Abstract
Purpose
The treatment of recurrent thyroid cancer with critical organ invasion is challenging. The combination of radiofrequency ablation (RFA) and external beam radiation therapy (EBRT) has been proposed as an effective option. This study evaluates outcomes for inoperable residual/recurrent differentiated thyroid cancer (rDTC) patients treated with RFA followed by EBRT.
Materials and Methods
Patients with rDTC treated with RFA followed by EBRT were retrospectively studied. RFA was performed using a free‐hand, ‘moving‐shot’ technique under US or CT guidance. For lesions invading critical structures intolerant to ‘en bloc’ high-temperature RFA, limited-field EBRT using 6‐ or 10‐MV photons was used for adjuvant treatment at a dose of 66 Gy in 33 daily fractions. Toxicities and outcomes were reviewed.
Results
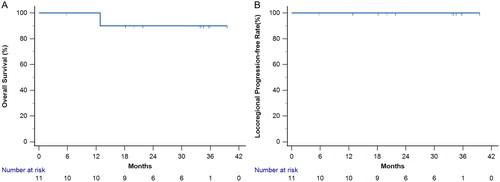
Between April 2020 and January 2022, 11 patients with 14 rDTC lesions underwent RFA followed by EBRT. Five patients had metastatic lesions at rDTC diagnosis. With a median follow-up period of 33.7 months, all patients maintained locoregional control, while achieving a 2-year survival rate of 90.9%. This combined treatment achieved a volume reduction ratio of 92.1% ± 5.1%. The mean nadir thyroglobulin level in patients without initial distant metastases after treatment was 1.40 ± 0.81 ng/ml. Regarding treatment-related complications, one patient (9%) experienced temporary hoarseness after RFA, grade 2 radiation dermatitis occurred in 3 patients (27.2%), and grade 2 dysphagia was noted in 4 patients (36.4%). No grade 3 or greater toxicities occurred.
Conclusions
Salvage RFA followed by EBRT is feasible, effective and safe for patients with rDTC.
Introduction
Thyroid cancer is the 8th most common malignancy in Taiwan, with approximately 5000 newly diagnosed patients diagnosed in 2019 [Citation1]. Differentiated thyroid cancer (DTC) is the most common thyroid cancer and comprises more than 90% of cases. Although patients with DTC have excellent disease-specific survival, approximately 30% of patients experience recurrence during long-term follow-up, and two-thirds of these patients have local recurrence [Citation2,Citation3]. The mainstay treatment for locally recurrent tumors is salvage surgical resection. However, repeat surgery presents a considerable challenge—because of the distortion of tissue planes and severe fibrosis from scar tissue—and leads to complications, including hypocalcemia and recurrent laryngeal nerve injury [Citation4,Citation5].
Recently, radiofrequency ablation (RFA) has been reported to be a minimally invasive nonsurgical option for treating locally recurrent differentiated thyroid cancer (rDTC) [Citation4,Citation6,Citation7]. It results in a significant reduction in tumor volume and is considered an alternative treatment for patients with locally recurrent thyroid cancers or neck lymph node metastases after thyroidectomy, especially in cases where there is a high surgical risk or the patient refuses surgery [Citation4,Citation8]. Recommended by the Korean, Taiwanese, and European Thyroid Associations in their guidelines for managing recurrent thyroid cancers, especially in high-risk surgical patients, RFA offers considerable symptom relief and disease control [Citation9–12]. The advantages of RFA include fewer comorbidities and fewer complications, suitability for real-time guidance, and the possibility of being performed on an outpatient basis [Citation13]. However, RFA has limitations in achieving en bloc ablation of tumors invading critical structures, such as the trachea in the central compartment, with a lower tumor disappearance rate than for other lesions not in contact with the trachea [Citation14].
External beam radiotherapy (EBRT) has been reported to provide durable locoregional control in unresectable and gross residual DTC patients in retrospective studies [Citation15–18]. Locally invasive thyroid cancer may involve the recurrent laryngeal nerve, trachea, and esophagus, which are not amenable to complete resection, especially in recurrent disease [Citation19]. The aim of adjuvant EBRT is to decrease local relapse without compromising organ function. In a prior study [Citation14], six out of 119 patients with recurrent thyroid cancers invading the airways in the central compartment underwent a combination of RFA and EBRT. This approach, which combines RFA with RT, was employed for the treatment of recurrent thyroid cancer. However, for patients with rDTC invading critical structures who initially undergo RFA, the optimal application of adjuvant radiotherapy remains unclear. To our knowledge, there is a paucity of literature evaluating salvage RFA followed by EBRT in patients with inoperable recurrent thyroid cancer.
