Abstract
Purpose
To investigate the feasibility, safety and efficacy of high intensity focused ultrasound ablation (HIFU) as a preoperative treatment for challenging hysteroscopic myomectomies.
Materials and methods
A total of 75 patients diagnosed with types 0–III of uterine fibroids were enrolled. Based on the Size, Topography, Extension of the base, Penetration and lateral Wall position (STEPW) classification scoring system, 25 cases with a score ≥ 5 points were treated with HIFU followed by hysteroscopic myomectomy (HIFU + HM group), whereas 50 cases with a score < 5 points were treated with hysteroscopic myomectomy (HM group).
Results
The median preoperative STEPW score was 7 in the HIFU + HM group and 2 in the HM group. The average non-perfused volume (NPV) ratio achieved in fibroids after HIFU was 86.87%. Patients in the HIFU + HM group underwent hysteroscopic myomectomy one to four days after HIFU, and downgrading was observed in 81.81% of fibroids. The operation time for patients in the HIFU + HM group was 73 min and the success rate of myomectomy in a single attempt was 60%. The volume of distention medium used during the operation was greater in the HIFU + HM group than in the HM group (15,500 ml vs. 7500 ml). No significant difference was observed between the two groups in terms of intraoperative blood loss, the incidence of intraoperative and postoperative complications, menstrual volume score, or uterine fibroid quality of life score.
Conclusion
HIFU can be utilized as a preoperative treatment for large submucosal fibroids prior to hysteroscopic myomectomy. HIFU offers a novel approach in the management of this subset of patients.
Introduction
Uterine fibroids, also known as uterine leiomyomas or myomas, are the most common benign tumors in women of childbearing age [Citation1,Citation2]. Although the prevalence of uterine leiomyomas varies among races and different age groups, it is generally reported that the incidence rate is 20–40% in women of reproductive age [Citation3]. The symptoms associated with uterine leiomyomas depend on the location and size of the leiomyomas. Uterine fibroids may lead to heavy menstrual bleeding, prolonged menstrual periods, anemia, infertility, miscarriage and premature delivery. The International Federation of Obstetrics and Gynecology (FIGO) categorized uterine fibroids into nine types based on their relationship with the myometrium. Among them, type 0–II are submucosal fibroids and type III fibroids are intermural fibroids [Citation4]. Different treatment methods are employed based on the location of the uterine fibroids [Citation5–8]. Hysteroscopic myomectomy (HM) is the most effective conservative treatment for submucosal fibroids [Citation9]. The size and location of the fibroids influence the surgical difficulty and require evaluation before the procedure. The Size, Topography, Extension of the base, Penetration and lateral Wall position (STEPW) classification scoring system is utilized to evaluate the surgical complexity, myoma resection rate, operation duration and intravasation syndrome during hysteroscopic myomectomy (HM) [Citation10]. A score of 0–4 indicates a low-difficulty level for hysteroscopic surgery, which can be safely performed. A score of 5–6 indicates a relatively challenging hysteroscopic surgery, requiring the application of gonadotropin-releasing hormone (GnRH) as a pre-surgical treatment. A potential secondary surgery may also be necessary. A score higher than 7 indicates an extremely challenging hysteroscopic myomectomy, where hysteroscopy is not recommended. Currently, there is no consensus on whether GnRH facilitates the complete resection of submucosal fibroids, reduces operation time and fluid absorption, or prevents major complications [Citation10].
As a noninvasive treatment, high intensity focused ultrasound (HIFU) ablation has been widely used in the treatment of uterine leiomyomas. Many studies have shown that HIFU is a safe and effective treatment for uterine fibroids. It selectively ablates the fibroids without damaging the surrounding structures, thereby reducing the waiting time for pregnancy after surgery [Citation11]. Several studies have shown that HIFU treatment can downgrade submucosal fibroids from type II to type I or type 0 in patients who are unsuitable for hysteroscopic treatment [Citation12–14]. Previous research conducted by our team demonstrated that HIFU pretreatment can induce coagulation and necrosis in certain types II and III fibroids, resulting in rapid downgrading [Citation15]. HIFU treatment offers an opportunity for patients with types II and III fibroids who do not meet the criteria for hysteroscopic myomectomy to undergo this procedure.
