 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
Psoriasis is characterized by an increase in the proliferation of keratinocytes and nerve fiber activity, contributing to the typical skin lesions. Pulsed Dye Laser (PDL) treatment is effective for the treatment of psoriatic lesions but its mechanism remains unclear. One hypothesis is that PDL causes thermal damage by the diffusion of heat to neighboring structures in lesional skin. There is limited information on the thermal sensitivity of these neighboring skin cells when exposed to hyperthermia for durations lasting less than a minute. Our study aimed to investigate the cell-specific responses to heat using sub-minute exposure times and moderate to ablative hyperthermia.
Materials and Methods
Cultured human endothelial cells, smooth muscle cells, neuronal cells, and keratinocytes were exposed to various time (2–20 sec) and temperature (45–70 °C) combinations. Cell viability was assessed by measuring intracellular ATP content 24 h after thermal exposure and this data was used to calculate fit parameters for the Arrhenius model and CEM43 calculations.
Results
Our results show significant differences in cell survival between cell types (p < 0.0001). Especially within the range of 50–60 °C, survival of neuronal cells and keratinocytes was significantly less than that of endothelial and smooth muscle cells. No statistically significant difference was found in the lethal dose (LT50) of thermal energy between neuronal cells and keratinocytes. However, CEM43 calculations showed significant differences between all four cell types.
Conclusion
The results imply that there is a cell-type-dependent sensitivity to thermal damage which suggests that neuronal cells and keratinocytes are particularly susceptible to diffusing heat from laser treatment. Damage to these cells may aid in modulating the neuro-inflammatory pathways in psoriasis. These data provide insight into the potential mechanisms of PDL therapy for psoriasis and advance our understanding of how thermal effects may play a role in its effectiveness.
Introduction
Laser treatment is used to treat vascular deformities, inflammatory skin diseases, and early-stage skin cancers. It is also applied for hair removal, and to induce skin rejuvenation [Citation1]. The common denominator of these therapies is that incident (laser) light is absorbed by a certain chromophore in the skin, such as oxyhemoglobin, melanin, or water, resulting in a transient temperature rise. Although the underlying evidence of the exact biological mechanism is not fully understood, pulsed dye laser (PDL) treatment, which was originally developed to treat port-wine stains, also has been shown to be effective for psoriasis treatment [Citation2–4]. In short, PDL is based on the absorption of laser light (585 nm) by hemoglobin in red blood cells. The absorbed energy is converted into heat [Citation1]. Depending on the settings of the laser (typically in the range of 6-9 J/cm2, with a pulse duration of 0.45 or 1.5 ms, and spot size of 5 to 10 mm), the temperature may rise locally to 80–200 °C and cause coagulation and rupture of the blood vessels, which could be an essential component of the treatment [Citation2,Citation5]. However, there are few studies focusing on the effect of heat diffusion into the surrounding tissue. The heat likely diffuses from PDL-targeted red blood cells to blood vessel wall, including endothelium, smooth muscle cells, vascular nerves, and possibly even further to keratinocytes, inducing temperatures in the range of 45-70 °C. Previous work from Hern et al. (2001) showed that PDL treatment reduces endothelial cell proliferation and endothelial surface area, as well as a significant reduction of lymphocytic infiltration and a slight reduction in keratinocyte proliferation in psoriatic skin [Citation6]. Other studies have shown that laser heating may block nerve function and individual case reports have shown that inhibition of nerve function through capsaicin or denervation improves the psoriatic lesions. Furthermore, it was suggested that moderate hyperthermia (43–47 °C) can increase metabolic activity in tissues and cause depletion of enzymes and subsequent overload on synthetic pathways, which may inflict cell chaos and injury [Citation7]. In a recent study, we showed by image-derived finite element modeling of PDL treatment of a psoriatic skin that the laser light absorbed by the microvasculature leads to heating of collateral tissue up to 60° and 43 °C at distances of 18 and 350 µm, respectively, from the blood vessel wall [Citation8]. Our hypothesis is that diffusing heat, albeit not uniform, can damage neuronal cells in the vicinity of blood vessels (vascular and perivascular nerves) and that this damage is essential in the effectiveness of PDL for psoriasis treatment [Citation9]. Understanding cell sensitivity to heat can shed light on cell death and survival in the vicinity of blood vessels and the subsequent biological effects triggered by damage after PDL therapy. In the current study, we investigated the survival rate after exposure to moderate and ablative hyperthermia in cultured endothelial cells, smooth muscle cells, neuronal cells, and keratinocytes. We performed experiments in a time range of 2, 3, 5, 10, 15, and 20 s, with heat exposure (approximating the temperature range caused by energy diffusion in the tissue) of 45 °C, 50 °C, 55 °C, 60 °C, 65 °C, and 70 °C. The viability of cells was assessed by measuring ATP content 24 h after thermal exposure as an estimation for cell survival and analyzed with the Arrhenius model for thermal damage.