Therefore, the purpose of this study was to assess the efficacy and toxicity of these two combined modalities in treating rDTC.
Materials and methods
Patients and salvage treatment
Our cohort study was reviewed and approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (IRB No. 202200774B0). Between April 2020 and January 2022, 11 patients who had a pathological diagnosis of differentiated thyroid cancer and unresectable recurrent or residual tumors were retrospectively reviewed. Patients who previously underwent total thyroidectomy, therapeutic neck dissection, thyrotropin-suppression therapy and radioactive iodine (RAI) before recurrence or residual tumor were detected. Four patients had a history of more than two repeated surgeries, and all patients had radioiodine refractory DTC. Fourteen locoregional lesions in 11 patients were detected, and these patients were ineligible for surgical resection.
Before salvage RFA was performed, the patients were evaluated by ultrasound (US). The presence of recurrent tumors was confirmed by ultrasound-guided fine needle aspiration biopsy. Additionally, all patients underwent contrast-enhanced neck computed tomography (CT) and pretreatment laboratory tests. Positron emission tomography/computed tomography (PET-CT) can be used for the detection of recurrent tumors. Laboratory examinations included serum thyroglobulin levels, thyroid function tests, platelet counts and blood coagulation tests (partial thromboplastin time and prothrombin time). All patients, with a total of 14 lesions, initially underwent salvage treatment with RFA. Considering incomplete ablation due to lesions attaching to critical structures (such as carotid arteries, trachea, or esophagus) or invading the recurrent laryngeal nerve leading to voice changes, which cannot tolerate ‘en bloc’ high-temperature radiofrequency ablation (RFA), external beam radiotherapy was performed as adjuvant treatment after RFA. Two patients had two additional recurrent lymph nodes in the retropharyngeal and retrotracheal regions that could only be treated with RT due to the difficulty of access for RFA. For patients with initial distant metastases, in addition to local salvage therapy, lenvatinib was used for systemic control.
Radiofrequency ablation technique
The patients underwent the procedure on an outpatient basis. After anesthetizing locally with 2% lidocaine at the puncture site for the subcutaneous and thyroid capsules, we performed hydrodissection with 5% dextrose solution between the tumor and the dangerous triangle or other vital organs [Citation9,Citation20]. Radiofrequency generators (VIVA, STARmed and M2004, RF Medical) and an 18-gauge internally cooled electrode with a 5 mm, 7 mm, or 1 cm active tip depending on the tumor size were used. The moving shot technique was used to ablate the gross tumor slice by slice under sonographic guidance after placing an electrode at the deepest portion of the tumor as previously described [Citation21–23]. We terminated ablation when all visual fields of the tumors had changed to transient hyperechoic zones. Specific care for the surrounding regions, including the carotid space, trachea and esophagus, was taken during the procedure.
Radiotherapy technique
The patients underwent CT simulation scans with 2.5 mm slices in the supine position and were immobilized using thermoplastic head-neck-shoulder casts. For patients with gross tumors, the gross target volume (GTV) was defined as the lesion. The clinical target volume (CTV) was defined as at least a 5 mm margin from the GTVs and/or potential areas at risk for microscopic tumor involvement, generally a 5 mm margin encompassing the pre-RFA gross tumor volume and the post-RFA tumor bed. As all patients were treated with only a limited field, elective nodal irradiation was not applied. The planned target volume (PTV) margin was set to 3 mm to account for setup errors, and the prescribed dose was uniformly 66 Gy in 33 daily fractions to the PTVs, based on previous studies in the postoperative radiotherapy setting [Citation24,Citation25]. Regarding dose constraints to the PTVs, our protocol was set such that at least 95% of the PTV was covered by the prescribed dose and 100% of the PTV was covered by the 95% isodose surface; the dose constraints to the organs at risk were based on our institutional protocol for fractionated radiotherapy. All treatments were delivered with volumetric-modulated arc therapy using 6 or 10 megavoltage photons.
