?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To investigate the long-term efficacy of ultrasound-guided high-intensity focused ultrasound (USgHIFU) for multiple uterine fibroids and the factors associated with recurrence.
Materials and methods
Five hundred and forty-nine patients with multiple uterine fibroids treated with USgHIFU from June 2017 to June 2019 were retrospectively analyzed. The Pictorial Blood Loss Assessment Chart (PBAC) was used to assess menstrual blood loss. The patients were asked to undergo pre- and post-USgHIFU magnetic resonance imaging (MRI) and complete routine follow-up after USgHIFU. Cox regression analysis was used to investigate the risk factors associated with recurrence.
Results
The median number of fibroids per patient was 3 (interquartile range: 3–4), and a total of 1371 fibroids were treated. Among them, 446 patients completed 3 years follow-up. Recurrence, defined as PBAC score above or equal to 100 and/or the residual fibroid volume increased by 10%, was detected in 90 patients within 3 years after USgHIFU, with a cumulative recurrence rate of 20.2% (90/446). The multi-factor Cox analysis showed that age was a protective factor for recurrence. Younger patients have a greater chance of recurrence than older patients. Mixed hyperintensity of fibroids on T2WI and treatment intensity were risk factors for recurrence. Patients with hyperintense uterine fibroids and treated with lower treatment intensity were more likely to experience recurrence than other patients after USgHIFU. No major adverse effects occurred.
Conclusions
USgHIFU can be used to treat multiple uterine fibroids safely and effectively. The age, T2WI signal intensity and treatment intensity are factors related to recurrence.
Introduction
Uterine fibroids are the most common benign tumors of the reproductive system in childbearing age women. The prevalence of uterine fibroids is generally considered to be 20–40%, and half of the patients have multiple uterine fibroids [Citation1–3]. Patients with multiple uterine fibroids are more often symptomatically than patients with solitary fibroid and presented with a chief complaint of increased menstrual blood volume, lumbosacral pain, and even infertility. Treatment options for patients with uterine fibroids include non-surgical and surgical approaches. Patients with uterine fibroids can be treated with different modalities based on their age, desire of fertility, and fibroid characteristics (size, number and locations). Medical treatment is effective in the management of patients with uterine fibroids, but symptoms often return after withdrawal of medication. Uterine artery embolization (UAE) is also used in the treatment of multiple uterine fibroids, but its adverse effects on ovarian function have limited its clinical application. Currently, myomectomy remains the treatment of choice for those patients with uterine fibroids who wish to preserve fertility, but the cumulative recurrence rate for multiple uterine fibroids after myomectomy was still high [Citation4–7]. Therefore, new treatment approaches for effective management of multiple uterine fibroids are needed.
Over the last 10 years, ultrasound-guided high-intensity focused ultrasound (USgHIFU) has been widely used in the treatment of uterine fibroids. This noninvasive therapeutic technique has its significant advantages of a few complications, short recovery time, and no damage to ovarian function [Citation8–11]. Several studies have shown the long-term outcomes of USgHIFU treatment for patients with a solitary uterine fibroid [Citation12, Citation13]. However, we didn’t find any study on the long-term outcomes and the factors affecting long-term recurrence of multiple uterine fibroids treated with USgHIFU. The recurrence rate is a main concern to both physicians and patients. Therefore, the aims of this study were to evaluate the long-term outcomes of patients with multiple uterine fibroids treated with USgHIFU and further analyze the factors influencing recurrence after USgHIFU treatment.
Materials and methods
The protocol for this retrospective study was approved by the ethics committee at our institute (No. KLL-2023-581) and the requirement for informed consent was waived.
Patients
Patients with multiple uterine fibroids, who didn’t have myomectomy previously, underwent USgHIFU treatment in the First Affiliated Hospital of Zunyi Medical College and the First Affiliated Hospital of Southwest Medical University from June 2017 to June 2019 were included in this study.