Materials and methods
Cell culture
Heat exposure experiments were performed using four different cell lines. Human Umbilical Vein Endothelial Cells (HUVEC) were cultured in endothelial cell growth medium 2 (Promocell, C-22011) supplemented with 100 µg/ml Primocin (Invivogen, cat. No. 20A17, Toulouse, France). Human keratinocytes (Hacat) were generously provided by the lab of fertility (Amsterdam UMC, The Netherlands) and grown in RPMI1640 (Gibco, 21875-034, Thermo Fisher, Landsmeer, The Netherlands) with 8% Fetal Bovine Serum (FBS) (Gibco,10270-106, Thermo Fisher, The Netherlands), 5% L-glut (Lonza, BE17-605E, Geleen, The Netherlands) and 100 µg/ml primocin. Human BE2-M17 neuroblast cells (ECACC − 95011816, Merck, Amsterdam, The Netherlands) were cultured in DMEM high glucose and Nutrient Mix F12 Ham’s (Sigma, N6658, Amsterdam, The Netherlands) (1:1 ratio) with 1% non-essential amino acids (Sigma, M7145), 15% FBS, 1% L-glut, and 100 µg/ml primocin. Human primary aortic smooth muscle cells (ATCC, PCS-100-012) were cultured in vascular cell basal medium (ATCC - PCS-100-030) supplemented with Vascular Smooth Muscle Growth Kit (ATCC - PCS-100-042) as recommended by the provider. All cell cultures were maintained in T75 culture flasks (Corning, 43072OU), and grown at 37 °C, 5% CO2. The medium was refreshed every three to four days. For the heating experiments, about 4 · 105 cells were seeded on Ø12 mm coverslips (Thermo Scientific, 0980) that were placed in 24-well plates (Costar, 3524) with 500 µl of cell medium and grown to full confluence. For each temperature-time combination, one 24-well plate contained three biological replicates, i.e. three separate wells within a plate that were exposed to the same thermal conditions. These biological replicates all had the same passage number of the cell culture. In addition, the experiments were performed at three consecutive passages.
Hyperthermic shock and cell viability assessment
A glass beaker was filled with ± 200 ml Dulbecco PBS (dPBS) and placed on a thermal plate with a magnetic stirrer (Carl Roth, IKA C-MAG HS 7, Karlsruhe, Germany). A magnetic stir bar (4 cm) was placed at the bottom of the beaker to ensure homogenization of temperature. The thermometer of the heating plate was placed at the bottom of the beaker. Additionally, two thermocouples were placed to observe temperatures at a central position at 1/2th and 3/4th depth in the dPBS-filled beaker. The temperature of the thermocouples was measured with a calibrated Amprobe TMD-56 (Fluke Europe, Eindhoven, The Netherlands). The dPBS was serially heated to 37 °C, 45 °C, 50 °C, 55 °C, 60 °C, 65 °C and 70 °C. The cells grown on coverslips were immersed in the heated dPBS using sterile forceps for 2, 3, 5, 10, 15, or 20 s. Then, coverslips were returned to fresh medium and cultured for 24 h at 37 °C, 5% CO2. Experiments were performed under sterile conditions in a flow hood.