Follow-up, outcomes, and complications
Patient follow-up was scheduled every 3 months in the first two years after treatment and then every 6 months thereafter; it involved measuring serum thyroglobulin (Tg) levels and administering regular imaging surveys, including thyroid US and contrast-enhanced CT scans. As PET-CT was not reimbursed by our National Health Insurance for post-treatment patients, only those who were willing to pay out-of-pocket were followed with PET-CTs. In addition, a radiation oncologist contoured and measured the volumes of the thyroid tumors or lymph nodes via MIM-facilitated contrast-enhanced CT (MIM Software, Cleveland, Ohio) before and after treatment. The equation volume reduction ratio (VRR) (%) = initial volume (mL)–final volume (mL) × 100/initial volume was used for calculations at the time of follow-up [Citation12]. In addition, to evaluate the correlation between tumor volume and the Tg level, these two parameters were normalized. The relative tumor volume was defined as the tumor volume/initial tumor volume. The absolute values of the serum Tg were normalized to the relative Tg levels calculated as the serum Tg/baseline Tg level. Toxicity and clinical status were also evaluated during radiotherapy on a weekly basis and during follow-up. Complications during the RFA procedure or follow-up were evaluated using standard terminology from the Society of Interventional Radiology [Citation26]. In addition, adverse events of radiotherapy were assessed and scaled according to the Common Terminology Criteria for Adverse Events (version 4.0) [Citation27]. Late complications of radiotherapy, including laryngeal edema and dysphagia, were defined as those occurring three months or more after the completion of RT [Citation28,Citation29].
Statistics
Statistical analysis was performed by using SPSS, version 25 (SPSS, Inc., Chicago, IL, USA). The data are presented as the mean ± standard error of the mean. The primary outcome was locoregional control, which was defined as no progression of symptoms, critical structure or cosmetic problems. The secondary outcomes were VRR, change of serum Tg, and overall survival. Kaplan–Meier estimates were performed for overall survival and locoregional control. One-way analysis of variance (ANOVA) was used to assess differences in tumor volume at each follow-up. The relationships between relative volume and relative Tg level were studied in patients with localized disease and patients with detectable Tg levels using Pearson correlation. When the p value was <0.05, differences were considered statistically significant.
Results
The median follow-up duration was 33.7 months (range 5.8–39.5 months). The baseline characteristics of the 11 patients are summarized in . The median age was 61 years (range, 30-72 years) in this group, with 1 man and 10 women included. Before treatment, positron emission tomography-computed tomography (PET-CT) was used to detect recurrent or residual lesions in 10 patients (90%). Five patients had metastatic lesions at the initial diagnosis of rDTC. The mean serum Tg concentrations were 133.26 ± 60.18 ng/ml and 27.13 ± 16.04 ng/ml in patients with and without initial distant metastases, respectively. The lesion characteristics and treatment outcomes are shown in . Among the 14 recurrent or residual tumors, five lesions (36%) occurred in the thyroid surgical bed, and 9 (64%) lesions occurred in the paratracheal or lateral neck lymph nodes. The mean largest diameter of the tumor was 2.1 ± 0.3 cm, and the mean tumor volume was 11.8 ± 5.9 ml.
Table 1. Baseline patient characteristics.
Table 2. Characteristics of lesions and treatment outcomes.
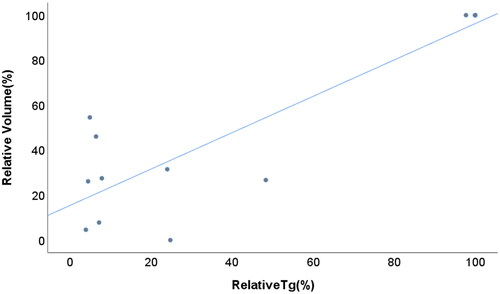
The relative tumor volumes before and at each follow-up are shown in . Initially, the tumor volume decreased from 100% to 81.0% of the baseline tumor volume, with a significant trend (p = 0.068) at the 3-month follow-up. Thereafter, the relative tumor volume gradually decreased to 50.4% (p < 0.001), 29.6% (p < 0.001), 21.2% (p < 0.001), 11.8% (p < 0.001) and 7.9% (p < 0.001) at the 6-, 9-, 12-, 18- and 24-month follow-ups, respectively. The two-year volume reduction ratio was 92.1% ± 5.1% (range, 67.6-100%). Six lesions (43%) in the 6 patients had completely resolved at the final follow-up CT (). For these tumors, the median interval from RFA to complete disappearance was 16.4 months (range, 10.8–24.8 months). The other 8 lesions that did not completely disappear showed no enhancement with fat stranding around the post-treatment region, and no tumor regrowth was detected at the last follow-up. In addition, we observed that the two lesions treated with RT alone had a mean volume reduction ratio of 16.7%. Two patients with localized disease had an undetectable low Tg level (<0.2 ng/ml). The other four patients without distant metastases had decreased thyroglobulin levels from 2.63 ng/ml, 4.14 ng/ml, 76.4 ng/ml and 79.2 ng/ml to 0.65 ng/ml (18 months), 0.2 ng/ml (24 months), 4.88 ng/ml (9 months) and 3.05 ng/ml (24 months), respectively, during the last follow-up. The median interval from RFA to the nadir Tg was 5.4 months (range, 4.7–14 months). The mean nadir Tg in patients without distant metastases after treatment was 1.40 ± 0.81 ng/ml. In addition, the relative tumor volumes in patients with localized disease and initial detectable Tg levels significantly correlated with the relative Tg levels (p < 0.001) ().
