Abstract
Objective
To analyze and summarize the types, incidence rates and relevant influencing factors of adverse events (AEs) after high-intensity focused ultrasound ablation of gynecological diseases and provide reference and basis for handling such events in clinical practice.
Method
We searched PubMed, Cochrane Library, Web of Science and Embase databases to retrieve all literature since its establishment until February 2024. We evaluated the quality of included literature and publication bias and conducted a meta-analysis of single group rates for various AEs using Stata 17.0.
Results
This systematic review finally included 41 articles. We summarized 34 kinds of AEs in 7 aspects and conducted a single group rate meta-analysis and sub-group analysis of 16 kinds of AEs. Among the common AEs of High-Intensity Focused Ultrasound (HIFU), the incidence of lower abdominal pain/pelvic pain is 36.1% (95% CI: 24.3%∼48.8%), vaginal bleeding is 20.6% (95% CI: 13.9%∼28.0%), vaginal discharge is 14.0% (95% CI: 9.6%∼19.1%), myoma discharge is 24% (95% CI: 14.6%∼34.8%), buttock pain is 10.8% (95% CI: 6.0%∼16.5%) and sacral pain is 10% (95% CI: 8.8%∼11.2%). Serious complications include uterine rupture, necrotic tissue obstruction requiring surgical intervention, third degree skin burns and persistent lower limb pain or movement disorders.
Conclusion
The common AEs after HIFU surgery are mostly mild and controllable, and the incidence of serious complications is extremely low. By reasonable prevention and active intervention, these events can be further reduced, making it a safe and effective treatment method. It is a good choice for patients who crave noninvasive treatment or have other surgical contraindications.
Introduction
The idea of applying heat to treat solid tumors in affected area generated by focalized ultrasonic wave was initially proposed by Fry et al. in the 1950s in the United States [Citation1]. Based on decades of efforts by countless researchers and institutions, it was ultimately transformed to the approach of focused ultrasound ablation technology and applied to clinical practice afterward. Currently, the High-Intensity Focused Ultrasound (HIFU) ablation has been used for various benign and malignant solid tumors, including those in the bone, uterus, prostate, liver, kidney, breast and pancreas, etc., [Citation2] since it was successfully indicated to a case of osteosarcoma for the first time in the world in 1999. Generally HIFU is indicated to uterine fibroids and adenomyosis in the field of gynecology. In 2002, the outcome of preliminary research by Wang Zhi Biao et al. demonstrated that ultrasound-guided high-intensity focused ultrasound ablation for uterine fibroids was safe and effective [Citation3]. In 2004, magnetic resonance-guided focused ultrasound (MRgFUS) was approved by the Food and Drug Administration (FDA) for the treatment of uterine fibroids. In 2008, HIFU was applied to adenomyosis. Nowadays it has been intensively indicated in more and more diseases in obstetrics and gynecology, such as abdominal wall endometriosis, placental implantation, incision pregnancy, cornual pregnancy, ovarian cancer, cervicitis, vulvar diseases, etc. as its development is gradually accepted.
Tissue damage caused by ultrasound is mainly initiated by two biological mechanisms, namely the conversion of mechanical energy into thermal energy and cavitation effects [Citation4]. High intensity focused ultrasound waves can precisely couple with the skin and aim at the target tissue, causing the tissue temperature in the focal area to rise above 60 °C within seconds [Citation5] and inducing protein denaturation and irreversible coagulation necrosis of the target tissue. Theoretically, by accurate monitoring the location and scope of treatment with real-time ultrasound, its damage range of HIFU can be limited to the expected ablation area and relatively speaking, the tissue in the ultrasound propagation path is not affected [Citation6]. As the widespread application of this technology in the whole world grows, the effectiveness of HIFU surgery has been recognized, but its safety remains an issue worthy of discussing. Although a large number of clinical studies have confirmed that HIFU is a primarily safe treatment with slight injury, its postoperative adverse events and complications are observed in its application and follow-up studies, including tissue damage in the ultrasound propagation path, skin burn, pain, nerve damage, acute renal failure, etc [Citation7]. At present, meta-analysis on its safety in gynecological application is found, with comparison to none-hysterectomy surgical treatment. The study by Ji et al. [Citation8] shows that compared to myomectomy, HIFU has significant advantages in reducing pain/discomfort, fever, transfusion, reproductive, gastrointestinal and anesthesia-related complications. Other studies have also reached similar conclusions, that compared to conventional surgery (myomectomy), the incidence of major complications and adverse events in HIFU is predominantly lower [Citation9,Citation10]. Two meta-analyses compared the incidence of adverse events between HIFU and uterine artery embolization (UAE). In the study by Xiaoyi Xiao et al. [Citation11], the incidence of adverse events in HIFU is lower than that in UAE. In the study by Yu Liu et al. [Citation12], there is no significant difference in the incidence of adverse events between the two treatment methods.
Although HIFU has certain advantages over other surgical methods in reducing adverse events (AEs), the types of AEs after surgery are still diverse. Some retrospective studies [Citation13,Citation14] have conducted multicenter, large-scale data statistics, which have good reference value. However, more rigorous evidence-based support is still needed to provide more valuable information for clinical doctors, researchers and others. Therefore, it is necessary to reassess the incidence of its AEs, explore its possible solutions, and later on, to improve the perioperative risk management plan and to avoid AEs caused by factors such as improper surgical operation by HIFU surgeons. Hopefully, this study might increase the safety of HIFU when treating gynecological diseases. The purpose of this article is to provide a systematic review on common AEs and complications after HIFU treatment for common gynecological diseases, including uterine leiomyoma, adenomyosis, endometriosis, placental implantation, as well as ectopic pregnancy. Potentially, it could provide evidence-based data and information when taking corresponding measures for early prevention and improving the safety of HIFU surgery.
Materials and methods
Retrieval method
This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analysis) writing process updated in 2020 and registered in the International prospective register of systematic reviews (PROSPERO registration number is CRD42023453839). Two reviewers (Ms. Lu Shanshan and Ms. Yang Wei) used Boolean logic operations to search all literature in PubMed, Cochrane library, Web of Science and Embase databases since their establishment to February 2024. The search terms included: High-Intensity Focused Ultrasound Ablation, Leiomyoma, Adenomyosis, Endometriosis, Placenta Accreta, Pregnancy, Ectopic and related entry terms. All search terms and specific search logic are shown in Annex 1 (Supplementary Material), with the last search date of 25 February 2024.
Inclusion and exclusion criteria
The inclusion criteria for this systematic review are prospective studies, language limitations in English, without limitations on the guidance method of HIFU. In the study comparing HIFU with other interventions, data from a single HIFU intervention group was included. Exclusion criteria were: case reports, studies that did not report the occurrence of AEs or complications, non-English literature, reviews, animal studies, studies of tissue in vitro, editorials, conference abstracts/conference proceedings, guidance or comments, letters, articles unrelated to the research topic and studies without a full text (). Due to the fact that most studies had reported two or more types of AEs in the same patient, there are few studies that specifically reported the total number of AEs. Through team discussions, this review ultimately used the incidence rate of various AEs as the statistical result. The tolerable mild skin burning sensation, lower abdominal pain and nerve stimulation during HIFU surgery are warning signals for tissue damage. Those whose symptoms disappeared after the surgery were not included in the scope of AEs.
Table 1. Inclusion and exclusion criteria.