The effect of hyperthermal exposure was quantified 24 h after the hyperthermic shock by determining intracellular ATP as an estimation for cell survival [Citation10,Citation11]. Plates with hyperthermia-treated cells were gently rinsed with pre-warmed dPBS. Then, CelltiterGlo® luminescent Cell viability assay (Celltiter Glo G7571, Promega, Leiden, The Netherlands) was added in a 1:1 ratio with dPBS on every coverslip, protected from light, and placed on a shaker (IKA-Vibrax VXR, VWR, Amsterdam, The Netherlands) for 30 min. Finally, the samples were pipetted into a 96-well plate and the luminescent signal was quantified in a microplate reader (Biotek synergy, Agilent, United States).
Data analysis
Luminescent signal represents cellular ATP content (reflecting cell viability) which was measured with a plate reader and normalized to the ATP content of cells exposed to 37 °C (no thermal injury). Cell data are displayed as median ± interquartile range that was derived from a total of nine individual measurements (one for each coverslip) resulting in a dataset of relative viability defined as , where S is survival as a function of time (t) and temperature (T). Due to experimental errors, six measurements out of a total of 1296 measurements were excluded. Our goal was to test the hypothesis that cell survival after thermal damage differs between cell types. To this end, we estimated the lethal temperature (LT50; the temperature that causes 50% damage of the cell population) of the four cell types using an initial approximation via linear interpolation. We statistically analyzed the difference in LT50 by cell type using median values in a nonparametric Friedman Test with Dunn’s correction for multiple comparisons, with a significance level α of 0.05. All statistical analyses were performed with GraphPad Prism Software (version 9.5.1).
Modeling Thermal cell damage
The Arrhenius damage model was used to analyze the cell data and predict thermal damage and thus the survival per cell type [Citation12]. In this Arrhenius damage model, the temperature T K dependent) cell death rate is defined as:
(1)
(1)
With ,
is the activation energy, and
is the frequency factor. Assuming a constant temperature during the exposure time
, the cell survival can be described as:
(2)
(2)
In order to obtain and
for each cell type, all (∼324)
measurements for that cell type were simultaneously fitted with
. In order to obtain confidence intervals for the fitted parameters, bootstrapping was employed 10000 times [Citation13] to the measured
for each time-temperature combination. For each of the 10000 times fitted
and
,
and the LT50 were calculated (the latter by setting
), resulting in a range of cell survival and LT50 for each time-temperature combination. For each exposure time, the median and the 95% confidence intervals (based on the 2.5th and 97.5th percentiles) for the cell survival and LT50 were determined. Furthermore, the extrapolation of the LT50 curves per cell type allowed to determine the CEM43 values, which indicate the exposure time needed to cause a similar damage with a temperature of 43 °C. Here, we again statistically analyzed whether CEM43 values were significantly different amongst cell types using a nonparametric Kruskal Wallis Test with Dunn’s correction for multiple comparisons test was performed where a p-value <0.05 was considered statistically significant.
Results
Survival fractions of cells upon hyperthermia
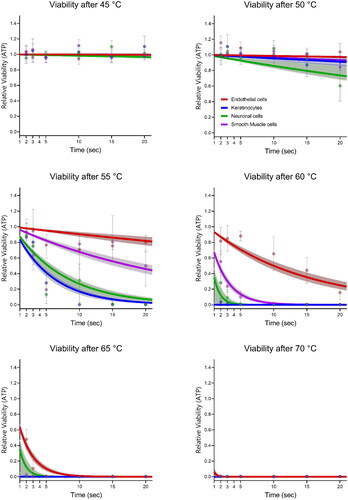
Thermal cell damage was investigated across different cell types through the examination of various combinations of time (2, 3, 5, 10, 15, and 20 s) and temperature (45, 50, 55, 60, 65, and 70 °C). Threshold levels of thermal cell damage were observed for each specific cell type, as depicted in . Generally, all cell lines exhibited maintained levels of intracellular adenosine triphosphate (ATP) at 37 °C and 45 °C indicating unaffected cell viability. The earliest decline in cell viability was observed at 20 s of exposure to 50 °C in neuronal cells. At 55 °C, a reduction in cell viability commenced at 5 s, resulting in decreased survival for neuronal cells, smooth muscle cells, and keratinocytes, while endothelial cells did not exhibit such decline. Smooth muscle cells reacted inconsistently, with an initial decrease in survival at 5 s, followed by an increase in viability at 10 s. A considerable decrease in viability was observed for keratinocytes, neuronal cells, and smooth muscle cells at 2, 3, and 5 s when exposed to 60 °C. Only endothelial cells exhibited substantial cell survival at 10 s and 60 °C and displayed a near-complete decline in survival fractions at 3 s and 65 °C.