Inclusion criteria: (1) non-menopausal women who didn’t undergo myomectomy previously, older than 18 years; (2) symptomatic patients with the number of fibroids equal to or greater than 3 based on magnetic resonance imaging (MRI); (3) patients completed USgHIFU ablation.
Exclusion criteria: (1) patients with incomplete information; (2) patients without pre- or post-USgHIFU MRI; (3) patients who lost to follow-up.
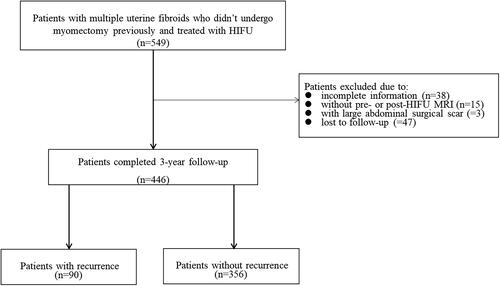
As shown in , we retrospectively reviewed a total of 549 patients with multiple uterine fibroids who didn’t undergo myomectomy and completed USgHIFU. Among them, 38 patients were excluded due to incomplete information, 18 patients were excluded due to no pre- or post-USgHIFU MRI, 47 patients were lost during the follow-up period. Finally, 446 patients were enrolled in the study.
Pre-USgHIFU MRI evaluation
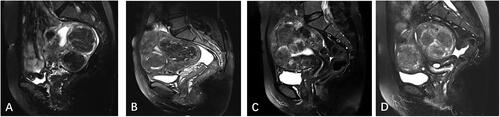
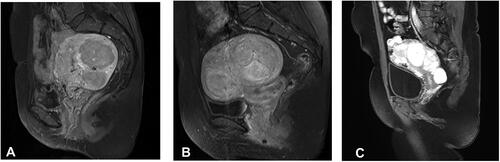
The standard pelvic MRI was performed using a 1.5 T MRI system (Siemens Healthcare, Magnetom Avanto Dot) before USgHIFU treatment. The uterine position, fibroid location, number of fibroids, size of fibroids, abdominal wall thickness, subcutaneous fat thickness in abdominal wall, distance from the superficial surface of the fibroid to the skin, T2WI signal intensity of the fibroids and degree of enhancement of the fibroids were recorded. The signal intensity of the fibroids on T2WI of MRI was defined as follows: hypointensity (the signal intensity of the fibroids was lower than that of the myometrium); isointensity (the signal intensity of the fibroids was equal to that of the myometrium); hyperintensity (the signal intensity of the fibroids was higher than that of the myometrium); mixed intensity (heterogeneous mixed signal within the fibroids) (). The degree of enhancement of the fibroids was defined as follows: mild enhancement (the overall enhancement degree of the fibroids was weaker than that of the myometrium); moderate enhancement (the overall enhancement degree of the fibroids was similar to that of the myometrium); and significant enhancement (the enhancement degree of the fibroids was stronger than that of the myometrium) ().
USgHIFU treatment
All patients were treated using a Focused Ultrasound Tumor Therapeutic System (Model JC200 or JC, Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). The USgHIFU treatment protocol was described in our earlier studies [Citation12, Citation13]. Briefly, the procedure of USgHIFU treatment was performed under conscious sedation and guided by real-time ultrasound. Every patient was first positioned prone on USgHIFU table with the abdominal wall in contact with degassed water in the reservoir under USgHIFU table. Then, a degassed water balloon was made and placed between the transducer and the abdominal wall to compress and push the bowel away from the acoustic pathway. Afterwards, the treatment plan was made using the sagittal ultrasound scanning mode and the targeted fibroids were divided into slices at a distance of 5 mm between the two slices. A point scan energy delivery with the treatment power of 300–400 W was used. During the treatment, the focus was set at least 1.5 cm from the endometrium and 1 cm from the serosa of the uterus. The treatment was terminated when the grayscale in the fibroid significantly increased and the contrast-enhanced ultrasound (CEUS) was then performed to assess the ablated volume of the fibroids. Additional treatment could be performed if the CEUS showed an unsatisfactory non-perfused volume (NPV) ratio. The treatment power, treatment time, and sonication time were recorded, and the treatment dose (sonication time × average treatment power), treatment intensity (the time of sonication of the fibroids per hour, treatment intensity = sonication time/treatment time) and energy efficiency factor (EEF) (the ultrasound energy required to ablate a unit volume of lesion, EEF = η × treatment power × sonication time/non-perfused area volume, where η is the focus factor of 0.7) were calculated. During the procedure, the patients were requested to report any discomfort and respire rate, heart rate, oxygen saturation, and blood pressure were monitored.