Screening of literature and data extraction
Two reviewers (Ms. Lu Shanshan and Ms. Yang Wei) screened the literature based on inclusion and exclusion criteria. First, the title and abstract of the article were screened, then full content of the included literature was reviewed, finally excluded literature that did not meet the inclusion criteria. Ms. Yang Liu and Ms. Zhang Xia Lin were responsible for extracting literature data. EndNote X9 was used to manage the literature and Excel was used to extract and organize the data. The extracted information included: title, first author, publication year, study type, HIFU system used in the study, whether other interventions are merged and the type of intervention, patient’s age, number of included patients, type and occurrence of AEs. According to Practice Guidelines for Interventional Radiology (Society of Interventional Radiology, SIR) [Citation15], complications were classified into categories A-F, category A: no treatment, no consequences; category B: nominal treatment, or no consequences, including overnight admission for observation only, category C: require treatment, mild hospitalization (<48 h), category D: require to stay in hospital for large-scale treatment (>48 h), nursing level increased and hospitalization duration extended, category F: permanent adverse sequelae, category G: Death, among which categories A-B referred to minor complications, while categories C-F to major complications. When researches did not classify AEs according to the SIR complication grading system, but they described the duration, corresponding treatment, hospitalization time and other information of the AEs, we categorized them based on relevant information. Any disagreement arising during literature screening and data extraction was negotiated and agreed upon by the reviewer or data extractor. When not being resolved, they were consulted from the corresponding author, Professor Wen Yi. The areas of disagreement included the definition of AEs (whether they were normal intraoperative warning signals, whether they were related to HIFU, etc.), such as the discharge of necrotic tissue after HIFU surgery. We excluded this part of the data in the early stage, and after literature review and consultation with the corresponding authors, we included it in the statistical analysis due to careful consideration of professionalism. For specific descriptions, please refer to the discussion section. Other aspects that need to be discussed included the research type of literature, SIR grading of adverse events, etc.
Quality assessment of included literature
This review was independently conducted by Ms. Wang Jing and Ms. Yang Lei lei to evaluate the quality of the included literature. The new version of Cochrane’s bias risk assessment tool RoB 2.0 [Citation16] was used to evaluate the bias risk of RCT. The domains of bias included bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result. The Newcastle Ottawa Scale (NOS) [Citation17] was used to assess the risk of bias in non-RCT cohort studies. The scale consists of three modules with eight items, including selection, comparability and exposure/outcome. The Joanna Briggs Institute (JBI) Critical Appraisal Checklist [Citation18] was used for quality assessment of single arm studies, where the quality of each item is assessed as ‘Yes’, ‘No’, ‘Unclear’, or ‘Not applicable’. Disagreements were solved by the two individuals. If it cannot be resolved, it was resolved through consultation with the corresponding author, professor Wen Yi.
Statistical analysis
The Metaprop program in Stata 17.0 was used for single group rate meta-analysis [Citation19] of AEs with more than three articles, and subgroup analysis was performed by disease type, HIFU system type, and other subgroups. Using the Freeman-Tukey double inverse sine transform method for data consolidation, and selecting a random effects model. Funnel plots and Egger test were used to evaluate publication bias in literature, and publication bias was considered when p < .05.
Results
Literature screening and overall situation
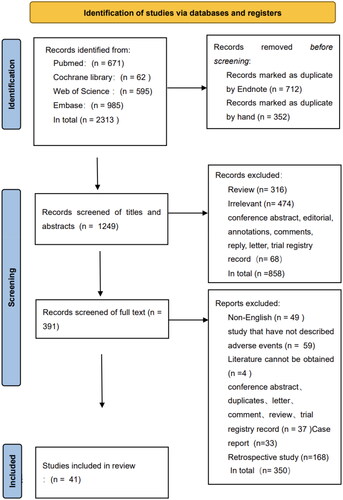
After a preliminary search on the four databases, 2313 articles in total were retrieved, and 1249 articles were kept after duplicate articles were removed. Through independent screening by two reviewers (Ms. Lu Shan shan and Ms. Yang Wei) on the titles, abstracts and full text of the literature, a total of 41 articles from 2007 to 2022 were included. The specific screening process is shown in . Among the 41 articles, there were 8 randomized controlled trials, 13 non-RCT cohort studies and 20 single arm studies. Among them, 10 articles included HIFU intervention in both groups. Therefore, this review treated these articles as 2 studies, and a total of 51 studies were included in this review. The benign gynecological diseases adopted in the literature include uterine leiomyoma (33 articles), adenomyosis (5 articles), endometriosis (1 article) and ectopic pregnancy (2 articles). The titles, first author, publication year, study design, HIFU system, HIFU treatment parameters, with/without other intervention measures combined and the type of intervention measures, average age of patients, number of included patients, types and occurrence of AEs of all literature are presented in the form of a table in Annex 2 (Supplementary Material).
Quality assessment of included literature
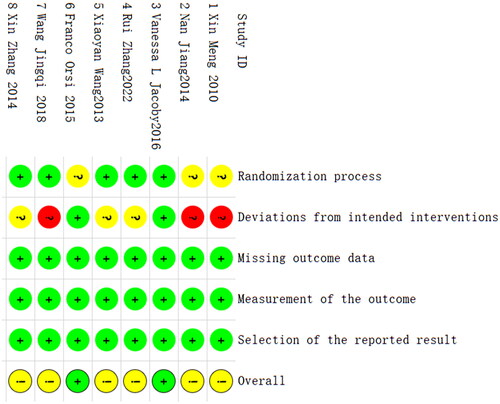
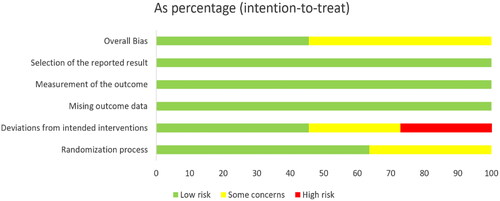
The literature quality assessment table of RCT is shown in Annex 3 (Supplementary Material). The risk of bias is mainly concentrated in the deviation from intended interventions ( and Citation3). Two studies did not describe the method of random allocation sequence in detail, the carers and workers delivering the interventions cannot be blinded in six studies, and five studies did not mention the deviation from the intended intervention caused by the experimental context. Overall, the deviation risk of RCT is relatively low. The literature quality assessment of non-RCT cohort studies is shown in Annex 4, with a NOS scale score of 7.5 ± 1.1 points (range 6–9 points). The quality assessment of single arm studies is shown in Annex 5 (Supplementary Material). Overall, the quality of the literature is from medium to high.
Publication bias
The publication bias funnel plot and Egger test of various AEs are shown in Annex 6 (Supplementary Material). The incidence of lower abdominal pain/pelvic pain (p = .006), burns (p = .039), fever (p = .029), low back pain (p = .034) suggested publication bias, while nausea/vomiting/anorexia (p = .074), abdominal distention/diarrhea (p = .15), uterine fibroids discharge (p = .091), vaginal discharge (p = .903), vaginal bleeding (p = .066), urinary tract infection/urinary irritation symptoms (p = .328), hematuria (p = .123), sacrum/sacrococcygeal pain (p = .209), lower limb pain/numbness (p = .876), lower limb weakness/foot droop (p = .528), buttock pain (p = .659) and urinary retention (p = .108) had no publication bias.
Statistical results of various AEs
Among 51 studies included, the total number of patients was 4645 and 2633 AEs were recorded. We divided them into 7 aspects and 34 types. According to the SIR classification, in 2526 cases they were classified as mild complications (category A-B), in 7 cases they were classified as major complications (category C-D), no E-F complications were found, and in 100 cases, it was unable to be classified due to lack of relevant information, as shown in . All minor complications resolved spontaneously after treatment or without treatment. The major complications included one case of uterine rupture, one case of uterine fibroid discharge, four cases of burns and one case of lower limb pain and numbness. Among the seven major complications, one patient with uterine rupture underwent an emergency cesarean section at 36 + 5 weeks of pregnancy and recovered well after surgery. A patient discharged most of the fibroid tissue from the vagina six months after HIFU surgery and ultimately underwent hysteroscopic surgery to completely remove the remaining fibroid tissue. A patient developed III degree skin burns in two areas after HIFU surgery, which were ultimately repaired by surgery. The remaining three burn patients defined as major complications were not described in the literature regarding their management and prognosis. One patient experienced persistent lower limb pain for two months after HIFU surgery, which was defined as a major adverse event. The forest plots of the meta-analysis of the incidence rates of various AEs is shown in Annex 7 (Supplementary Material).
Table 2. Summary of AEs after HIFU treatment for benign gynecological diseases.