Figure 1. Cell survival after hyperthermia of endothelial cells, smooth muscle cells, keratinocytes, and neuronal cells. Fits for survival data is also shown through the line plots. Survival fractions are relative to the ATP levels at exposure to 37 °C. Dots indicates the median of the cell data (n = 9), with error bars indicating the full range. Line plots indicate the Arrhenius damage model for thermal damage, error bands show the 95% CI. bars indicate the standard deviation of the median.
Temperature thresholds for 50% of viability
To determine the temperatures at which cell survival declined with 50%, the data was interpolated and fitted. illustrates the lethal temperatures at which a decline in ATP content of 50% (LT50) occurs for each cell line per exposure time. These data show that endothelial cells exhibit the highest thermal damage thresholds, followed by smooth muscle cells, keratinocytes, and neuronal cells. For endothelial cells, a temperature of 65 °C for 2 s is required to damage 50% of the cells, while neuronal cells experience a 50% decline in viability after only 2 s at approximately 58 °C (). The Friedman test demonstrated significant differences (p < 0.0001) between endothelial cells and all three other cell types and between smooth muscle cells and keratinocytes and neuronal cells. No significant difference (p = 0.68) was found in the LT50 between neuronal cells and keratinocytes. Fitting the data using the Arrhenius model for thermal damage and bootstrapping the data resulted in LT50 values with 95% CI as depicted in .
Figure 2. Temperature and time combinations that result in reduction of cell viability by 50% (LT50) of the linear interpolated points (symbols, n = 9, median with 95%CI) and the fitted LT50s using the Arrhenius model (lines, median, 95% CI, n = 5000). Significance levels of differences between the cell types are indicated with * for p < 0.0001 at α = 0.05.
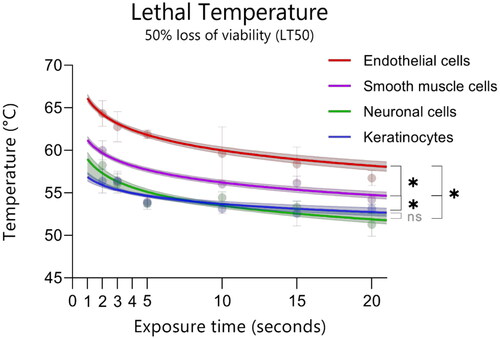
Extrapolating the Arrhenius model for each cell type also allowed the time it would take to inflict 50% damage at 43 °C, CEM43. The median CEM43 values and the 25th (Q1) and 75th (Q3) percentile were (from small to large): neuronal cells (0.28 h [0.22;0.40 h]), smooth muscle cells (1.8 h [1.4;2.2 h]), endothelial cells (2.6 h [1.9; 3.6 h), and lastly keratinocytes (8.6 h [5.0;15.9 h). A Kruskal Wallis test showed that the CEM43 values varied significantly (p < 0.0001) amongst all cell types.
Discussion
This study examined the sensitivity to thermal exposure among different cell types present in the skin. To achieve this goal, we tested four skin-relevant cell types under various hyperthermic conditions. We tested a range of short exposure times of 2 to 20 s, with temperatures that could represent the temperatures after diffusion of laser treatment in the skin (40–70 °C). Our results indicate that keratinocytes and neuronal cells are the most vulnerable to thermal stress. On the other hand, endothelial cells were the most resistant cell type to thermal exposure under the tested conditions.