Building on the original study, this research expanded the sample size, incorporated a control group and further investigated the safety and efficacy of HIFU as a preoperative pretreatment for challenging and extremely challenging hysteroscopic myomectomies in patients with uterine fibroids, as assessed using the STEPW classification.
Materials and methods
The research protocol of this retrospective study was approved by the Ethics Committee of Suining Municipal Hospital of Traditional Chinese Medicine (No.:SNTCM-2021072) and the requirement for informed consent was waived.
Patients
Between January 2020 and September 2022, a total of 75 patients with uterine fibroids diagnosed as type 0–III based on pelvic magnetic resonance imaging (MRI) (), color Doppler ultrasound and hysteroscopy () were treated at Suining Hospital of Traditional Chinese Medicine. The patients who scored ≥ 5 points using the STEPW classification ( and ) were treated with HIFU followed by HM, while those with a score < 5 points were treated with HM alone. Clinical data, including age, body mass index (BMI), liver and kidney function, intrauterine adhesions and medical history were collected. The STEPW classification scoring system is currently applicable for assessing submucosal fibroids for HM. Due to the absence of a hysteroscopic scoring system for type III fibroids, we also utilized the STEPW classification for type III fibroids.
Figure 1. Pelvic magnetic resonance image of a type II–Ⅵ uterine fibroids. (A) Sagittal view of the T2-weighted image reveals the fibroid located in the posterior wall and fundus of the uterus, extending more than 50% into the muscular layer. (B) Transverse images demonstrate the fibroid on the posterior wall protruding into the uterine cavity, with an anteroposterior diameter of 8 cm.
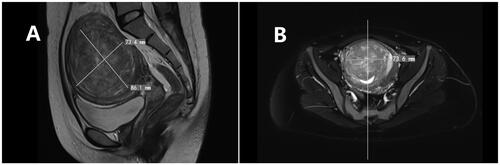
Figure 2. Hysteroscopic image of a single type II–Ⅵ fibroid before and after HIFU. (A) Preoperative hysteroscopy shows that 20% of the fibroid protrudes into the uterine cavity. (B) On the second day after HIFU, hysteroscopy reveals that 60% of the fibroid now protrudes into the uterine cavity.
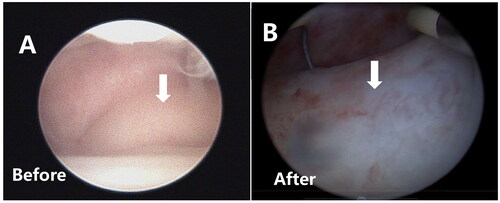
Table 1. STEPW classification scoring system.
Table 2. Score and treatment recommendations according to STEPW classification scoring system.
Inclusion criteria for the HM group: (1) Type 0–II uterine fibroids; (2) STEPW score < 5 points; (3) Presence of symptoms associated with uterine fibroids, such as prolonged menstruation, increased menstrual flow and anemia.
Exclusion criteria: (1) Type 0–II uterine fibroids with STEPW score ≥ 5 points; (2) Contraindications for HM, such as inability to fully dilate the cervix and acute reproductive tract infection; (3) Severe medical or surgical conditions that prevent surgery; (4) Suspected or confirmed malignant tumors.
Inclusion criteria for the HIFU + HM group: (1) Type 0–II uterine fibroids with a STEPW score of ≥ 5 points; (2) Symptomatic type III fibroids; (3) Presence of symptoms related to uterine fibroids, such as increased menstrual flow, prolonged menstrual periods and anemia.
Exclusion criteria: (1) STEPW score < 5 points, asymptomatic III-Ⅷ fibroids; (2) Contraindications for MRI, HIFU, or hysteroscopy, such as claustrophobia, inability to establish a safe acoustic channel, insufficient cervical dilation and acute stage of genital tract infection; (3) Severe medical or surgical conditions that prevent surgery; (4) Adenomyoma; (5) Suspected or confirmed malignant tumors.