Figure 1. Change in tumor volume percentage before RFA and RT and at each follow-up.
Error bars represent the standard error of the mean; ***p ≤ 0.001
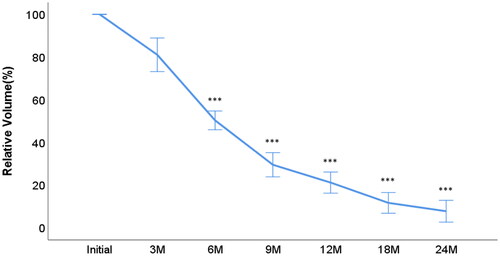
Figure 2. A 63-year-old man with recurrent thyroid cancer.
(a) contrast-enhanced CT before RFA and RT, showing one enhanced mass invading the tracheal wall and esophageal wall; (b) PET-CT before RFA and RT, showing increased FDG uptake in the paratracheal mass lesion (SUV max: 22.25); (c) contrast-enhanced CT 39 months after RFA and RT, showing that the recurrent tumor had almost completely disappeared; (d) PET-CT 6 months after RFA and RT, showing background FDG avidity with complete metabolic response in the left paratracheal region; CT, computed tomography; RFA, radiofrequency ablation; RT, radiotherapy; PET-CT, positron emission tomography and computed tomography; FDG: fludeoxyglucose F-18; SUV max, maximum standardized uptake value
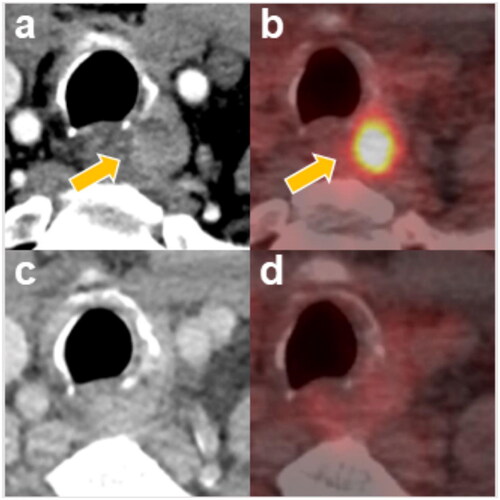
Figure 3. The relationship between relative tumor volume and relative Tg in patients with localized recurrent disease and initial detectable Tg (Pearson correlation coefficient, 0.886; p < 0.001).
The 2-year locoregional control was 100%. Following treatment, all patients exhibited no symptoms of compression, including neck pain, dysphagia, foreign body sensation, discomfort, and cough. Additionally, no palpable masses or cosmetic concerns were observed. The 2-year overall survival was 90.9% (), 10 patients were alive, and one patient died of cardiovascular disease. However, 5 patients who had metastatic disease before treatment experienced distant lesion progression. Two patients experienced progression of lung metastatic lesions, and the other patient experienced progression of soft tissue metastatic lesions. Six patients with initial localized tumors had no distant recurrence.
The incidence of toxicity resulting from salvage treatment is shown in . All patients tolerated the RFA procedure and subsequent radiotherapy well. The overall complication rate was 9% (1/11) after RFA. One patient developed voice changes during RFA. This patient with voice changes recovered within 1 month after ablation. No life-threatening or delayed complications during the follow-up period were reported. Acute grade 2 dermatitis was observed in three (27.2%) of the eleven patients after radiotherapy. Four patients (36.4%) had acute grade 2 pharyngeal mucositis or dysphagia. No patient experienced acute grade 3 or higher toxicity, and no elective tracheostomy or tube feeding was needed. There were no late complications, including laryngeal edema or dysphagia with tube feeding dependence, after salvage treatment.