Burn
A total of 27 studies were included, and the overall incidence of burns was 2.2% (95% CI: 0.4%∼0.51%, I2=79.05%, p < .01). We conducted subgroup analysis based on the types of diseases, and the probability of burns occurring varied among different diseases (p = .019). The incidence of uterine fibroids (23 studies) was 3.0% (95% CI: 0.5%∼6.9%, I2=81.95%, p < .01) and the incidence of adenomyosis (4 studies) was 0.5% (95% CI: 0–2.3%, I2=0%, p = .54). The incidence of burns varies among different HIFU systems (p = .008), with 27 studies including 6 HIFU systems. JC 200 was the most commonly used system (18 studies) with an incidence rate of 2.2% (95% CI: 0.2 ∼ 5.4%, I2=81.0%, p < .01), followed by ExAblate (3 studies) with an incidence rate of 1.4% (95% CI: 0 ∼ 5.3%), JM 2.5 C (2 studies) with an incidence rate of 15.6% (95% CI: 4.0%∼31.5%), and Sonalleve (2 studies) with an incidence rate of 2.7% (95% CI: 0%∼15.7%). ALPIUS and HIFU-2001 are only one study, with incidence rates of 0% and 15%, respectively. We attempted to conduct subgroup analysis using USgHIFU and MRgHIFU as groups, but the difference was not statistically significant (p = .39).
Lower abdominal pain/pelvic pain
Among the 25 studies included, the overall incidence of lower abdominal/pelvic pain after HIFU surgery was 36.1% (95% CI: 24.3%∼48.8%, I2=94.86%, p < .01), and the incidence of lower abdominal/pelvic pain varied among different diseases (p = .025). Among them, 19 studies were uterine fibroids, with an incidence of 40% (95% CI: 26.6%∼54.1%, I2=93.89%, p < .01), and 4 studies were adenomyosis, with an incidence of 31.4% (95% CI: 6.0%∼65.0%, I2=96.21%). p < .01). There is only one study on endometriosis and cesarean scar pregnancy, with incidence rates of 8.7% and 18.8%. We also compared the incidence of abdominal pain/pelvic pain under different HIFU system (p < .01), and 25 studies included 10 HIFU systems. Two studies were not included in the statistics because the HIFU system was not described. JC-200 is the most commonly used system (13 studies in total), with an incidence rate of 41.1% (95% CI: 23.7%∼59.6%, I2=94.75%, p < .01) for abdominal/pelvic pain, followed by Sonalleve (2 studies in total) with an incidence rate of 55.8%. Other systems mentioned was independently seen in one article, including HIFUNIT 9000 (12%), JM 2.5 C (30%), JM 5100 (7.0%), FEP-BY02 HIFU (26.3%), RODIN HIFU (30.8%), HIFU-2001 (35.0%), Focal One (8.7%), Model FEU P-BY-01 (18.8%).
Vaginal bleeding
Among the 15 studies included, the overall incidence of vaginal bleeding was 20.6% (95% CI: 13.9%∼28.0%, I2=92.60%, p < .01). The incidence of vaginal bleeding varied among different diseases (p < .01), uterine fibroids (9 studies) was 12.8% (95% CI: 7.3%∼19.5%, I2=91.55%, p < .01), adenomyosis (4 studies) was 37.9% (95% CI: 28.2%∼48.0%, I2=49.36%, p = .115), endometriosis (1 study) was 8.7%, and cesarean scar pregnancy was (1 study) 56.3%.
Vaginal discharge
Among the 15 studies included, the overall incidence of vaginal discharge was 14.0% (95% CI: 9.6%∼19.1%, I2=73.22%, p < .01). We attempted to compare the incidence of vaginal discharge among different diseases, but the difference was not statistically significant (p = .26).
Necrosis tissue excretion
Four studies described the excretion of necrotic tissue after HIFU, all of which were uterine fibroids, containing three submucosal fibroids, and one dominant fibroid of intramural fibroids, with an overall incidence rate of 24% (95% CI: 14.6%∼34.8%, I2=27.33%, p = .248).
Sacral/sacrococcygeal pain
Among the four studies included, three studies were uterine fibroids and one study was adenomyosis. The incidence of sacral/sacrococcygeal pain was 10.0% (95% CI: 8.8%∼11.2%, I2=0%, p = .975).
Lower limb pain/numbness
Among the 13 studies included, 11 studies were uterine fibroids, 1 study was adenomyosis, and 1 study was endometriosis. The incidence of lower limb pain/numbness was 1.5% (95% CI: 0.5%∼3.0%, I2=49.83%, p = .021).
Lower limb weakness/foot droop
Four studies were included, with three uterine fibroids and one adenomyosis. The incidence of lower limb weakness/foot droop was 0.3% (95% CI: 0.1%∼0.6%, I2=0%, p = .43).
Lower back pain
A total of five studies were included, with four uterine fibroids and one endometriosis. The incidence of Lower back pain was 5.4% (95% CI: 0–16.2%, I2=70.77%, p = .008).
Buttock pain
A total of 11 studies were included, with 9 uterine fibroids and 2 adenomyosis. The incidence of buttock pain was 10.8% (95% CI: 6.0%∼16.5%, I2 = 62.75%, p = .003). We attempted to conduct subgroup analysis on uterine fibroids and adenomyosis, but the difference was not statistically significant (p = .173).
Nausea/vomiting
A total of seven studies were included, with six uterine fibroids and one adenomyosis. The incidence of nausea/vomiting was 1.2% (95% CI: 0.2%∼2.6%, I2 = 58.08%, p = .026).
Abdominal distension/diarrhea
A total of six studies were included, among which two were uterine fibroids, three were adenomyosis, and one was endometriosis. The incidence of abdominal distension and diarrhea is 3.0% (95% CI: 0.6%∼6.7%, I2=31.72%, p = .198).
Urinary tract infection/urinary irritation symptoms
A total of six studies were included, with three studies of uterine fibroids, two studies of adenomyosis and one study of endometriosis. The incidence of urinary tract infections/urinary irritation symptoms was 4.5% (95% CI: 1.8%∼8.0%, I2=0%, p = .637).
Hematuria
Four studies were included, among which three were uterine fibroids and one was adenomyosis. The incidence of hematuria was 1.8% (95% CI: 0%∼7.9%, I2=80.28%, p = .002).
Urinary retention
Four studies were included, all of which were uterine fibroids. The incidence of urinary retention was 0.002% (95% CI: 0%∼0.7%, I2=73.09%, p = .01).
Fever
A total of 10 studies were included, with 6 uterine fibroids and 4 adenomyosis. The incidence of fever was 3.3% (95% CI: 0.3%∼8.3%, I2=85.76%, p < .01). We attempted to perform subgroup analysis by grouping uterine fibroids and adenomyosis, but the difference was not statistically significant (p = .93).
Discussion
We accomplished this literature review on various types of AEs on a systematic basis, exploring and analyzing their risk factors and summarizing their conventional solutions. In the process of literature searching, we also discovered some considerably rare complications of HIFU that were not included in the statistics, such as intestinal injury, abdominal hernia formation, renal function injury, etc. and supplemented it in the form of a literature review.
Thermal damage to skin and abdominal wall
The optimal ablation temperature threshold for achieving coagulation necrosis without carbonization on the lesion was 60–100 °C [Citation20]. During the process of lesion ablation, the temperature of the skin, subcutaneous tissue and deep abdomen along the ultrasound propagation path increased accordingly [Citation21], When the skin temperature was rising to 43 °C, patients feel local pain, and when temperature exceeds 44 °C, skin burns occurred [Citation22]. Thermal damage to the skin and abdominal wall is one of the adverse events that we need to focus on. Early studies have reported a serious complication of full-thickness skin necrosis after HIFU surgery [Citation23]. After more than a decade of clinical practice and exploration by researchers, many risk factors that can increase the risk of skin burns after HIFU surgery have been discovered, such as abdominal skin scars. Due to reduce blood supply to scar tissue, decreased skin sensation and high degree of scar fibrosis, consequently, ultrasound energy was easily absorbed by scar tissue and difficult to penetrate, leading to increased temperature in local skin and abdominal wall [Citation24,Citation25]. Besides the factor of skin scar, Yin et al. [Citation26] studied the factors related to abdominal wall thermal injury caused by USgHIFU treatment on uterine fibroids. The results showed that thickness of fat in abdominal wall, fibroid volume, distance from the ventral side of the fibroid to the abdominal wall, ultrasound time and total energy were significant risk factors for the thermal injury, which caused by heat absorption by adipose tissue, higher abdominal wall thickness and fat content. Meanwhile prolonged compression of the dehydration balloon reduced skin circulation and heat dissipation, later increasing the risk of skin burns. The study by Zhao et al. [Citation27] showed that liposuction could alter the tissue structure of abdominal wall, causing subcutaneous tissue fibrosis and scar formation, thereby increasing the incidence of heat injury.