Thermal damage is a combination of the temperature and the time a cell is exposed, and dependent on the time point at which cellular damage is measured. This temperature and time dependence makes it challenging to compare thermal damage thresholds among different studies. Still, the observations in the current study align with previous research on hyperthermia in three aspects:
Exposure to temperatures of 45 °C for 20 seconds does not show a decrease in viability in all our tested cell lines
The onset of thermal damage is 55 °C–60 °C, depending on the cell type and the exposure time (2–20 sec)
Exposure to 2 seconds of 70 °C did not show cell survival for all cell lines
In order to substantiate our observations, we evaluated the measurements of cell viability with the Arrhenius damage model. In this model, it is assumed that the thermal cell damage process is limited by one rate-limiting step which exposure time and temperature dependence are given by EquationEquation (1)(1)
(1) . In order to obtain an indication of 95% confidence intervals of the by EquationEquation (2)
(2)
(2) fitted cell survival data, we applied bootstrapping of our original data. In this way, we obtained 10000 a and E values. Although it is tempting to give the median and range of the fitted a and E values, the fitted a and E values were dependent on each other, which has been observed in many other studies [Citation12,Citation14]. The bootstrapping did facilitate the depiction of the range of fitted cell survival. In and the different susceptibilities to thermal stress for the different cell lines are clearly visible,. By extrapolation of the fitted LT50 to 43 °C, the so-called CEM43 time could be determined. As depicted in , the different cell types also differ in their estimated CEM43 values.
Table 1. CEM43 Values of thermal damage per cell type. Time (h) to reach 50% loss of survival at 43 °C was extrapolated from the Arrhenius curves for LT50.
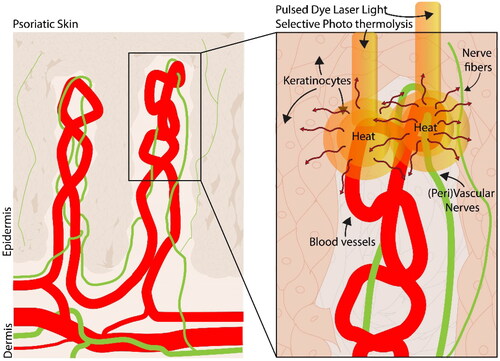
Despite the limited number of studies utilizing sub-minute exposure times in our study design, our results are consistent with the existing body of literature. Henderson et al. (2022) reported a decline in cell viability of human fibroblasts starting at 46 °C and cell viability declined to ∼ 25% after 60-min exposure to 54 °C [Citation15]. For an exposure time of 60 min, the LT50 of human fibroblasts was determined to be 48 °C. This matches our results in part since no decrease in cell viability was seen after 20 s 45–50°C, and that a first decline in cell viability was seen at ∼55 °C. However, there are two main differences between Henderson et al. (2022) and our current work: exposure time (60 min [Citation15] versus 2–20 s) and the time of viability measurement (1 h [Citation15] versus 24 h after hyperthermia). These differences in experimental design may explain why the LT50 of 48 °C is lower than our calculated LT50’s. In Chinese hamster lung cells, the survival fraction was already below 50% (∼45%) within 10 min at 46 °C with a recovery time of one week after hyperthermia exposure [Citation16]. Another study found that cell viability significantly decreased from 89% to 20% after one minute at 45 °C and stayed at 16% after 2 min at 50 °C [Citation17]. Another study, albeit on hepatic carcinoma cells, showed that metabolic activity was reduced by 50% after exposure to ± 80 °C for 5 min [Citation18]. An LT50 of 80 °C (exposure for 5 min) is a much greater survival rate compared to our findings. However, the method for heating in Mayrhauser et al. comprised placing the 96-well plates into a pre-heated oven (55, 65, 75, 85, 100 °C) for 5, 10, and 15 min, therefore creating a situation of gradual heating where insulation of the plastic and cell medium elongates the time to reach the stated temperature. Our study used block heating in a bath, which means that the cells are directly exposed to the stated temperature. Even so, the exposure times in the heating cabinet for 10 and 15 min are compatible with our data, showing a complete loss of viability between 65 °C and 75 °C. Within the setting of selective photothermolysis therapy, thermal damage of the vasculature and nearby tissue is influenced by multiple factors. Vascular heating depends on factors like laser wavelength, radiant exposure and the consequent laser fluence in the skin, tissue optics, chromophore volume, and pulse duration. Currently, we have a poor understanding of the local spatial and temporal temperature gradient in the skin following pulsed dye