Table 3. Incidence of toxicity.
Discussion
Our results demonstrated that RFA followed by local RT was an effective salvage treatment for patients with recurrent unresectable thyroid cancer. A previous study [Citation14] also revealed the feasibility and concept of combining RFA and RT. Chung et al. [Citation14] reported the use of RFA and adjuvant RT to treat recurrent thyroid cancer invading the airways. In one patient who received the combination approach, the recurrent tumor with intraluminal tracheal invasion completely disappeared. In our study, all 14 lesions that were treated with combined RFA and RT showed marked volume reduction and symptom relief. Notably, all these lesions were deep-seated tumors with invasion or attachment to critical structures such as carotid arteries, trachea, or esophagus that limited en bloc ablation by RFA. Although RFA, a single modality, has been demonstrated to be an effective treatment for recurrent thyroid cancer [Citation30], local control for lesions with incomplete ablation has not yet been studied. The rationale for combining RFA with RT in our series was based on the principle of adjuvant therapy for head and neck cancer patients with macroscopic/microscopic residual tumors or unclear margins after surgical resection. Based on this rationale, the dose that we chose for our patients was 66 Gy in 33 fractions for those with high-risk, margin-positive patients [Citation24,Citation25,Citation31]. None of our patients experienced locoregional recurrence, and we did not observe any severe acute or late side effects.
Radiofrequency ablation (RFA) is recognized as an effective alternative for managing recurrent thyroid cancers, particularly in patients at high surgical risk or preferring non-surgical options. Supported by the Korean, Taiwanese, and European Thyroid Associations, RFA is recommended for its efficacy in cases with high surgical complications [Citation9–12]. The Korean guidelines strongly advocate for RFA in recurrent cancers at the thyroidectomy bed and cervical lymph nodes, highlighting its role in avoiding complications from repeat surgeries. Studies show RFA is particularly beneficial for patients with fewer and smaller recurrent tumors [Citation8,Citation32,Citation33], especially in cases where patients had fewer than three or four locally recurrent tumors, and the largest tumor measured between 1.5 and 2 cm in diameter [Citation7,Citation20,Citation34]. The Taiwanese consensus also supports RFA for managing recurrent thyroid cancers and neck lymph node metastases post-thyroidectomy, noting its lower complication rates and effectiveness in reducing tumor burden [Citation4]. The consensus reflects on studies showing high volume reduction rates (VRRs) and low recurrence rates with RFA, positioning it as a robust option for symptom relief and as a bridge to other treatments [Citation7,Citation20,Citation34]. Meanwhile, the European guidelines suggest RFA and other minimally invasive techniques as alternatives to surgery, especially in managing radioiodine refractory cervical recurrences, achieving similar survival rates with fewer complications [Citation7,Citation35]. Overall, RFA is valued across guidelines for its minimal invasiveness and effectiveness, providing significant symptom relief and disease control, making it a suitable choice for patients with high surgical risks or those who decline surgery.
Despite the advantages of RFA, the rate of complete ablation by RFA alone could be reduced when recurrent tumors invade critical organs such as the trachea, esophagus, nerves or vessels [Citation14,Citation36,Citation37], and local heat may cause considerable morbidities or even life-threatening events. In such a clinical scenario in which the presence of residual tumor may be inevitable after RFA, adjuvant local therapy to reduce the risk of local recurrence should be considered. The rationale follows the concept of using adjuvant RT for locally advanced head and neck cancer after surgery based on the high-risk pathological features of positive margins in the EORTC trial by Bernier et al. [Citation24]. Our results demonstrated that this combined modality of local treatment to eradicate microscopic disease was highly effective and should be applied for patients with suspicious residual tumors after local RFA.