Post-USgHIFU MRI evaluation
Post-USgHIFU MRI was performed 1 day after USgHIFU to evaluate the treatment results. The NPV, which was defined as the volume of coagulative necrosis, was measured and calculated. The fibroid volume and NPV were obtained by measuring in three dimensions: longitudinal diameter (a), transverse diameter (b), and antero-posterior diameter (c). Both the NPV and fibroid volume were calculated according to the following equation: V = 0.5233 × a × b × c. The NPV ratio was calculated as follows: NPV ratio = NPV/fibroids volume × 100%. The adverse events were also recorded.
Follow-up
All patients were requested to undergo a follow-up every 3 months after USgHIFU treatment for three years to evaluate symptom improvement and changes of fibroids. The Pictorial Blood Loss Assessment Chart (PBAC) method was used to assess menstrual blood loss. Scores of 1, 5, or 20 were assigned based on the proportion of the blood-stained area on sanitary pads (≤1/3, 1/3 ∼ 3/5, 1). The recurrence was defined as PBAC score returned to above or equal to 100 and/or the residual volume of fibroid increased by 10%. The patients were asked to undergo ultrasound examination at each follow-up point. If recurrence was suspected, the patients were also referred for MRI. If patients had performed other treatments (hysterectomy, myomectomy, UAE, medication, etc.) again for fibroids during follow-up period, we defined them as re-intervention. Patients who experienced recurrence of fibroids were included in the recurrence group, while those who did not experience recurrence of uterine fibroids were included in the non-recurrence group.
Statistical analysis
R (4.2.1) statistical analysis software was used for data analysis. Normally distributed variables were expressed as mean ± standard deviation (±s) and t-test was used to compare the variables between different groups. Skewed variables were expressed as median with interquartile range and non-parametric tests were conducted to compare the variables between different groups. Count data were expressed as frequency and percentage and analyzed with the Chi-square test. The survival package was used to perform univariate and multivariate Cox regression analysis to screen factors related to recurrence. The R (RMS) package was used for prognostic Calibration analysis and visualization. The test level α was set at 0.05, and differences were considered statistically significant when the P value was less than 0.05.
Results
In this study, 446 patients completed 3 years follow-up after USgHIFU treatment. The total number of fibroids was 1517 (range: 3–18 per patient), the median number of uterine fibroids per patient was 3 (interquatile range: 3–4), and a total of 1371 uterine fibroids were treated. During 3-year follow-up, recurrence occurred in 90 of 446 (20.2%) patients, and 356 of 446 (79.8%) patients didn’t experience recurrence. The cumulative recurrence rate at 3 years after USgHIFU was 20.2% (90/446). Among the 90 patients with recurrence, 59 patients received re-intervention treatments included 11.9% (7/59) of patients received medical treatment, 50.8% (30/59) of patients underwent myomectomy, 37.3% (22/59) of patients received hysterectomy, and 31 out of 90 patients chose expectant management. The 3-year overall re-intervention rate was 13.2% (59/446).
Comparison of baseline characteristics of patients between the group with recurrence and the group without recurrence
We further compared the baseline characteristics between patients with recurrence and patients without recurrence. As shown in , the median age of patients in the recurrence group was 42 (38–44) years, while the median age of patients in the non-recurrence group was 44 (42–47) years, the patients in the recurrence group was significantly younger than patients in the non-recurrence group (p < 0.05). The proportion of fibroids with hyperintensity and mixed hyperintensity in the recurrence group was significantly higher than that of patients in the non-recurrence group (p < 0.05). No other significant differences in baseline characteristics were observed ().