laser treatment. After radiofrequency ablation (7 min ∼100 °C), a temperature increase was observed at 5, 10, and 15 mm away from the probe area in a pig model, even 30 s after the treatment. The temperature ranged from 70.6 °C (5 mm distance) to 46.7 °C (15 mm distance) [Citation19]. Thus, this supports the idea that diffusing heat is sufficiently high to damage nearby cell types and structures. In the current study, we show that at 60 °C cell viability of endothelial cells is much higher compared to the other cell types. However, LT50 calculations showed significant differences even though they were only small around 1 °C amongst the different cell types. Nonetheless, our data suggests that diffusing heat from PDL could potentially damage neuronal cells and keratinocytes. This finding could imply that an important part of the therapeutic effects of PDL are related to damage to adjacent structures. Thus, we hypothesize that in PDL treatment, keratinocytes and (peri)vascular nerves can be damaged by heat (), but it is unlikely that nerves and keratinocytes are damaged to a greater extent than endothelial cells or smooth muscle cells in PDL treatment. Furthermore, it is important to consider the effect of cooling the skin (e.g. cryospray), which is a commonly used in laser treatment to reduce blistering and scarring. It was previously suggested that cooling in between hyperthermia may change the thermo-tolerance, which may lead to increased cell survival [20]. Thermal cell damage may be altered depending on the use and length of cooling applied to the skin during laser treatment [Citation16].
Figure 3. Illustration of heat diffusion of pulsed dye laser light from the blood vessels where the chromophore oxyhemoglobin resides toward the surrounding areas. These could include the (peri)vascular nerves, free nerve fiber endings, and keratinocytes.
Our study reports on the effect of short exposure to heat doses on multiple skin-relevant cell types. However, it is not without limitations. Despite efforts to culture human-derived cells, not all cell lines used in this study were derived from skin due to availability constraints. Moreover, an inherent problem with intracellular ATP measurement in cell cultures is the possibility of certain cells detaching from the wells at higher temperatures. This detachment could influence cell viability calculations, as there would be fewer cells available for counting in the assay. However, if cells lose adherence to the extracellular matrix in an in vivo setting, it is probable that they would not survive and might already be undergoing cell death [Citation17]. Finally, although ATP levels are a global assessment of cell viability, they do not fully account for potential differences in the ATP maintenance of different cells. In this context, it is important to carefully interpret small differences in thermal damage between neuronal cells, keratinocytes, and smooth muscle cells. Ideally, future research could validate the current findings by employing alternative methods to measure cell viability, such as colony formation assays. Furthermore, it would be valuable to verify whether the current differences between cells in thermal dose hold out in 3D skin culture settings and/or functional measurements of thermal damage to blood vessels.
Conclusions
The results of this study showed that thermally induced cell death of nerves and keratinocytes occurs at lower temperatures than in endothelial cells and smooth muscle cells. We also found that the LT50 values are significantly different in a range of 2 to 20 s of exposure to hyperthermia. Future research should focus on further investigating the spatial and temporal aspects of heat distribution within the skin after laser therapy. Understanding the cell-specific thermal damage in the skin after laser treatment could lead to more efficient and refined clinical practices in laser use in dermatology.
Acknowledgments
We would like to thank J. de Vos and R. Otto for setting up the culture of human smooth muscle cells. We would also like to thank N.B. van der Beek for fruitful discussions. We would also like to thank C. de Winter-Korver from the lab of fertility (AMC) for generously providing the keratinocytes. Lastly, we would like to thank T. Kaptein from the lab of experimental immunology (AMC) and K.W. Geijtenbeek from Medical Biology (AMC) for the use of their equipment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in figshare at 10.6084/m9.figshare.24467440 (data under embargo till publication).