With the increasing cesarean section rate and demand for noninvasive surgery, more and more clinical studies explored how to safely and effectively perform HIFU surgery on women with abdominal scars. A previous study [Citation28] used an intravenous contrast agent containing paramagnetic iron oxide particles to visualize scars on MRI and successfully treated 25 patients with uterine fibroids with abdominal scars by optimizing the surgical plan to avoid ultrasound energy beams passing through the scars. A case report [Citation29] revealed the use of abdominal scar patches in MR-HIFU ablation treatment, with no serious adverse events. Our research shows that the incidence of burns varies among different HIFU systems, with higher burn rates observed in JM 2.5 C (15.6%) and HIFU-2001 (15.0%). Our study also suggests that the burn rate of patients with uterine fibroids after HIFU surgery is higher than that of adenomyosis. From a technical perspective, we are currently unable to explain the reason for this difference, which may be related to factors such as the HIFU system, the location and quantity of fibroids and the operation by technicians.
Overall, the probability of skin burns occurring after HIFU surgery is relatively low. Effective measures can be taken to further reduce the probability and improve the safety of HIFU surgery, such as offering strict preoperative skin preparation, degreasing and degassing to avoid residual air bubbles. Furthermore, during surgery, doctors or technicians should maintain a temperature of 10–20 °C with degassed water to cool down, regularly lower the energy converter to reduce abdominal pressure, promptly check the skin condition and observe for local hardening. After surgery, they should continue to apply ice compress for 2 h, and the ice application time can be appropriately extended according to the skin condition. However, for patients with scar width greater than 1.5 cm and depth that are difficult to avoid or being protected through alteration of technical means, as well as obese patients with thick abdominal walls and local skin damage treated, HIFU treatment is not recommended.
Pelvic and reproductive system related regions
Lower abdominal pain and pelvic pain are the most common AE after HIFU surgery, which have a lower incidence than postoperative pain caused by UAE (about 90%) [Citation30]. Energy deposition during HIFU surgery, inflammatory response after lesion necrosis and swelling on surrounding tissue might be major causes of lower abdominal pain [Citation31]. Most cases were mild, with short duration, and can improve on their own without treatment. Patients with obvious pain can receive symptomatic treatment such as oral anti-inflammatory drugs and painkillers. We have not found any serious adverse events related to persistent lower abdominal pain or pelvic pain in our literature review. For patients with persistent lower abdominal pain that does not improve after symptomatic treatment, they should be alert to the occurrence of serious complications such as peripheral tissue damage and infection.
Vaginal bleeding caused by endometrial ablation injury is another common AE of HIFU. To protect the myometrium and avoid adjacent organs from heat damage, the ablation site should be at least 5 mm away from the endometrium and serosa layer [Citation32]. Kim et al. [Citation33] evaluated the integrity of the endometrium after HIFU treatment for the first time. Although the ultrasound beam was difficult to avoid the endometrium, the degree of postoperative endometrial damage was still relatively low. A study [Citation34] on enhanced MRI scanning three months after HIFU treatment for submucosal fibroids showed that 50 patients experienced endometrial damage after HIFU treatment. In follow-up after three months, MRI images showed a decrease in the number of injured individuals to 29, indicating that the damaged endometrium repaired by itself to a certain extent after HIFU surgery. Our study shows that the incidence of vaginal bleeding in adenomyosis is higher than that in uterine fibroids, as uterine fibroids can compress surrounding muscle wall fibers to form a pseudocapsule [Citation35], which contains abundant blood vessels and the accumulated heat is easily carried away by the blood, resulting in less damage to the endometrium. In patients with adenomyosis, the lesions show diffuse growth in the uterine myometrium with unclear boundaries, and some ectopic lesions in patients with adenomyosis are connected to the normal endometrium, which can easily damage the endometrium and cause bleeding during ablation of the lesions.
Vaginal discharge and existence of pelvic fluid accumulation were caused by aseptic inflammation after HIFU and faded off without special treatment [Citation13]. For patients with adenomyosis combined with heavy menstrual bleeding (HMB) and have no fertility requirements, the combination of lesion and partial endometrial ablation is beneficial for reducing menstrual flow [Citation36]. Our previous study [Citation37] showed that an overall endometrial ablation rate of ≥ 30% can further reduce menstrual flow and improve dysmenorrhea in patients with adenomyosis on the basis of lesion ablation alone (p < .05). This ablation method did not increase the incidence of adverse events such as lower abdominal pain, lumbosacral pain and lower limb pain (p > .05), but prolonged vaginal bleeding and fluid flow time (p < .05), increasing the potential risk of postoperative infection. We effectively avoided the occurrence of infection through postoperative prevention, health education and active follow-up measures, which not only maintained the patient’s quality of life but also did not reduce their satisfaction with treatment.
In some studies, it has been observed that uterine fibroid necrosis tissue discharged after HIFU treatment is often accompanied by significant abdominal pain or vaginal bleeding, which increases the risk of postoperative intervention and infection. Therefore, previous studies have classified it as an adverse event [Citation38,Citation39]. Due to the fact that fibroids can be spontaneously expelled without invasive surgery such as hysteroscopy, it can be considered as one of the advantages of HIFU [Citation40]. In fact, for patients with some submucosal fibroids and placental implantation, the clearance of necrotic tissue after HIFU surgery itself is included in the overall treatment plan. Therefore, the author believes that whether the clearance of necrotic tissue should be regarded as an adverse event after HIFU surgery needs further discussion.
The occurrence of pelvic infection after HIFU surgery may be due to ablation breaking through the endometrial layer, resulting in longer vaginal bleeding and exudation time, or due to the retention of necrotic tissue in the uterine cavity, increasing the risk of secondary infection in the uterine cavity [Citation41]. There were two studies in which one patient developed symptoms of pelvic infection such as fever and lower abdominal pain after HIFU surgery, both of which were considered secondary infections and fully recovered after antibiotic treatment [Citation42,Citation43]. He et al. [Citation44] retrospectively analyzed the early (2.7 ± 1.4 days) and late (34.7 ± 15.0 days) removal of necrotic tissue after HIFU treatment for placental implantation. During postoperative follow-up, one patient in the late stage group developed intrauterine infection. There is no consensus on the interval time from the removal of damaged intrauterine dead tissue to HIFU surgery. After ultrasound ablation, tissue absorption or excretion requires a certain period of time, but long-term residual can increase the risk of infection and bleeding. Therefore, in clinical practice, it is necessary to comprehensively evaluate the relationship between them and to keep a balance between them is a demanding technical issue that needs to be solved. Based on the clinical experience of the author’s team, the waiting time for necrotic tissue to be discharged is usually not more than four weeks, and for patients at risk of infection, prophylactic antibiotic treatment can be used.
At present, there is no evidence to confirm that HIFU increases the risk of postoperative pelvic adhesion. A clinical study on the treatment of tubal pregnancy with HIFU [Citation45] showed that after 12 months of follow-up after HIFU surgery, only one of 33 successfully treated patients developed tubal adhesion. Researchers believed that the cause of tissue adhesion might be induced by combination of fibrosis after embryonic necrosis and the thermal effects of HIFU. However, as the necrotic substances gradually absorbed, the function of the fallopian tubes has been partially restored. Liu et al. [Citation46] conducted a retrospective study on whether HIFU treatment for uterine fibroids could cause pelvic adhesion. The results revealed that the risk of pelvic adhesion after HIFU surgery was related to the total energy of ablation, ultrasound time and treatment time, NPV ratio and energy response factors. However, there was no statistically significant difference in adhesion rate compared to the group that did not receive HIFU or other surgical treatments. There is no consensus on whether postoperative HIFU will increase the risk of pelvic adhesion, and more well-designed, large sample and multicenter clinical trials are needed for evaluation.