Table 1. Comparison of baseline characteristics between the recurrence group and the non-recurrence group.
Comparison of USgHIFU therapeutic parameters between patients with recurrence and patients without recurrence
As shown in , no significant statistical difference was observed between patients with recurrence and patients without recurrence in number of treated fibroids, sonication power, treatment time, sonication time, treatment intensity, and treatment dose.
Table 2. Comparison of treatment results between patients with recurrence and patients without recurrence.
The patients tolerated the treatment well. During the procedure of USgHIFU treatment, the patients in both groups experienced skin burning sensation, transient leg pain, and mild lower abdominal or sacrococcygeal pain. The pain score was ranged from 1 to 4 points. There was no significant difference in the incidence of adverse events during the treatment between patients with recurrence and patients without recurrence. All the treatment related adverse effects disappeared in 3 days after HIFU. No severe adverse effects occurred in any of these patients. No any patient reported sciatic pain lasting longer than 3 and longer than 6 months during the follow-up. We further analyzed the rate of recurrence in the different medical centers, no significant difference was observed in this study ().
Table 3. Comparison of recurrence rate and adverse events between two medical centers.
Analysis of factors influencing recurrence
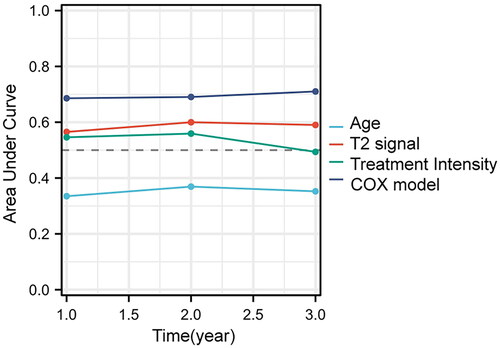
The univariate and multivariate Cox regression analysis was used to screen the recurrence-related factors after USgHIFU treatment for multiple uterine fibroids. The univariate analysis showed that age, fibroid location, signal intensity of uterine fibroids on T2WI and treatment intensity were the main factors influencing the recurrence of fibroids after USgHIFU treatment (p < 0.05). The hazard ratio (HR) for age was 0.931(95% confidence interval 0.899–0.963), which was less than 1, and representing age was a protective factor for recurrence of uterine fibroids after USgHIFU. It indicated that the patients with older age had lower probability of recurrence than that of younger patients. In contrast, retro-position of the uterus, mixed hyperintensity signal on T2WI and treatment intensity were risk factors for recurrence after USgHIFU treatment, with a HR higher than 1. The HR for fibroids in the posterior wall of the uterine was 1.792 (95% confidence interval 1.040–3.087 with reference to fibroids in the anterior wall). The HR for mixed signal intensity of fibroids on T2WI was 1.924 (95% confidence interval 1.157–3.199 with reference to hypointensity fibroids). The HR for treatment intensity was 1.001 (95% confidence interval 1.000–1.002). The fibroids in the posterior wall, with mixed hyperintensity signal, with higher treatment intensity were more likely to relapse after USgHIFU ablation. The multifactorial analysis showed that only age, signal intensity of fibroids on T2WI and treatment intensity were included in the multifactorial model (p < 0.05). The HR for age was 0.927 (95% confidence interval 0.894–0.960). The HR for T2WI mixed hyperintensity signal was 1.916 (95% confidence interval 1.137–3.227 with reference to hypointensity). The HR for treatment intensity was 1.001 (95% confidence interval 1.000–1.001). Therefore, age was a protective factor for post-USgHIFU recurrence (HR < 1), which indicated that the patients with older age had a lower risk of recurrence than that of younger patients after USgHIFU treatment. Mixed hyperintensity of fibroids on T2WI and treatment intensity were risk factors for post-USgHIFU recurrence (HR > 1) (), which indicated that the fibroids with hyperintensity and with high treatment intensity were more likely to relapse. Finally, we evaluated the multi-factor Cox model using Prognostic Calibration analysis, which showed that the model was optimal for predicting the probability of recurrence of multiple fibroids after USgHIFU ().