Additional information
Funding
References
- Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol. 2003;49(1):1–34. quiz 31-4. doi: 10.1067/mjd.2003.582.
- Henning JP, Gemert M, Lahaye CTW. Clinical and histological evaluation of portwine stain treatment with a microsecond-pulsed dye-laser at 577 NM. Lasers Surg Med. 1984;4(4):375–380. doi: 10.1002/lsm.1900040410.
- Zelickson BD, Mehregan DA, Wendelschfer-Crabb G, et al. Clinical and histologic evaluation of psoriatic plaques treated with a flashlamp pulsed dye laser. J Am Acad Dermatol. 1996;35(1):64–68. doi: 10.1016/S0190-9622(96)90498-3.
- Erceg A, de Jong EM, van de Kerkhof PC, et al. The efficacy of pulsed dye laser treatment for inflammatory skin diseases: a systematic review. J Am Acad Dermatol. 2013;69(4):609–615 e8. doi: 10.1016/j.jaad.2013.03.029.
- Hohenleutner U, Hilbert M, Wlotzke U, et al. Epidermal damage and limited coagulation depth with the flashlamp-pumped pulsed dye laser: a histochemical study. J Invest Dermatol. 1995;104(5):798–802. doi: 10.1111/1523-1747.ep12606996.
- Hern S, Allen MH, Sousa AR, et al. Immunohistochemical evaluation of psoriatic plaques following selective photothermolysis of the superficial capillaries. Br J Dermatol. 2001;145(1):45–53. doi: 10.1046/j.1365-2133.2001.04280.x.
- Johnson HA, Devaney KO. Thermal instability in the endoplasmic reticulum of the rat hepatocyte. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40(3):357–364. doi: 10.1007/BF02932877.
- Wilk LS, Doppegieter M, van der Beek N, et al. Modeling pulsed dye laser treatment of psoriatic plaques by combining numerical methods and image-derived lesion morphologies. Lasers Surg Med. 2024. doi: 10.1002/lsm.23781.
- Doppegieter M, van der Beek N, Bakker ENTP, et al. Effects of pulsed dye laser treatment in psoriasis: a nerve-wrecking process? Exp Dermatol. 2023;32(7):1165–1173. doi: 10.1111/exd.14816.
- Ulukaya E, Ozdikicioglu F, Oral AY, et al. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol in Vitro. 2008;22(1):232–239. doi: 10.1016/j.tiv.2007.08.006.
- Petty RD, Sutherland LA, Hunter EM, et al. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995;10(1):29–34. doi: 10.1002/bio.1170100105.
- Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia. 2013;29(4):262–280. doi: 10.3109/02656736.2013.786140.
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Taylor and Francis; 1994.
- Qin Z, Balasubramanian SK, Wolkers WF, et al. Correlated parameter fit of arrhenius model for thermal denaturation of proteins and cells. Ann Biomed Eng. 2014;42(12):2392–2404. doi: 10.1007/s10439-014-1100-y.
- Henderson E, Kempf M, Yip C, et al. The lethal heat dose for 50% primary human fibroblast cell death is 48 degrees C. Arch Dermatol Res. 2022;314(8):809–814. doi: 10.1007/s00403-021-02217-y.
- Chinn SB, Lee FT, Kennedy GD, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176(3):789–795. doi: 10.2214/ajr.176.3.1760789.
- Jung H. Interaction of thermotolerance and thermosensitization induced in CHO cells by combined hyperthermic treatments at 40 and 43 degrees C. Radiat Res. 1982;91(3):433–446. doi: 10.2307/3575883.
- Assi HTI, Arsenault MG, Whelan WM, et al. A new thermal dose model based on Vogel-Tammann-Fulcher behaviour in thermal damage processes. Int J Hyperthermia. 2022;39(1):697–705. doi: 10.1080/02656736.2022.2065367.
- Chaabane W, User SD, El-Gazzah M, et al. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp (Warsz). 2013;61(1):43–58. doi: 10.1007/s00005-012-0205-y.