Uterine rupture is a rare and serious long-term complication of HIFU, and there are currently no literature reports on patients who died from uterine rupture after HIFU surgery. Lai et al. [Citation47] described a patient with diffuse forearm adenomyosis combined with uterine fibroids who developed uterine rupture at 37 weeks of pregnancy after receiving HIFU treatment. The patient underwent emergency cesarean section and recovered well after surgery. In another study comparing pregnancy outcomes of HIFU and laparoscopic myomectomy [Citation48], both the HIFU group (1/178) and the myomectomy group (1/173) experienced incomplete uterine rupture in one patient respectively, and there was no statistically significant difference in the incidence of uterine rupture between the two groups (p = 1.0). Both patients underwent emergency cesarean section and both mother and baby were healthy. The study also compared the differences in the incidence of other childbirth complications between the two groups, such as amniotic fluid embolism, postpartum hemorrhage, postpartum infection, etc., and the differences were not statistically significant (p > .05). As a noninvasive treatment method, HIFU has been proven to effectively improve the fertility outcomes of patients with adenomyosis [Citation49]. Compared with laparoscopic resection, patients with adenomyosis and infertility have a higher pregnancy rate after HIFU surgery [Citation50]. Therefore, HIFU is still a good choice for women who desire to retain fertility and plan for pregnancy. Due to the low incidence and unknown risk factors of uterine rupture after HIFU surgery, Liu et al. [Citation51] suggested that patients with unexpected pregnancy after HIFU surgery should reduce their daily activities in the late pregnancy, and late pregnancy pelvic MRI examination be helpful to evaluate the thickness of the uterine wall at the HIFU ablation site, and be aware of the possibility of uterine rupture when unexplained abdominal pain and fetal distress occur.
Spine and nervous system
The occurrence of sacrococcygeal pain, lower limb pain, buttock pain and perineal numbness after HIFU treatment was accompanied to local tissue edema and nerve stimulation at the ablation site. Zhang et al. [Citation52] compared the postoperative adverse event of HIFU treatment with anterior and posterior leaning uterus. Patients with posterior leaning uterus had a higher incidence of sciatica, buttock pain and inguinal pain compared to those with anterior leaning uterus, as posterior leaning uterus was adjacent to the sacrum. In the study by Gong et al. [Citation53], the lesion of external adenomyosis was adjacent to the sacrum, resulting in a higher incidence of neuropathic sciatica or hip pain in those patients compared to other types of adenomyosis. One study reported postoperative pain or distension around the anus in HIFU patients [Citation54], which was a mild AE and considered to be caused by nerve stimulation. The study by Cun et al. [Citation55] on sacral injury after USgHIFU surgery showed that, in addition to the remarkable correlation between the distance from the dorsal side of fibroids to the sacrum and the type of fibroids with sacral injury, there was also a correlation between fibroid volume. This study also suggested that high signal intensity on magnetic resonance imaging (MRI) was an independent risk factor for sacral injury in terms of fibroid features, meanwhile, in terms of treatment parameters, energy efficiency factor (EEF) and therapeutic dosimetry (TD) were relevant risk factors for sacral injury. Numbness and pain in the lower limbs were associated with stimulation on the sacral nerve [Citation56]. Severe nerve damage could lead to weakness in the lower limbs and foot droop [Citation57]. Current reports indicated that this adverse event was reversible and could be recovered by symptomatic treatment such as nerve maintenance, and there was no case with existence of sequelae. Besides nerve stimulation, prolonged prone position might also be considered with lower back pain [Citation58].
In addition, nerve damage is a problem that needs to be taken seriously and severe damage may lead to permanent sequelae, inevitably affecting patients’ quality of life. First, patients with a history of lower limb pain, such as intervertebral disk herniation and spinal stenosis, should be excluded before surgery to avoid terminating treatment due to position induced pain. Second, carefully monitor the patient’s condition during surgery to avoid excessive analgesia and sedation. Once the patient experiences radiating pain, such as pain and numbness in the bilateral inguinal ligaments, lateral buttocks, lower limbs, perineum, anus and perianal area, the irradiation should be stopped immediately and the irradiation site should be changed. For patients who experience radiation pain during HIFU treatment and disappear after treatment, dexamethasone 30 mg can be administered intravenously in batches (total dose ≤ 30 mg) during or after surgery. Nonsteroidal anti-inflammatory drugs should be administered for another two weeks after surgery, and close follow-up should be conducted. For patients who experience radiation pain during surgery and still experience discomfort in the radiation pain area within 14 days after surgery, dexamethasone 30 mg can be administered intravenously in batches (total dose ≤ 30 mg) for 3 days during or after surgery. Nonsteroidal anti-inflammatory drugs can be taken orally until symptoms disappear for one week. If the symptoms do not disappear, the course of treatment can be extended to six weeks, and nerve nourishing drugs can be added.
Digestive system
Minor adverse events in the digestive system included nausea, vomiting, regurgitation, bloating and diarrhea, which were resulted from the use of sedatives during surgery and inflammatory reactions. Most symptoms were mild and self-limited and relieved after symptomatic treatment. Rectal bleeding and constipation only appeared in one study [Citation59], correlative with the HIFU system and the site of ablation. Eleven participants experienced gastrointestinal related adverse events after transrectal ultrasound ablation on deep endometriosis, with 4 patients experiencing minor rectal bleeding and 6 patients experiencing constipation. According to the Clavien Dindo grade classification, all patients were classified as Class I (mild) and received no treatment. Some studies have reported patients with intestinal injury after HIFU surgery [Citation14,Citation60,Citation61], in which they had uterine fibroids and adenomyosis, and the onset time varied from 2 to 14 days after HIFU treatment. Through retrospective analysis of the cases, the reasons included intestinal tract being located in the ultrasound beam path, the lesion being too close to the intestine, the ablation area being too large and energy reflection caused by lesion calcification. All patients reported recovered after intestinal repair surgery and did not experience permanent sequelae. Abdominal hernia formed after surgery was one of the postoperative complications, and in recent years, there were a few literature reports about abdominal hernia after HIFU surgery. A case report described a patient with no history of either pelvic inflammatory disease or abdominal surgery, experienced small intestinal incarceration caused by a fibrous band defect between left adnexal area and peritoneum two years after HIFU [Citation62]. In another case report [Citation63], the patient developed partial atrophy and defect of the left rectus abdominis muscle one year after receiving USgHIFU treatment. As the patient did not have the history of previous surgery or corresponding diseases, we believed that her abdominal hernia was induced by HIFU.
Urinary system
Common urinary complications include hematuria, urinary retention and urinary tract infection. Zhao et al. attributed hematuria to the damage of the urethral mucosa caused by the catheter [Citation64], and other researchers analyzed that the occurrence of hematuria was caused by the appearance of bubbles in the bladder during the ablation process [Citation65], which was due to tissue damage caused by the cavitation effect of ultrasound [Citation66]. Under normal circumstances, flushing the bladder with 4 °C saline solution could alleviate the symptom of hematuria. Urinary tract infections were associated with catheterization [Citation67,Citation68], and all patients recovered by antibiotic treatment. In a study [Citation42], a patient developed pyelonephritis during follow-up after HIFU surgery and fully recovered after 14 days of oral administration of ciprofloxacin. Due to insufficient analysis in this literature, it was unclear whether this adverse event was related to HIFU. A patient with a 10 cm sized fibroid developed hydronephrosis after HIFU surgery [Citation13], and analysis suggested that the compression on the ureter was aggravated by tissue edema after ablation where previous large fibroid was located. Postoperative urinary retention was usually regarded as a minor adverse event of surgeries, which might be seen in gynecological surgery, hernia repair, anorectal surgery and other surgical operations [Citation69]. During HIFU, it is necessary to use normal saline solution to fill up the patient’s bladder to push the intestinal tract away from the acoustic channel, which interferes with the contraction of the detrusor muscle. Furthermore, the impact of the operation itself and the use of narcotic drugs could also lead to urinary retention [Citation70], which usually was relieved after symptomatic treatment such as catheterization. Acute renal failure was a rare and serious complication of HIFU, with unclear causes. A case report [Citation71] described a 38 year old woman who experienced thrombocytopenia and renal function damage after 10 h of HIFU treatment. The authors speculated that the mechanical damage and thermal degeneration of red blood cells in HIFU led to short-term massive hemolysis, increasing the burden on the kidneys and impairing kidneys. Moreover, the use of ultrasound contrast agents was another suspected risk factor. In a retrospective study [Citation72], two patients who used ultrasound contrast agents (SonoVue) experienced renal function damage, and researchers speculated that SonoVue was the main cause of this complication. Both patients had a history of hypertension, and one had a history of long-term use of non-steroidal anti-inflammatory drugs. These two factors might increase the risk of renal function damage. In addition, tumor lysis syndrome was considered as another factor [Citation73]. After tumor necrosis, cells dissolved and released a large amount of substances into the bloodstream, causing a series of electrolyte disorders, including hyperkalemia, hyperphosphatemia, hyperuricemia and hypocalcemia. As a result, uric acid crystals and phosphates deposited and blocked in the renal tubules, ultimately leading to renal function damage [Citation74]. However, tumor syndrome was rare in solid tumors, and there was currently no relevant evidence to support this conclusion. In the future, more solid research is acquired to continue the exploration about pathological process of acute kidney injury after HIFU surgery.