Figure 4. Prognostic calibration analysis. The blue line represented the model line, and the gray line represented the optimal prognosis model. The more similar the linear fit between the two lines, the better the model fits.
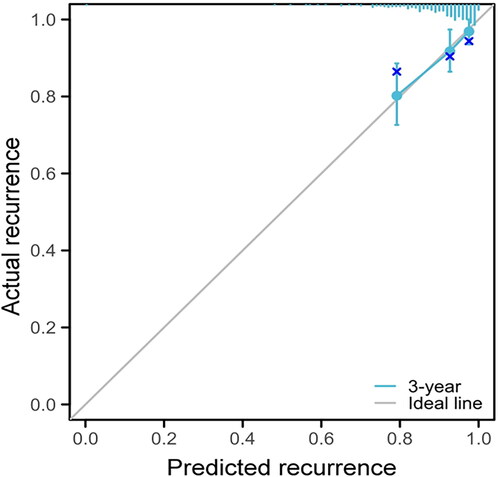
Table 4. Univariate and multivariate Cox analysis of factors that affecting the recurrence of uterine fibroids after USgHIFU treatment.
Establishment of combined scoring system
Based on the results of Cox analysis, we further established a combined scoring system to predict recurrence. It was that each variable was multiplied by the correlation coefficient (B value), and finally added to get the score. The B value of age was 1, the B values of other variables were transformed (divided by the B value of age respectively), and the following equation was finally obtained. Risk score = 39.268 – (age × 1) – (isointensity × 0.776) + (hyperintensity × 3.039) + (mixed hyperintensity × 8.552) + (treatment intensity × 0.008). Among them, isointensity, hyperintensity, and mixed hyperintensity were recorded as 1 (if present) or 0 (if absent).
ROC curve analysis
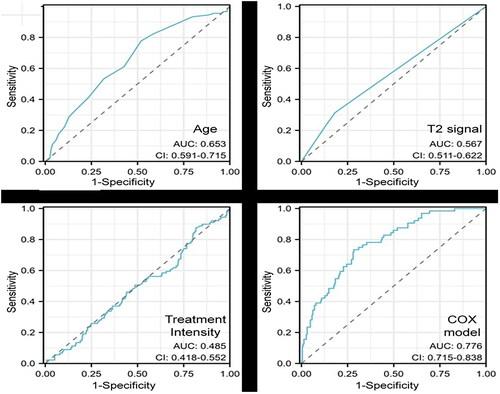
We evaluated the accuracy of each independent influencing factor and the combined Cox scoring model by ROC curve analysis. As shown in and , when the threshold of age was 44.5 years, the sensitivity, specificity, and accuracy of the recurrence prediction were 77.7%, 48.0%, and 54.0%, respectively. When the threshold of T2WI signal intensity was 2.5 (the value of hypointensity of fibroids on T2WI was 1, the value of isointensity of fibroids on T2WI was 2, the value of hyperintensity on T2WI was 3, the value of mixed signal value was 4), the sensitivity of the recurrence prediction was 31.4%, the specificity was 81.8%, and the accuracy was 71.6%, respectively. When the threshold of the treatment intensity was 591.2, the sensitivity, specificity, and accuracy of recurrence prediction were 87.6%, 18.3%, and 32.2%, respectively. When the threshold of Cox combined scoring model was 3.01, the sensitivity, specificity and accuracy of the recurrence prediction were 73.4%, 71.6% and 72.0%, respectively.
Table 5. ROC curve analysis.