HIFU treatment
The procedure of HIFU treatment was described previously [Citation16]. Briefly, the HIFU treatment procedure was conducted using a Focused Ultrasound Tumor Therapeutic System (Model JC200; Chongqing Haifu Medical Technology Co. Ltd., China) with an ultrasound imaging device for treatment guidance. Before the treatment, the patients were asked to complete a specific bowel preparation prior to HIFU treatment. Patients were instructed to consume semi-liquid or liquid food two days before the HIFU treatment and then to have an enema on the morning of the HIFU treatment day following a 12-h fasting. Routine skin preparation was mandatory and every patient was requested to shave their abdominal wall from the lower edge of the umbilicus to the upper margin of the pubic symphysis and then degrease and degas the skin with 70% of ethanol and degassed water. Before the HIFU treatment, a urinary catheter was inserted to adjust the bladder volume by infusing normal saline during the procedure of HIFU treatment procedure to ensure a safe acoustic pathway.
Each of the patients was positioned prone on the HIFU table, with their anterior abdominal wall in contact with degassed water. The treatment was performed under sedation and analgesia. The respiratory rate, heart rate, blood pressure and oxygen saturation were monitored during the procedure. Sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. The locations of the fibroids and surrounding tissues were identified on ultrasound imaging, and the targeted fibroids were divided into several sections by real-time ultrasound, each approximately 5 mm wide. A point scan was used, and the power was set between 300 and 400 watts. The distance from the focal point to the endometrium is at least 1.5 cm. The treatment was terminated when the fibroid exhibited an increased grayscale change or if there was an absence of blood supply, as assessed by contrast-enhanced ultrasound immediately after HIFU ablation. The ablation range was evaluated, and the size of the immediate non-perfusion area was measured: ‘L’ stands for upper-lower diameter; ‘W’ stands for left-right diameter; ‘D’ stands for front-back diameter. Pre- and postoperative contrast sonograms were compared (). If the ablation was unsatisfactory, additional treatment was safely administered. Throughout the procedure, vital signs such as respiration, oxygen saturation, heart rate and blood pressure were closely monitored. Fibroid volume was calculated using the formula V = 0.5223 × L × W × D (cm3); non-perfusion area volume (NPV) was calculated as V = L × W×D × 0.5233; non-perfusion area volume ratio (NPVR) was calculated as NPV/V lesion immediately after treatment × 100%.
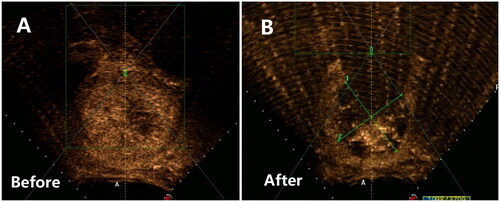
Figure 3. Contrast-enhanced ultrasound image obtained from a patient with a solitary type II–Ⅵ fibroid. (A) Pre-HIFU contrast-enhanced ultrasound showed significant perfusion of the fibroid. (B) Post-HIFU contrast-enhanced ultrasound showed no perfusion of the fibroids. The fibroid was completely ablated.
HM surgery
Patients in the HM group underwent a routine HM. In the HIFU + HM group, HM was performed within one to four days after the HIFU treatment. Before the procedure of HM surgery, a single-use cervical dilation mold was placed in the cervical canal. The treatment of HM was performed under general anesthesia, and the patient was placed in the lithotomy position. After routine disinfection and applying surgical drapes, the cervical dilation mold was removed, and the cervix was dilated to a size of 9.5–10 mm. A hysteroscopic resection device was then inserted using a Storz monopolar high-frequency electrode. Patients without a history of diabetes received a 5% glucose solution as the distending medium, while patients with diabetes received 20% mannitol as the distending medium, both under color Doppler ultrasound monitoring. Intrauterine pressure was maintained at 80–100 mmHg (1 mmHg = 0.133 kPa) or below the patient’s mean arterial pressure. The perfusate flow rate ranged from 260 ml/min to 400 ml/min. The cutting power was approximately 80 W, and the electrocoagulation power was approximately 60 W.