Systemic symptoms
Post ablation syndrome, which refers to a series of flu-like symptoms such as fever, nausea and general discomfort during the ablation process, is mainly attributed to inflammatory reactions caused by tissue necrosis and is a common adverse event after microwave ablation, radiofrequency ablation and cryoablation [Citation75]. Park et al. [Citation67] also classified symptoms of fear of cold, low fever (<38 °C) and general discomfort after HIFU surgery as part of this category. Mild symptoms, such as low fever, fatigue and lassitude are self-limited and do not require management. Severe high fever requires vigilance to evaluate possible accompany with other infections and timely detection of potential serious complications as well. Intraoperative dizziness and headache [Citation76], temporary blurred vision [Citation77] and severe changes of blood pressure [Citation78] were relatively rare. It was unclear whether these adverse events were related to HIFU, and it could not be ruled out that they were caused by intraoperative analgesics and sedatives.
Others
Thrombocytopenia is a rare complication of HIFU, and its cause remains unknown. Severe burned patients might experience platelet decline in the early stages, as the hemostatic reaction consumed a large amount of platelets [Citation79]. Some researchers believed that this phenomenon was able to explain the thrombocytopenia observed after HIFU ablation [Citation71], but this statement was not been powerfully confirmed. Due to the limited number of cases, the cause of thrombocytopenia after HIFU surgery is still unclear, and there are no effective preventive measures. In clinical practice, symptomatic treatment is often used. In addition, prolonged surgery duration increased the risk of deep vein thrombosis [Citation80]. A total of three patients developed deep vein thrombosis after HIFU surgery and received anticoagulant treatment [Citation81].
Compared with other uterine preserved surgical treatments, HIFU has many advantages, such as the protection of fertility [Citation82]. In addition, HIFU can significantly shortened hospital stay, reduced financial costs and saved medical resources [Citation83–85]. However, any surgery or procedure is risky. Laparoscopic surgery has a greater trauma compared to HIFU, leading to damage to the uterine muscle layer and formation of uterine scars, as well as higher postoperative pain scores and longer recovery time [Citation86]. UAE is a relatively safe surgery, but the rate of postoperative intervention is higher than surgical intervention [Citation87]. Clinical applications have also observed the occurrence of some serious complications, such as permanent amenorrhea, pulmonary embolism, severe uterine ischemia and infection, with a severe complication rate of 2.9% [Citation88]. Although a meta-analysis showed no significant difference in the incidence of major adverse events between UAE and HIFU [Citation89], but UAE carries a potential risk of ovarian function impairment [Citation90], and the reporting rate of permanent amenorrhea after UAE surgery is significantly higher in patients over 45 years old [Citation91]. while HIFU has been confirmed not to affect ovarian reserve function [Citation92,Citation93].
Conclusion
Among the AEs related to gynecological diseases that we have summarized, most of them are directly linked with the treatment site, in which the majority are damage to tissues and organs in the near-field and far-field of the sound channel, which is mainly related to the thermal/cavitation effects of HIFU technology and the treatment site. Early research suggests that different models of HIFU systems may also affect the occurrence of adverse events [Citation94], which is consistent with our research findings. In addition, factors of AEs also include the technical proficiency of the surgeon, the type of disease, the condition of the patient’s abdominal wall, the position of the patient’s uterus, the location and size of the lesion, treatment parameters, the use of ultrasound contrast agents, the use of anesthetic drugs and other unknown reasons. Our research provides certain reference value for the incidence of common adverse events and their interventions. Firstly, before surgery, it is necessary to fully inquiry the patient’s medical history (such as hypertension history, abdominal surgery history, lower abdominal radiotherapy history, etc.), medication history (such as nonsteroidal anti-inflammatory drugs) and clarify the size, location and blood flow signal of the patient’s lesion. By assessing the difficulty of surgery, the energy administered during surgery, the duration of treatment and the range of ablation should be rationally evaluated, and excessive treatment should not be allowed. Secondly, for patients with high-risk factors, estimate the potential AEs or complications that may occur during or after surgery and take timely preventive measures. Finally, the postoperative patients should be reevaluated and discreetly followed up to promptly detect warning signals of serious complications, such as skin erythema (burns), lower limb motor disorders (nerve damage), persistent abdominal pain (organ damage), oliguria or anuria (acute renal function injury) and unexplained abdominal pain in late pregnancy (uterine rupture) and timely intervention should be taken.
Due to individual discrepancies and complexity of diseases, adverse events are difficult to be completely avoided. Although minor adverse events may occur, the probability of serious complications in HIFU remains significant low. In recent years, machine learning models have been widely used in the medical field for prediction. Some prediction models can accurately predict the ablation dose [Citation95] and efficacy [Citation96] of HIFU, providing surgeons with more reasonable treatment plans, improving surgical safety and further reducing the incidence of AEs. In summary, we believe that HIFU is considerately a safe and effective therapeutic method and an ideal choice for patients who desire noninvasive treatment or have other surgical contraindications.
Nevertheless, our review has certain limitations, such as not including non-English literature, which may cause some deviation in the statistical results. On the other hand, different researchers may have slightly different comprehensions about complications, leading to lack of unified standards for the definition and description of AEs after HIFU [Citation97]. Overreporting or underreporting may cause some bias in statistical analysis of the data. Apart from that, the description of AEs in a few articles is very brief, and factors such as uncertain variation of follow-up time can also give some impact on results of this study. Due to the limitation of literature quantity, some AEs are only descriptive analyses and cannot provide accurate statistical information. Due to the lack of controlled studies in the meta-analysis of single group rates, some results exhibit significant heterogeneity and require careful reference. In the future, more large-scale, rigorously designed, multicenter clinical trials are hopefully carried out to furtherly validate these conclusions.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Yi Wen], upon reasonable request.
Additional information
Funding
References
- ter Haar G. Ultrasound focal beam surgery. Ultrasound Med Biol. 1995;21(9):1–15. doi: 10.1016/0301-5629(95)02010-1.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327. doi: 10.1038/nrc1591.
- Wang W, Liu WY, Zhou JM, et al. Preliminary clinical study of high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Chin J Ultrasound Imaging. 2002;11(3):161–163. (Chinese).
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76(909):590–599. doi: 10.1259/bjr/17150274.
- Jenne JW, Preusser T, Günther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z Med Phys. 2012;22(4):311–322. doi: 10.1016/j.zemedi.2012.07.001.
- Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi: 10.1080/02656730601186138.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284. doi: 10.3109/02656736.2014.996790.
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181–1188. doi: 10.1007/s00404-017-4548-9.
- Tsai MC, Chang LT, Tam KW. Comparison of high-intensity focused ultrasound and conventional surgery for patients with uterine myomas: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(10):1712–1724. doi: 10.1016/j.jmig.2021.06.002.
- Liu L, Wang T, Lei B. High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis. Eur Radiol. 2022; 32(2):1195–1204. doi: 10.1007/s00330-021-08156-6.