In addition, the results of the Area Under Curve (AUC) of the time-dependent ROC curve showed that the AUC of the combined Cox scoring model was significantly greater than that of all independent factors (). The combined Cox scoring model had a better sensitivity, specificity and accuracy than independent factors and can be used as a prediction model for recurrence in patients with multiple uterine fibroids after USgHIFU treatment.
Discussion
Earlier studies reported the re-intervention rates ranged from 0 to 21% at 3 to 33.6 months of follow-up after USgHIFU treatment. The risk of re-intervention was higher in long-term follow-up than that of short-term follow-up in patients with both solitary and multiple fibroids [Citation14–16]. In the present study, the recurrence rate at 3 years after USgHIFU was 20.2% and the post-USgHIFU re-intervention rate was 13.3%, which is similar to the recurrence rate in patients with uterine fibroids reported in earlier studies [Citation15, Citation16]. Previous studies have demonstrated that multiple factors such as age, T2WI signal intensity of fibroids, degree of contrast enhancement of fibroids, fibroids size, and NPV ratio are important factors influencing recurrence and re-intervention after USgHIFU treatment for uterine fibroids [Citation17–19]. In this study, the Cox analysis revealed that age, T2WI signal intensity of fibroids and USgHIFU treatment intensity were the main factors affecting the recurrence of multiple fibroids after USgHIFU treatment. Of these, age was a protective factor, while T2WI hyperintensity or mixed hyperintensity signal, and treatment intensity were risk factors for recurrence of uterine fibroids after USgHIFU ablation. However, we didn’t find the correction between the NPV ratio and post-USgHIFU recurrence in this study. This phenomenon may be explained by the difference in the distribution of NPV ratio between studies. In this study, the median NPV ratio was 76.4% (interquartile range: 59.3%∼90.7%) in the group without recurrence and 75.4% (interquartile range: 61.2%∼90.3%) in the group with recurrence. The median NPV ratio in both groups was higher than 70% and no significant difference in NPV ratio was observed between the two groups. Therefore, even if a high NPV ratio is achieved, other factors may cause recurrence.
The results of this study showed that age was the only protective factor. It indicated that the older patients have lower chance of recurrence than that of younger patients after USgHIFU treatment. This finding can be explained by that uterine fibroids are an estrogen dependent disease, and as increases in women’s age, their estrogen level decreased. In this study, the median age of patients with recurrence was 42 years old, while it was 44 years old in patients without recurrence. Experimental studies have found that estradiol and progesterone can induce mitosis of uterine smooth muscle cells and promote the proliferation of smooth muscle cells [Citation20], while the clinical application of Mifepristone as adjuvant therapy, antagonizing the stimulation of progesterone, can effectively inhibit the growth of fibroids and reduce the recurrence rate of post-USgHIFU fibroids [Citation21, Citation22]. Therefore, we speculated that low-dose mifepristone may be effective in improving the long-term outcomes of USgHIFU treatment in high-risk patients, but this hypothesis needs to be further explored.
This study also found that both mixed hyperintensity signal of fibroids on T2WI and USgHIFU treatment intensity were risk factors for fibroid recurrence after USgHIFU ablation, and there was a significant correlation between mixed hyperintensity signal of fibroids on T2WI and USgHIFU treatment intensity. In the group without recurrence, most of the patients had hypointense fibroids. However, more patients had fibroids with mixed hyperintensity on T2WI in the group with recurrence. This finding indicated a correlation between histological characteristics of uterine fibroids and recurrence after USgHIFU treatment. A previous study demonstrated that histological characteristics such as heterogeneity and vascular distribution of uterine fibroids were key factors affecting the treatment of uterine fibroids with USgHIFU ablation [Citation23]. Uterine fibroids with mixed hyperintensity on T2WI have a high variability of density within the fibroid tumor, so the scattering, reflection and refraction of ultrasound was more significant in this type of fibroids, which reduced the deposition of ultrasound energy at the treated area. In addition, fibroids with mixed hyperintensity on T2WI may have large blood vessels in the internal septum, and the blood flow may also take away some energy, reducing the energy deposition at the treated area, affecting the ablation effect, making ablation more difficult and resulting in a low NPV ratio [Citation24].