For patients with recurrent thyroid cancer invading the trachea or esophagus who underwent RT alone and/or tyrosine kinase inhibitor, previous studies have suggested the possibility of fistula formation [Citation37,Citation38]. Combination therapy has the potential to prevent this complication, as RFA administered before RT induces fibrosis in treated recurrent tumors [Citation14,Citation37]. Additionally, using RFA before RT can effectively reduce the tumor burden by decreasing tumor volume. Previous studies demonstrated that the local control and efficacy of RT were better when only a microscopic residual tumor remained (R1 resection) than when a macroscopic residual tumor remained (R2 resection) in patients who underwent surgery [Citation18,Citation39]. Reducing the tumor burden by RFA aligns with the concept of achieving resection with only microscopic tumors remaining after surgery, thereby improving the efficacy of RT in eradicating tumors and ensuring local control. Furthermore, this combined treatment can significantly alleviate compressive symptoms caused by tumors near critical organs, including difficulties in swallowing, shortness of breath, neck discomfort, sensation of a foreign object, and pain. Additionally, there are no significant cosmetic problems detected after treatment. In terms of comparing RT doses between combination treatment and RT alone, the addition of RFA can lead to a reduction in the RT dose. Previous studies have shown that the total dose of RT alone for gross tumors is typically 70 Gy [Citation40,Citation41]. The RT dose in our study was reduced to 66 Gy (similar to the postoperative RT doseCitation24] because RFA led to a reduction in tumor burden. Additionally, RFA can efficiently decrease Tg levels [Citation6,Citation8,Citation12]. In the present study, we found that a decrease in the serum Tg concentration was associated with a decrease in the tumor volume in patients with localized tumors and a decrease in the Tg concentration after treatment, comparable to the findings of a previous study [Citation42]. Therefore, considering the prevention of fistula formation, potential improvement in local control with volume reduction, symptom improvement and reduced RT dose, RFA combined with RT could be considered an option for patients with rDTC, especially those with tumors invading critical structures.
The optimal irradiation field for rDTC has long been debated, especially in cases of prophylactic irradiation. A previous study conducted by Kim et al. [Citation43] compared the difference between extensive field and limited field RT among 23 patients. The results of their study showed that more than 40% of 11 patients who underwent limited field RT experienced out-field recurrence at a median of 41.1 months, whereas only 8.3% of those who underwent extensive field RT experienced locoregional recurrence. However, in our study, all 11 patients were treated with limited-field RT, and none of the patients experienced locoregional in-field or out-of-field failure. There were two explanations for the different outcomes as follows. First, the patients in the Kim study were treated from 2004 to 2008. As diagnostic techniques have advanced over the years, the accuracy of pretreatment examinations has increased. All patients in the present study underwent ultrasound and contrast-enhanced CT for locoregional evaluation before treatment. Furthermore, PET-CT was employed for detecting recurrence in the majority of patients (91%) with DTC despite its limitations in detecting small tumors [Citation38]. Furthermore, all patients in our study were treated with RFA followed by RT. RFA substantially reduced the tumor burden with the aim of reducing locoregional recurrence. Thus, along with both comprehensive pretreatment evaluation and the curative intent of RFA, limited RT fields are effective and have a low risk of locoregional recurrence.
In addition to locoregional control, treatment-related complications were another consideration regarding RT. In previous studies, elective RT with extensive fields caused more than 50% grade 2 and 8-30% grade 3 acute toxicity, including pharyngeal mucositis and dysphagia, in patients with rDTC15 [Citation40,Citation43,Citation44],. Severe acute toxicity may lead to treatment breaks and short-term enteral tube feeding for nutritional support [Citation15,Citation40,Citation44]. In addition, permanent tube-feeding dependence, a late complication, was also reported under extensive RT in one retrospective study [Citation15]. In our study, none of the patients experienced grade 3 or higher side effects, and thus, no patients required tube feeding during or after the RT course. Although long-term complications have not yet been reported in our study, consequential late side effects may be minimized due to the amelioration of acute radiation reactions [Citation45].
Although the results of our treatment protocol revealed good locoregional control and low toxicity, systemic control still posed a challenge, especially in patients with initial metastatic disease. While no distant recurrence was found in the patients with only localized tumors treated with RFA + RT during follow-up, 3 patients with metastatic disease before treatment experienced distant progression, including lung or soft tissue metastases. Treatment strategies for patients with progressive and radioiodine-refractory metastatic differentiated thyroid cancer are limited. Lenvatinib, an antiangiogenic multikinase inhibitor, improved progression-free survival from 3.6 to 18.3 months in a phase III study [Citation46]. According to further subgroup analysis, although overall survival improved in patients older than 65 years of age receiving lenvatinib, more severe grade 3 toxicity occurred in these patients than in younger patients [Citation47]. The efficacy and safety of other mutation-specific kinase inhibitors related to targetable driver mutations (e.g. NTRK, ALK, RET and BRAF) have been investigated [Citation48]. There is an ongoing phase II trial evaluating the effect of lenvatinib combined with pembrolizumab in patients with progressive metastatic DTC [Citation49]. The preliminary results revealed that 62% of patients had a partial response, and 35% had stable disease, for a 97% clinical benefit rate. PFS at 12 months was 74%, and 10% of patients had >80% tumor shrinkage. The results of this combined treatment seemed promising but need further validation.