- Xiao X, Feng Z, Li T, et al. Comparing the efficacy and safety of high-intensity focused ultrasound and uterine artery embolization in caesarean scar pregnancy: a meta-analysis. Adv Ther. 2019;36(6):1314–1325. doi: 10.1007/s12325-019-00959-w.
- Liu Y, Wang L, Zhu X. Efficacy and safety of high-intensity focused ultrasound compared with uterine artery embolization in cesarean section pregnancy: a meta-analysis. J Minim Invasive Gynecol. 2023;30(6):446–454. doi: 10.1016/j.jmig.2023.02.021.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61. doi: 10.1080/02656736.2018.1473894.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676. doi: 10.1016/j.ultsonch.2015.05.031.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133.
- Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019.
- Jin X, Feng Y, Zhu R, et al. Temperature control and intermittent time-set protocol optimization for minimizing tissue carbonization in microwave ablation. Int J Hyperthermia. 2022;39(1):868–879. doi: 10.1080/02656736.2022.2075041.
- Fan X, Hynynen K. Ultrasound surgery using multiple sonications–treatment time considerations. Ultrasound Med Biol. 1996;22(4):471–482. doi: 10.1016/0301-5629(96)00026-9.
- Martin NA, Falder S. A review of the evidence for threshold of burn injury. Burns. 2017;43(8):1624–1639. doi: 10.1016/j.burns.2017.04.003.
- Leon-Villapalos J, Kaniorou-Larai M, Dziewulski P. Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns. 2005;31(8):1054–1055. doi: 10.1016/j.burns.2005.04.019.
- Lin Z, Gong C, Huang Q, et al. A comparison of results following the treatment of placenta accreta and placenta increta using high-intensity focused ultrasound followed by hysteroscopic resection. Int J Hyperthermia. 2021;38(1):576–581. doi: 10.1080/02656736.2021.1909149.
- Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(4):1–86.
- Yin N, Hu L, Xiao Z-B, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34(8):1298–1303. doi: 10.1080/02656736.2018.1433880.
- Zhao WP, Chen JY, Chen WZ. Effect of abdominal liposuction on sonographically guided high-intensity focused ultrasound ablation. J Ultrasound Med. 2014;33(9):1539–1544. doi: 10.7863/ultra.33.9.1539.
- Zaher S, Gedroyc W, Lyons D, et al. A novel method to aid in the visualisation and treatment of uterine fibroids with MRgFUS in patients with abdominal scars. Eur J Radiol. 2010;76(2):269–273. doi: 10.1016/j.ejrad.2009.07.004.
- Zhu Y, Keserci B, Viitala A, et al. Volumetric MR-guided high-intensity focused ultrasound ablation to treat uterine fibroids through the abdominal scars using scar patch: a case report. J Ther Ultrasound. 2016;4(1):20. doi: 10.1186/s40349-016-0064-9.
- Pisanie JLD, Commander CW, Burke CT. Management of postprocedural uterine artery embolization pain. Semin Intervent Radiol. 2021;38(5):588–594. doi: 10.1055/s-0041-1739161.
- Fan H-J, Zhang C, Lei H-T, et al. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine. 2019;98(10):e14566. doi: 10.1097/MD.0000000000014566.
- Yu J-W, Yang M-J, Jiang L, et al. Factors influencing USgHIFU ablation for adenomyosis with NPVR ≥ 50. Int J Hyperthermia. 2023;40(1):2211753. doi: 10.1080/02656736.2023.2211753.
- Kim Y-S, Kim T-J, Lim HK, et al. Preservation of the endometrial enhancement after magnetic resonance imaging-guided high-intensity focused ultrasound ablation of submucosal uterine fibroids. Eur Radiol. 2017;27(9):3956–3965. doi: 10.1007/s00330-017-4765-4.
- Xu J, Tang W, Lin L, et al. Post treatment and 3 month contrast enhanced MRI findings following HIFU of submucosal fibroids: a retrospective study. Int J Hyperthermia. 2023;40(1):2216897. doi: 10.1080/02656736.2023.2216897.
- Tinelli A, Malvasi A, Hurst BS, et al. Surgical management of neurovascular bundle in uterine fibroid pseudocapsule. JSLS. 2012;16(1):119–129. doi: 10.4293/108680812x13291597716302.
- Fernandez H. Update on the management of menometrorrhagia: new surgical approaches. Gynecol Endocrinol. 2011;27 Suppl 1:1131–1136. Erratum in: Gynecol Endocrinol. 2012;28(2):156. doi: 10.3109/09513590.2011.634261.
- Ruonan L, Leilei Y, Xiaoli J, et al. The efficacy of high-intensity focused ultrasound combined with endometrial ablation in the treatment of adenomyosis with excessive menstruation. J Pract Med. 2023;39(23):3093–3100. Chinese.
- Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100(5 Pt 1):873–880. doi: 10.1016/s0029-7844(02)02341-4.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251. doi: 10.1016/j.ejogrb.2014.09.039.
- Xie B, Zhang C, Xiong C, et al. High intensity focused ultrasound ablation for submucosal fibroids: a comparison between type I and type II. Int J Hyperthermia. 2015;31(6):593–599. doi: 10.3109/02656736.2015.1046406.
- Mao SH, Tan XY, Wang J, et al. Adverse reactions and preventive measures of ultrasound ablation for adenomyosis. J Clin Ultrasound Med. 2012;14(09):634–636. Chinese. doi: 10.16245/j.cnki.issn1008-6978.2012-09.032.
- Thiburce AC, Frulio N, Hocquelet A, et al. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015;31(7):764–770. doi: 10.3109/02656736.2015.1063169.
- Zhao Y, Luo S, Liu Y, et al. High intensity focused ultrasound treatment for adenomyosis: comparison of efficacy based on MRI features. Int J Hyperthermia. 2023;40(1):2197574. doi: 10.1080/02656736.2023.2197574.
- He S, Xue M, Jiang J. Early versus late hysteroscopic resection after high-intensity focused ultrasound for retained placenta accreta. Int J Hyperthermia. 2021;38(1):257–262. doi: 10.1080/02656736.2021.1887943.
- He G-B, Luo W, Zhou X-D, et al. A preliminary clinical study on high-intensity focused ultrasound therapy for tubal pregnancy. Scott Med J. 2011;56(4):214–219. doi: 10.1258/smj.2011.011161.
- Liu X, Dong X, Mu Y, et al. High-intensity focused ultrasound (HIFU) for the treatment of uterine fibroids: does HIFU significantly increase the risk of pelvic adhesions? Int J Hyperthermia. 2020;37(1):1027–1032. doi: 10.1080/02656736.2020.1811903.
- Lai THT, Seto MTY, Cheung VYT. Intrapartum uterine rupture following ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroid and adenomyosis. Ultrasound Obstet Gynecol. 2022;60(6):816–817. doi: 10.1002/uog.24983.
- Wu G, Li R, He M, et al. A comparison of the pregnancy outcomes between ultrasound-guided high-intensity focused ultrasound ablation and laparoscopic myomectomy for uterine fibroids: a comparative study. Int J Hyperthermia. 2020;37(1):617–623. doi: 10.1080/02656736.2020.1774081.
- Zhou CY, Xu XJ, He J. [Pregnancy outcomes and symptom improvement of patients with adenomyosis treated with high intensity focused ultrasound ablation]. Zhonghua Fu Chan Ke Za Zhi. 2016;51(11):845–849. Chinese. doi: 10.3760/cma.j.issn.0529-567X.2016.11.009.
- Huang YF, Deng J, Wei XL, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with High-Intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia. 2020;37(1):301–307. doi: 10.1080/02656736.2020.1742390.
- Liu Y, Fu N, Lv B, et al. Uterine rupture after high-intensity focused ultrasound ablation of adenomyosis: a case report and literature review. Int J Hyperthermia. 2023;40(1):2212885. doi: 10.1080/02656736.2023.2212885.
- Zhang W, He M, Huang G, et al. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int J Hyperthermia. 2016;32(6):623–629. doi: 10.1080/02656736.2016.1191680.
- Gong C, Wang Y, Lv F, et al. Evaluation of high intensity focused ultrasound treatment for different types of adenomyosis based on magnetic resonance imaging classification. Int J Hyperthermia. 2022;39(1):530–538. doi: 10.1080/02656736.2022.2052366.