For type 0 submucosal fibroids, the pedicle of the fibroids was identified using a hysteroscope, and the fibroids were removed with forceps. If the fibroids were very large and could not be removed directly, they were cut into slices, and then the pedicle was subsequently removed.
For type I–II submucosal fibroids, electrodes were alternately used to create a groove-like structure on the lateral surface of the fibroids, and the tumor was removed using oval grasping forceps. If the fibroids did not protrude prominently into the uterine cavity, oxytocin, a pituitary hormone, or a resectoscope was used to induce uterine contractions and facilitate the protrusion of fibroids into the uterine cavity before the operation.
Continuous electrocardiography (ECG) monitoring was conducted throughout the procedure to track vital signs. Dynamic blood gas analysis was performed every 30 min to monitor the electrolyte levels and fingertip blood glucose levels. The intake and output of the distending medium were recorded. In case of electrolyte imbalance or water intoxication, the operation was immediately halted.
Adverse reaction evaluation and postoperative follow-up
The incidence of short-term adverse events during HIFU was evaluated according to the complication grading system of the Society of Interventional Radiology(SIR) [Citation17]. The Clavien-Dindo classification of postoperative complications for hysteroscopic surgery was utilized to assess the severity of postoperative complications by seven grades [Citation18]. Follow-up examinations were conducted in 1–12 months after surgery, including uterine accessory color ultrasound, pelvic magnetic resonance imaging, complete blood count (CBC), UFS-QOL questionnaires, hysteroscopy and whether there were long-term complications.
Evaluation of efficacy
The STEPW score evaluation was conducted under a microscope to observe the shape of the uterine cavity, determine the location, size and number of fibroids, assess the thickness of the fibroid base, evaluate the proportion of fibroids to the uterine cavity, and assess the extent of fibroids protruding into the uterine cavity. At the same time, the size, location and depth of fibroids expanding into the myometrium were reevaluated using ultrasound.
Excision rate of uterine fibroids: The intrauterine myomectomy rate was assessed immediately using color Doppler ultrasonography combined with hysteroscopy.
Improvement of symptoms: According to the scores of UFS-QOL questionnaires filled in by patients, the total scores were converted into a symptom severity score (SSS) and a quality of life score (HRQL) ranging from 0 to 100 points, respectively [Citation19]. The higher the SSS score is, the more severe the symptoms are, and the higher the HRQL score is, the better the quality of life is. Menstrual volume was assessed using the Pictorial Blood Assessment Chart (PBAC) scale [Citation20].
Statistical analysis
Statistical analyses were conducted using SPSS version 23.0. Normally distributed measurement data were expressed as mean ± standard deviation and analyzed using the t-test. Non-normally distributed data are represented as median (interquartile range P25, P75) and were analyzed using the rank sum test. Categorical data were presented as percentages (%) and compared using the chi-square test. Statistical significance was set at p < .05.
Results
Comparison of baseline characteristics between patients treated with HIFU followed by HM and patients treated with HM alone
As shown in , there were no significant differences in age, BMI, preoperative intrauterine adhesions, or underlying diseases between the two groups (p > .05) (). The lesion volume in the HIFU + HM group was 77.14 cm3 (interquartile range: 36.11, 134.55), and it was 4.56 cm3 (interquartile range: 1.03, 10.06) in the HM group. The lesion volume in the HIFU + HM group was significantly larger than that in the HM group (p < .01). The preoperative STEPW score in the HIFU + HM group was significantly higher than that in the HM group (7 vs. 2) (p < .01) ().
Table 3. Comparison of baseline characteristics between the two groups of patients.
Table 4. Comparison of intraoperative conditions between HIFU + HM group and HM group.
