.
- Nakato R, Sakata T. Methods for ChIP-seq analysis: a practical workflow and advanced applications. Methods. 2021;187:44–53. doi: 10.1016/j.ymeth.2020.03.005.
- Canete-Portillo S, Velazquez EF, Kristiansen G, et al. Report from the international society of urological pathology (ISUP) consultation conference on molecular pathology of urogenital cancers V: recommendations on the use of immunohistochemical and molecular biomarkers in penile cancer. Am J Surg Pathol. 2020;44(7):e80–e86. doi: 10.1097/PAS.0000000000001477.