Our study has several limitations. First, the retrospective chart review may have led to selection biases. However, the criteria for patient selection and salvage treatment procedures, including RFA and radiotherapy, were standardized in our hospital. For example, a uniform RT dose (66 Gy) was prescribed to treat the post-RFA tumor bed. Moreover, the relatively short follow-up period might require long-term validation of durable locoregional control. Furthermore, the small sample size (n = 11) led to a heterogeneous population with different recurrent or residual tumor statuses, including initial distant metastases. Finally, our study lacked a control group receiving RT alone and/or lenvatinib for comparison with the combination of RFA and RT. The significant differences and benefits of adding RFA need further validation through comparison with a control group. The current study is a proof-of-concept examination of treating unresectable recurrent disease with the combined modality of RFA and radiotherapy. Further clinical studies and trials are needed to assess the suggested strengths.
Conclusion
This study demonstrated that salvage RFA with RT is not only an effective treatment for reducing tumor volume but also a safe treatment modality. Moreover, distant control for rDTC patients remains an area for future exploration.
Acknowledgments
We appreciate the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data are available upon request to the corresponding authors.
Additional information
Funding
References
- Bureau of Health Promotion, Department of Health, Taiwan. Cancer Registry Annual Report. Published January 13, 2022. https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed May 12, 2022).
- Mazzaferri EL, Kloos RT. Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1–10. doi: 10.1210/jcem.86.4.7407.
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–428. doi: 10.1016/0002-9343(94)90321-2.
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36(1):359–367.
- Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19(12):1373–1380. doi: 10.1089/thy.2009.1606.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903. doi: 10.1007/s00330-019-06063-5.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918. doi: 10.1148/radiol.15140079.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and Meta-Analysis. Thyroid. 2016;26(3):420–428. doi: 10.1089/thy.2015.0545.
- Lin WC, Chen WC, Wang PW, et al. 2022 Taiwan clinical multicenter expert consensus and recommendations for thyroid radiofrequency ablation. Ultrasonography. 2023;42(3):357–375. doi: 10.14366/usg.22126.
- Mauri G, Hegedüs L, Cazzato RL, et al. Minimally invasive treatment procedures have come of age for thyroid malignancy: the 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Cardiovasc Intervent Radiol. 2021;44(9):1481–1484. doi: 10.1007/s00270-021-02870-w.
- Lee M, Baek JH, Suh CH, et al. Clinical practice guidelines for radiofrequency ablation of benign thyroid nodules: a systematic review. Ultrasonography. 2021;40(2):256–264. doi: 10.14366/usg.20015.
- Kim J-H, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655. doi: 10.3348/kjr.2018.19.4.632.
- Shin JE, Baek JH, Lee JH. Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: current status and challenges. Curr Opin Oncol. 2013;25(1):14–19. doi: 10.1097/CCO.0b013e32835a583d.
- Chung SR, Baek JH, Choi YJ, et al. Efficacy of radiofrequency ablation for recurrent thyroid cancer invading the airways. Eur Radiol. 2021;31(4):2153–2160. doi: 10.1007/s00330-020-07283-w.
- Terezakis SA, Lee KS, Ghossein RA, et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: memorial sloan-kettering cancer center experience. Int J Radiat Oncol Biol Phys. 2009;73(3):795–801.
- Brierley JD, Tsang RW. External beam radiation therapy for thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(2):497–509, xi. doi: 10.1016/j.ecl.2008.02.001.
- Keum KC, Suh YG, Koom WS, et al. The role of postoperative external-beam radiotherapy in the management of patients with papillary thyroid cancer invading the trachea. Int J Radiat Oncol Biol Phys. 2006;65(2):474–480.
- Schwartz DL, Lobo MJ, Ang KK, et al. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;74(4):1083–1091. doi: 10.1016/j.ijrobp.2008.09.023.