Therapeutic results in patients treated with HIFU
In the HIFU + HM group, 25 patients (33 fibroids) underwent 27 sessions of HIFU treatments. Among them, two patients with multiple uterine fibroids received two sessions of HIFU treatments. The median irradiation time for HIFU was 800 s (interquartile range: 520–1030), and the median treatment time was 107 min (interquartile range: 70–129). The median treatment energy was 320,400 J (interquartile range: 191,250–480,000), and the median treatment intensity was 500 s/h (interquartile range: 401–625). Contrast-enhanced ultrasonography performed immediately after HIFU showed an average ablation rate of 86.87% (interquartile range: 80–90.91%) for the fibroids. No severe complications occurred during or after the procedures. At one to four days after HIFU, the patients underwent a hysteroscopic examination and HM treatment under ultrasound guidance. Among them, the type II fibroids of 12 patients were downgraded to type I, the type III fibroids of 10 patients were downgraded to type II, the type I fibroids of 3 patients were downgraded to type 0, and the type III–V fibroids of 2 patients were downgraded to type II–V. Moreover, it was a significant challenge to assess the downgrading of large type II–V fibroids in six patients, which ranged from 108.85 cm3 to 377.61 cm3 and occupied the uterine cavity. However, the STEPW score decreased after the surgery, and it was completely resected after one or two hysteroscopic procedures. Statistical analysis revealed a downgrading rate of 81.81% at one to four days after HIFU. The pre-HIFU STEPW score for fibroids was 7 (interquartile range: 5.5, 8.5), which was significantly higher than the post-HIFU score of 6 (interquartile range: 5, 7) with a statistically significant difference (p < .01) (). The depth of myometrial extension and the proportion of fibroid base in the uterine cavity were the main indexes for the decrease in fibroid STEPW score after HIFU.
Table 5. Comparison of pre- and post-HIFU STEPW scores in the HIFU + HM group
Comparison of surgical outcomes between patients treated with HM and HIFU followed by HM
The median procedure time of HM in HIFU + HM group was significantly longer than that of the HM group (73 min (interquartile range: 56, 104.5) vs. 35 min [interquartile range: 27.5, 49.5]) (p < .01). The patients in the HIFU + HM group had a significantly higher uterine distention medium volume of 15,500 ml (interquartile range: 10,000, 21,500) compared to that of the HM group of 7500 ml (interquartile range: 5000, 10,000), with a statistically significant difference (p < .01). Intraoperative blood loss was 50 ml (interquartile range: 20–100) in the HIFU + HM group and 35 ml (interquartile range: 20–55) in the HM group, with no statistically significant difference (p > .05) ().
In the HM group, two patients experienced transurethral resection of the prostate (TURP) syndrome, leading to the immediate termination of the surgery, which resulted in the incomplete removal of the fibroids. In the HIFU + HM group, 22 out of the 33 fibroids were completely removed during a single operation. Among them, complete resection was achieved in one session in 15 patients with a solitary fibroid. However, four patients with multiple fibroids and a total fibroid volume exceeding 100 cm3 required a second operation for complete removal. Two patients in the group treated with HIFU followed by HM experienced hyponatremia, and one patient developed TURP syndrome, which led to the immediate termination of the surgery, leaving approximately 40–50% residual fibroid tissue. Additionally, three cases were unable to complete the surgery due to excessively large fibroid volumes (70.61 cm3, 189.49 cm3, 377.61 cm3), prolonged surgical time and the use of over 20,000 ml of uterine distension fluid. These patients opted for a second operation for complete removal at a later date. The results indicated a significant difference in the rate of complete removal in a single operation between the two treatment methods (96 vs. 60, p = .000) ().
Table 6. Comparison of myomectomy rate between the two treatment methods n (%).
PBAC scoring changes
The postoperative PBAC scores were significantly lower than the preoperative PBAC scores in both groups (p < .01), indicating that both treatment methods significantly improved menstrual volume in both patient groups. We also compared the preoperative PBAC scores between the two groups. The HIFU + HM group had a higher score than the HM group (p = .001). However, no statistically significant difference was observed in the postoperative PBAC score between the two groups (p = .431). This suggests that patients in the HIFU + HM group had a higher preoperative menstrual volume and more severe symptoms such as anemia. After surgery, no statistically significant difference in menstrual condition occurred between the two groups ( and ).