By evaluating the relationship between signal intensity on T2WI and the efficacy of USgHIFU ablation of uterine fibroids, Zhao et al. showed that the NPV ratio in hypointense fibroids was the highest in all fibroids, while hyperintense fibroids or mixed hyperintense fibroids were difficult to ablate [Citation25]. Therefore, the lower NPV ratio achieved in fibroids with mixed hyperintensity on T2WI was a main factor inducing fibroid recurrence. Due to the difficulty of ablation of fibroids with mixed hyperintensity on T2WI, in order to achieve better ablation results, we often increased the treatment intensity during the procedure to accumulate more ultrasound energy per hour and causing rapid warming of the target tissue and achieving coagulation necrosis. This phenomenon can be explained by the positive correlation between mixed hyperintensity of fibroids on T2WI and USgHIFU treatment intensity. Similarly, it can be assumed that a higher USgHIFU treatment intensity represents a greater difficulty in ablating the fibroid tissue and correspondingly increases the risk of post-USgHIFU recurrence.
This study is limited because it is a retrospective study and some bias may have occurred in the baseline characteristics and treatment outcomes. In addition, the study subjects were from two centers, and the skill levels of different physicians might be varied, which may also affect the treatment results. Furthermore, the imbalance in the number of patients with/without recurrence between the two groups might also affect the comparison results. Therefore, multicenter prospective studies with large sample size of subjects are necessary in the future to verify the results of this study.
Conclusions
In summary, USgHIFU is safe and effective in the treatment of multiple uterine fibroids. Multiple factors can affect the long-term recurrence after USgHIFU treatment. Age is a protective factor for post-USgHIFU recurrence of fibroids, while fibroids with mixed hyperintensity on T2WI and USgHIFU treatment intensity are risk factors for fibroid recurrence after USgHIFU. However, multicenter prospective studies with large sample size of subjects are necessary in the future to verify the findings of this study.
Disclosure statement
Lian Zhang is a senior consultant to Chongqing Haifu. The other authors have no conflict of interests to declare.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author, LZ. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
Additional information
Funding
References
- Stewart E, Laughlin-Tommaso S, Catherino W, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2(1):1. doi: 10.1038/nrdp.2016.43.
- Evans P, Brunsell S. Uterine fibroid tumors: diagnosis and treatment. Am Fam Phys. 2007;75(10):1503–10.
- Stewart E, Cookson C, Gandolfo R, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512. doi: 10.1111/1471-0528.14640.
- Singh, Shikha, Kumar, Praveen, Rathore, Saurabh Singh, et al. Contemporary approaches in the management of uterine leiomyomas. Eur J Obstet Gynecol Reprod Biol 2023;287:195–210. doi: 10.1016/j.ejogrb.2023.06.021.
- Cappelli A, Mosconi C, Cocozza M, et al. Uterine artery embolization for the treatment of symptomatic uterine fibroids of different sizes: a single center experience. J Pers Med. 2023;13(6):906. doi: 10.3390/jpm13060906.
- Tsakos E, Xydias E, Ziogas A, et al. Multi-port robotic-assisted laparoscopic myomectomy: a systematic review and meta-analysis of comparative clinical and fertility outcomes. J Clin Med. 2023;12(12):4134. doi: 10.3390/jcm12124134.
- Young R, Puma L, Latham M, et al. Radiofrequency ablation for treatment of leiomyomas: review of the manufacturer and user facility device experience (MAUDE) database. Obstet Gynecol. 2023;142(1):147–150. doi: 10.1097/AOG.0000000000005213.
- Qin S, Lin Z, Liu N, et al. Prediction of postoperative reintervention risk for uterine fibroids using clinical-imaging features and T2WI radiomics before high-intensity focused ultrasound ablation. Int. J. Hypertherm. 2023;40(1):2226847. doi: 10.1080/02656736.2023.2226847.