- Shindo ML, Caruana SM, Kandil E, et al. Management of invasive well-differentiated thyroid cancer: an American head and neck society consensus statement. AHNS consensus statement. Head Neck. 2014;36(10):1379–1390. doi: 10.1002/hed.23619.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170. doi: 10.1007/s00330-014-3405-5.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250. doi: 10.1007/s00330-008-0880-6.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194(4):1137–1142. doi: 10.2214/AJR.09.3372.
- Lin WC, Wang CK, Wang WH, et al. Multicenter study of benign thyroid nodules with radiofrequency ablation: results of 762 cases over 4 years in Taiwan. J Pers Med. 2022;12(1):63.
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641.
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk Squamous-Cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v.4.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed June 14, 2023.
- Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79–92.
- Ortigara GB, Bonzanini LIL, Schulz RE, et al. Late radiation effects in survivors of head and neck cancer: state of the science. Crit Rev Oncol Hematol. 2021;162:103335. doi: 10.1016/j.critrevonc.2021.103335.
- Tufano RP, Pace-Asciak P, Russell JO, et al. Update of radiofrequency ablation for treating benign and malignant thyroid nodules. The future is now. Front Endocrinol (Lausanne). 2021;12:698689. doi: 10.3389/fendo.2021.698689.
- National Comprehensive Cancer Network. Head and Neck Cancers (version 2. 2024). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Published 2024. Accessed.
- Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. The Journal of Clinical Endocrinology & Metabolism. 1992;75(3):714–720.
- Zhao Q, Tian G, Kong D, et al. Meta-analysis of radiofrequency ablation for treating the local recurrence of thyroid cancers. J Endocrinol Invest. 2016;39(8):909–916. doi: 10.1007/s40618-016-0450-8.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197(2):W331–336.
- Heilo A, Sigstad E, Fagerlid KH, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96(9):2750–2755. doi: 10.1210/jc.2010-2952.
- Park KW, Shin JH, Han BK, et al. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol. 2011;18(9):2564–2568. doi: 10.1245/s10434-011-1619-1.
- Lee MK, Baek JH, Chung SR, et al. Radiofrequency ablation of recurrent thyroid cancers: anatomy-based management. Ultrasonography. 2022;41(3):434–443. doi: 10.14366/usg.21221.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020.
- Groen AH, van Dijk D, Sluiter W, et al. Postoperative external beam radiotherapy for locoregional control in iodine refractory differentiated thyroid cancer. Eur Thyroid J. 2022;11(1):e210033. doi: 10.1530/ETJ-21-0033.
- Romesser PB, Sherman EJ, Whiting K, et al. Intensity-modulated radiation therapy and doxorubicin in thyroid cancer: a prospective phase 2 trial. Cancer. 2021;127(22):4161–4170. doi: 10.1002/cncr.33804.
- Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57(1):49–60. doi: 10.1016/s0360-3016(03)00405-x.
- Bachelot A, Cailleux AF, Klain M, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12(8):707–711. doi: 10.1089/105072502760258686.
- Kim TH, Chung KW, Lee YJ, et al. The effect of external beam radiotherapy volume on locoregional control in patients with locoregionally advanced or recurrent nonanaplastic thyroid cancer. Radiat Oncol. 2010;5(1):69. doi: 10.1186/1748-717X-5-69.
- Urbano TG, Clark CH, Hansen VN, et al. Intensity modulated radiotherapy (IMRT) in locally advanced thyroid cancer: acute toxicity results of a phase I study. Radiother Oncol. 2007;85(1):58–63.
- Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61(3):223–231. doi: 10.1016/s0167-8140(01)00429-7.
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470.
- Brose MS, Worden FP, Newbold KL, et al. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. 2017;35(23):2692–2699. doi: 10.1200/JCO.2016.71.6472.
- Aashiq M, Silverman DA, Na’ara S, et al. Radioiodine-Refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel). 2019;11(9):1382. doi: 10.3390/cancers11091382.
- Haugen B, French J, Worden FP, et al. Lenvatinib plus pembrolizumab combination therapy in patients with radioiodine-refractory (RAIR), progressive differentiated thyroid cancer (DTC): results of a multicenter phase II international thyroid oncology group trial. J Clin Oncol. 2020;38(15_suppl):6512–6512. doi: 10.1200/JCO.2020.38.15_suppl.6512.