Table 7. PBAC score before and after HM treatment or after HIFU + HM treatment.
Table 8. PBAC score before and after the two treatment methods, health-related quality of life questionnaire for uterine fibroids (UFS-QOL form).
Quality of life
We compared the preoperative and postoperative UFS and QOL scores of patients in each group. A significant difference was observed (p < .01), indicating that both treatment methods improved SSS and HRQL scores. In terms of comparing the preoperative SSS and HRQL scores between the two groups, there was no statistically significant difference (p > .05). After applying various treatment methods, there were no statistically significant differences in the postoperative SSS and HRQL scores between the two groups (p > .05) ().
Complications
During the HIFU procedure, the patients experienced varying degrees of discomfort, including lower abdominal and lumbosacral pain and a burning sensation of the skin. The pain scores (visual analog scale) ranged from 1 to 3. These adverse reactions were evaluated as SIR A-B, and the symptoms significantly improved when treatment was discontinued. The short-term and long-term complications of HM were evaluated using the Clavien-Dindo classification system. No significant differences were observed in the incidence of complications (TURP, intraoperative electrolyte disturbance, uterine perforation, postoperative infection, and postoperative intrauterine adhesions) between the two groups (p = .706) ().
Table 9. Statistics of complications of treatment methods in the two groups n (%).
Discussion
With the development of hysteroscopic technology and improvements in instruments, HM has become the most effective conservative and minimally invasive treatment for submucosal fibroids [Citation21]. Additionally, it could significantly shorten the waiting time for pregnancy after HM for patients who want to have children and greatly reduce the incidence of adverse events during pregnancy and postpartum periods [Citation22]. The guidelines of the International Society for Gynecologic Endoscopy (ISGE) on HM recommended pretreatment before HM surgery for submucosal fibroids with a score ≥ 5 points in the STEPW classification [Citation10,Citation23]. For type II and type III fibroids larger than 60 mm in diameter, the risk of secondary anemia significantly increases [Citation24]. As the size of fibroids that require clinical intervention is large, the risks and complications of hysteroscopic surgery are higher for treating large fibroids than that of smaller fibroids. Moreover, if complications occur, they are often severe and may endanger the patient’s life. Therefore, the implementation of HM requires strict adherence to indications and contraindications.
In this study, 33 fibroids in 25 patients with a score of ≥ 5 points underwent hysteroscopy + HM under ultrasound guidance at one to four days after HIFU; 81.81% of the fibroids were downgraded after HIFU. A previous study demonstrated that HIFU treatment of FIGO type II–III uterine fibroids can rapidly downgrade their classification. In the study, seven type II fibroids downgraded to type I within one to four days after HIFU and five type III fibroids downgraded to type II fibroids in a short period of time, so the fibroid tumors can be removed through hysteroscopic surgery at one time or in stages [Citation24]. Our results are consistent with the previous study. Recently, Liao et al. conducted HIFU treatment on 31 cases of type II submucosal fibroids (with a diameter greater than 4 cm). It was shown that five fibroids were downgraded to type 0 and 10 fibroids were downgraded to type I three months after HIFU treatment, and the downgrading rate of fibroids was 48.4% [Citation14]. Overall, the downgrading rate of fibroids was lower than that being observed in this study. Further statistical analysis in this study showed that the STEPW score of 7 (interquartile range: 5.5, 8.5) for fibroids before HIFU was higher than the postoperative score of 6 (interquartile range: 5, 7), and this difference was statistically significant (p < .01). It further proves that HIFU, as a pretreatment method for difficult HM, can downgraded fibroids in a short time, making hysteroscopic surgery easier. This phenomenon may be explained by the fact that HIFU waves promote strong contraction of the myometrium via a mechanical effect. Furthermore, when fibroids are treated, the uterus is in a state of heat accumulation, which is also a factor that promotes strong contraction of smooth muscle cells. The uterine cavity is a natural passage in the human body with very receptive and extensible endometrium. Fibroids close to the endometrium are pushed toward the uterine cavity, thus being downgraded. However, it needs to be further explored when this clinical phenomenon is most evident after HIFU, which would be the optimal surgical timing for HM. This study confirmed that type II–III uterine fibroids downgraded in one to four days after HIFU. It was indicated that the location of fibroids is not fixed and can change under certain conditions, which needs to be clarified in future studies.