- Kociuba J, Łoziński T, Zgliczyńska M, et al. Occurrence of adverse events after magnetic resonance-guided high-intensity focused ultrasound (MR-USGHIFU) therapy in symptomatic uterine fibroids-a retrospective case-control study. Int. J. Hypertherm. 2023;40(1):2219436. doi: 10.1080/02656736.2023.2219436.
- Xu J, Tang W, Lin L, et al. Post treatment and 3 month contrast enhanced MRI findings following USGHIFU of submucosal fibroids: a retrospective study. Int. J. Hypertherm. 2023;40(1):2216897. doi: 10.1080/02656736.2023.2216897.
- Yuan Y, Xu W, Shen H, et al. Long-term outcomes of ultrasound guided high intensity focused ultrasound ablation for patients with uterine fibroids classified by T2WI: a multicenter retrospective study. Int. J. Hypertherm. 2023;40(1):2212887. doi: 10.1080/02656736.2023.2212887.
- Wang Y, Gong C, He M, et al. Therapeutic dose and long-term efficacy of high-intensity focused ultrasound ablation for different types of uterine fibroids based on signal intensity on T2-weighted MR images. Int. J. Hypertherm. 2023;40(1):2194594. doi: 10.1080/02656736.2023.2194594.
- Liu Y, Wu X, Wu A, et al. Ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids: long-term outcomes and factors affecting local recurrence. 2021;38(1):1341–1348.
- Verpalen I, Anneveldt K, Nijholt I, et al. Magnetic resonance-high intensity focused ultrasound (MR-USGHIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: A systematic review and meta-analysis. Eur J Radiol. 2019;120:108700. doi: 10.1016/j.ejrad.2019.108700.
- Liu L, Wang T, Lei B. High-intensity focused ultrasound (USGHIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis. Eur Radiol. 2022;32(2):1195–1204. doi: 10.1007/s00330-021-08156-6.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;127(11):1422–1428. doi: 10.1111/1471-0528.16262.
- Liu Y, Wu X, Wu A, et al. Ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids: long-term outcomes and factors affecting local recurrence. Int. J. Hypertherm. 2021;38(1):1341–1348. doi: 10.1080/02656736.2021.1973585.
- Li W, Jiang Z, Deng X, et al. Long-term follow-up outcome and reintervention analysis of ultrasound-guided high intensity focused ultrasound treatment for uterine fibroids. Int. J. Hypertherm. 2020;37(1):1046–1051. doi: 10.1080/02656736.2020.1807617.
- Li W, Yang Z, Gao B, et al. Comparison of ultrasound-guided high-intensity focused ultrasound ablation and hysteroscopic myomectomy for submucosal fibroids: a retrospective study. Int. J. Hypertherm. 2021;38(1):1609–1616. doi: 10.1080/02656736.2021.1995053.
- Wang H, Yue Y, Zhou X, et al. Expression of vascular endothelial growth factor-receptor 1 and progesteronez receptor in patient with uterine leiomyoma (In Chinese). Chin. J. Lab. Diagnos. 2005;9(6):906–909.
- Wei A, Ren M, Chen X, et al. Effects of mifepristone on expression of estrogen, progesterone receptor and epidermal growth factor receptor in cultured uterine fibroid cells (In Chinese). J. Southwest Univ. 2004;23(6):370–372.
- Pan X. Clinical research on prevention of mifepristone to recurrence of uterine fibroids after myomectomy (In Chinese). China Modern Med. 2010;17(10):13–14.
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196(2):184.e181-186–184.e6. doi: 10.1016/j.ajog.2006.08.030.
- Zhao W, Chen J, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82(1):e43-49–e49. doi: 10.1016/j.ejrad.2012.08.020.
- Zhao W, Zhang J, Han Z, et al. A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep. 2017;7(1):10812. doi: 10.1038/s41598-017-11486-5.