In this study, we found that the lesion volume in the HIFU + HM group-was significantly larger than that in the HM group, and the preoperative STEPW score of fibroids in the HIFU + HM group was also higher than that of the HM group. In addition, the procedure time of HM was significantly longer in the HIFU + HM group than in the HM alone group, and the amount of distension medium used in the HIFU + HM group was significantly higher than that in the HM group. However, there was no statistically significant difference in the intraoperative blood loss and the incidence of intraoperative and postoperative complications between the two groups of patients. It has been demonstrated that pretreatment with HIFU reduces the risk of intraoperative bleeding during HM and maintains a clear intraoperative field of view, thereby making hysteroscopic surgery somewhat easier. Compared with the HM alone group, the incidence of intraoperative complications in the HIFU + HM group is not increased. In this study, 60% of the fibroids (15/25) were completely resected in one session in the HIFU + HM group, which was statistically lower than that of the HM group due to the large size, but it was similar to that of 78.3% (18/23) in the study by Zhang et al. [Citation25]. Therefore, the completely resection rate may be increased when the treated fibroids shrank. This study also demonstrated that the symptoms associated with uterine fibroids (evaluated by UFS-QOL questionnaires and menstrual PBAC scores) were alleviated and the quality of patients’ life of both groups was significantly improved in the 1- to 12-month follow-up after surgery. Moreover, as HIFU can rapidly downgrade fibroids, patients do not need to wait for a long time for HM surgery, which reduces clinical symptoms such as bleeding, discharging, abdominal pains and tumor expulsion during the process of downgrading and mitigates the risk of endometrial infection and intrauterine adhesions during the process.
This study is limited because it was a retrospective study, and the patients were not randomly enrolled, which may lead to bias in patient selection and potentially affect the results. Other limitations arose from the small number of cases included, making it impossible to perform a multifactorial statistical analysis, as well as the statistical methods. In addition, the follow-up period after surgery was relatively short. In this way, we did have long-term endometrial recovery, particularly pregnancy rates and outcomes. Therefore, we plan to expand the sample size and conduct further statistical analyses and follow up for a longer period in subsequent studies.
Conclusions
This study suggested that HIFU can be used as a preoperative pretreatment for hysteroscopy on uterine fibroids (FIGO types 0 to III) assessed as difficult and extremely difficult by HM based on the STEPW classification scoring system. This modality could make HM easier, so that fibroids can be completely removed through hysteroscopic surgery without increasing the risk of complications. This is a safe and feasible treatment method, which provides a new option in the management of patients with submucosal fibroids assessed as challenging and extremely challenging by HM based on the STEPW classification scoring system.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author, TTL. The data are not publicly available because it contains information that can compromise the privacy of the research participants.
Additional information
Funding
References
- Schiffman M, Lamparello N. Uterine-artery embolization or myomectomy for uterine fibroids. N Engl J Med. 2020;383(22):1–9.
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512. doi: 10.1111/1471-0528.14640.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99.
- Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
- Puri K, Famuyide AO, Erwin PJ, et al. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am J Obstet Gynecol. 2014;210(1):38.e1–38.e7. doi: 10.1016/j.ajog.2013.09.038.
- Chabbert-Buffet N, Esber N, Bouchard P. Fibroid growth and medical options for treatment. Fertil Steril. 2014;102(3):630–639. doi: 10.1016/j.fertnstert.2014.07.1238.
- Radhika BH, Naik K, Shreelatha S, et al. Case series: pregnancy outcome in patients with uterine fibroids. J Clin Diagn Res. 2015;9(10):QR01–QR04. doi: 10.7860/JCDR/2015/14375.6621.
- Lu N, Wang Y, Su YC, et al. Effects of the distance between small intramural uterine fibroids and the endometrium on the pregnancy outcomes of in vitro fertilization-embryo transfer. Gynecol Obstet Invest. 2015;79(1):62–68. doi: 10.1159/000363236.
- Xu F, Deng L, Zhang L, et al. The comparison of myomectomy, UAE and MRgFUS in the treatment of uterine fibroids: a meta analysis. Int J Hyperthermia. 2021;38(2):24–29. doi: 10.1080/02656736.2021.1933216.
- Loddo A, Djokovic D, Drizi A, et al. Hysteroscopic myomectomy: the guidelines of the International Society for Gynecologic Endoscopy (ISGE). Eur J Obstet Gynecol Reprod Biol. 2022;268:121–128.
- Zheng AQ, Chen JY, Xiao ZB, et al. Sacral injury and influencing factors after ultrasonic ablation of uterine fibroids ≤30 mm from the sacrum. Diagn Interv Radiol. 2023;29(1):195–201. doi: 10.5152/dir.2022.21407.
- Xie B, Zhang C, Xiong C, et al. High intensity high intensity focused ultrasoundablation for submucosal fibroids: a comparison between type I and type II. Int J Hyperthermia. 2015;31(6):593–599. doi: 10.3109/02656736.2015.1046406.
- Wang W, Wang Y, Wang T, et al. Safety and efficacy of US-guided high-intensity high intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol. 2012;22(11):2553–2558. doi: 10.1007/s00330-012-2517-z.
- Liao P, Jiang J, Zeng YH, et al. Comparison of outcomes of hysteroscopic myomectomy of type 2 submucous fibroids greater than 4 cm in diameter via pretreatment with HIFU or GnRH-a. Int J Hyperthermia. 2021;38(1):183–188. doi: 10.1080/02656736.2021.1874546.
- Tingting L, Yuchun H, Jia H, et al. Preliminary clinical study on rapid reduction of type II.∼III. (FIGO) uterine fibroids by focused ultrasound ablation therapy. Cancer Prev Treat. 2021;34(8):743–751.
- Lei T, Guo X, Gong C, et al. High-intensity focused ultrasound ablation in the treatment of recurrent ovary cancer and metastatic pelvic tumors: a feasibility study. Int J Hyperthermia. 2021;38(1):282–287. doi: 10.1080/02656736.2021.1889698.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e.
- Clavien PA, Barkun J, Oliveira MLD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2.
- Spies J. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300. doi: 10.1016/S0029-7844(01)01702-1.
- Gudmundsdottir BR, Hjaltalin EF, Bragadottir G, et al. Quantification of menstrual flow by weighing protective pads in women with normal, decreased or increased menstruation. Acta Obstet Gynecol Scand. 2009;88(3):275–279. doi: 10.1080/00016340802673162.
- Piecak K, Milart P. Hysteroscopic myomectomy. Prz Menopauzalny. 2017;16(4):126–128. doi: 10.5114/pm.2017.72757.
- Mazzon I, Favilli A, Grasso M, et al. Is cold loop hysteroscopic myomectomy a safe and effective technique for the treatment of submucous myomas with intramural development? A series of 1434 surgical procedures. J Minim Invasive Gynecol. 2015;22(5):792–798. doi: 10.1016/j.jmig.2015.03.004.
- Laganà AS, Alonso Pacheco L, Tinelli A, et al. Management of asymptomatic submucous myomas in women of reproductive age: a consensus statement from the global congress on hysteroscopy scientific committee. J Minim Invasive Gynecol. 2019;26(3):381–383. doi: 10.1016/j.jmig.2018.06.020.
- Ricci G, Scrimin F, Sartore A, et al. Characteristics of submucous myomas and the risk of anemia. Medicina. 2022;58(11). doi: 10.3390/medicina58111652.
- Shengpeng Z, Yuting S, Luping Z, et al. Clinical efficacy analysis of hysteroscopic type II submucosal large fibroid resection. Chin Med J. 2022;57(12):3.