- Jiang L, Yu J-W, Yang M-J, et al. Ultrasound-guided HIFU for uterine fibroids of hyperintense on T2-weighted MR imaging with or without GnRH-analogue-pretreated: a propensity score matched cohort study. Front Surg. 2022;9:975839. doi: 10.3389/fsurg.2022.975839.
- Cun J-P, Fan H-J, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2019;36(1):21–28. doi: 10.1080/02656736.2018.1528391.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;127(11):1422–1428. doi: 10.1111/1471-0528.16262.
- Jindal S, Jung J, Lee K, et al. High-intensity focused ultrasound for the treatment of fibroids: a single-center experience in Singapore. Gynecol Minim Invasive Ther. 2023;12(1):15–25. doi: 10.4103/gmit.gmit_102_22.
- Keserci B, Duc NM. The role of T1 perfusion-based classification in predicting the outcome of magnetic resonance-guided high-intensity focused ultrasound treatment of adenomyosis. Int J Hyperthermia. 2018;34(3):306–314. doi: 10.1080/02656736.2017.1326634.
- Philip C-A, Warembourg S, Dairien M, et al. Transrectal high-intensity focused ultrasound (HIFU) for management of rectosigmoid deep infiltrating endometriosis: results of Phase-I clinical trial. Ultrasound Obstet Gynecol. 2020;56(3):431–442. doi: 10.1002/uog.21937.
- Ko JKY, Seto MTY, Cheung VYT. Thermal bowel injury after ultrasound-guided high-intensity focused ultrasound treatment of uterine adenomyosis. Ultrasound Obstet Gynecol. 2018;52(2):282–283. doi: 10.1002/uog.18965.
- Lee J-S, Hong G-Y, Lee K-H, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221. doi: 10.1016/j.ultrasmedbio.2019.08.022.
- Choi H, Ryu DH, Lee JY, et al. High-intensity focused ultrasound (HIFU) for uterine myoma ablation caused incarcerated internal hernia. Indian J Surg. 2022;84(5):1120–1122. doi: 10.1007/s12262-021-03214-1.
- Park JW, Choi HY. Ventral hernia after high-intensity focused ultrasound ablation for uterine fibroids treatment: a case report. World J Clin Cases. 2022;10(30):11204–11209. doi: 10.12998/wjcc.v10.i30.11204.
- Zhao L, Deng Y, Wei Q, et al. Comparison of ultrasound-guided high-intensity focused ultrasound ablation and surgery for abdominal wall endometriosis. Int J Hyperthermia. 2018;35(1):528–533. doi: 10.1080/02656736.2018.1511836.
- Peng Y, Dai Y, Yu G, et al. Analysis of the type of cesarean scar pregnancy impacted on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment. Int J Hyperthermia. 2022;39(1):1449–1457. doi: 10.1080/02656736.2022.2107715.
- Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2(1):8–27. doi: 10.5306/wjco.v2.i1.8.
- Park MJ, Kim Y-S, Rhim H, et al. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol. 2014;25(2):231–239. doi: 10.1016/j.jvir.2013.11.011.
- Kim Y-S, Kim J-H, Rhim H, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: initial clinical outcomes. Radiology. 2012;263(2):600–609. doi: 10.1148/radiol.12111707.
- Jackson J, Davies P, Leggett N, et al. Systematic review of interventions for the prevention and treatment of postoperative urinary retention. BJS Open. 2019;3(1):11–23. doi: 10.1002/bjs5.50114.
- Pomajzl AJ, Siref LE. Postoperative urinary retention [Internet]. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Ker C-R, Ou K-Y, Long C-Y, et al. Acute renal insufficiency and thrombocytopenia after high-intensity focused ultrasound (HIFU) ablation for uterine myomas. Taiwan J Obstet Gynecol. 2020;59(4):594–597. doi: 10.1016/j.tjog.2020.05.021.
- Cheng C-Q, Zhang R-T, Xiong Y, et al. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine. 2015;94(16):e729. doi: 10.1097/MD.0000000000000729.
- Lee J-S, Hong G-Y, Park B-J, et al. Ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroid & adenomyosis: a single center experience from the Republic of Korea. Ultrason Sonochem. 2015;27:682–687. doi: 10.1016/j.ultsonch.2015.05.033.
- Barbar T, Jaffer Sathick I. Tumor lysis syndrome. Adv Chronic Kidney Dis. 2021;28(5):438.e1–446.e1. doi: 10.1053/j.ackd.2021.09.007.
- Kawabata T, Hiraki T, Iguchi T, et al. Post-ablation syndrome after percutaneous cryoablation of small renal tumors: a prospective study of incidence, severity, duration, and effect on lifestyle. Eur J Radiol. 2020;122:108750. doi: 10.1016/j.ejrad.2019.108750.
- Leung JHY, Yu SCH, Cheung ECW, et al. Safety and efficacy of sonographically guided high-intensity focused ultrasound for symptomatic uterine fibroids: preliminary study of a modified protocol. J Ultrasound Med. 2014;33(10):1811–1818. doi: 10.7863/ultra.33.10.1811.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364. doi: 10.1111/1471-0528.14689.
- Li X, Zhu X, He S, et al. High-intensity focused ultrasound in the management of adenomyosis: long-term results from a single center. Int J Hyperthermia. 2021;38(1):241–247. doi: 10.1080/02656736.2021.1886347.
- Johnson BZ, Stevenson AW, Barrett LW, et al. Platelets after burn injury - hemostasis and beyond. Platelets. 2022;33(5):655–665. doi: 10.1080/09537104.2021.1981849.
- Hesley GK, Gorny KR, Henrichsen TL, et al. A clinical review of focused ultrasound ablation with magnetic resonance guidance: an option for treating uterine fibroids. Ultrasound Q. 2008;24(2):131–139. doi: 10.1097/RUQ.0b013e31817c5e0c.
- Gorny KR, Woodrum DA, Brown DL, et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol. 2011;22(6):857–864. doi: 10.1016/j.jvir.2011.01.458.
- Li Y, Hua C. Is high-intensity focused ultrasound superior to uterine artery embolization in cesarean scar pregnancy and subsequent pregnancy outcomes? A meta-analysis of the Chinese population. J Minim Invasive Gynecol. 2023;30(3):180–191. doi: 10.1016/j.jmig.2022.11.015.
- Gao H, Li T, Fu D, et al. Uterine artery embolization, surgery and high intensity focused ultrasound in the treatment of uterine fibroids: a network meta-analysis. Quant Imaging Med Surg. 2021;11(9):4125–4136. doi: 10.21037/qims-20-1331.
- O’Sullivan AK, Thompson D, Chu P, et al. Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care. 2009;25(1):14–25. doi: 10.1017/S0266462309090035.
- Lozinski T, Filipowska J, Pyka M, et al. Magnetic resonance-guided high-intensity ultrasound (MR-HIFU) in the treatment of symptomatic uterine fibroids - five-year experience. Ginekol Pol. 2021. doi: 10.5603/GP.a2021.0098.
- Liu Y, Ran W, Shen Y, et al. High-intensity focused ultrasound and laparoscopic myomectomy in the treatment of uterine fibroids: a comparative study. BJOG. 2017;124 Suppl 3:36–39. doi: 10.1111/1471-0528.14745.
- Pinto I, Chimeno P, Romo A, et al. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment–a prospective, randomized, and controlled clinical trial. Radiology. 2003;226(2):425–431. doi: 10.1148/radiol.2262011716.
- Toor SS, Jaberi A, Macdonald DB, et al. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(5):1153–1163. doi: 10.2214/AJR.11.8362.
- Wang Y, Geng J, Bao H, et al. Comparative effectiveness and safety of high-intensity focused ultrasound for uterine fibroids: a systematic review and meta-analysis. Front Oncol. 2021;11:600800. doi: 10.3389/fonc.2021.600800.
- Kim CW, Shim HS, Jang H, et al. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016;206:172–176. doi: 10.1016/j.ejogrb.2016.09.001.
- Kröncke T. An update on uterine artery embolization for uterine leiomyomata and adenomyosis of the uterus. Br J Radiol. 2023;96(1143):20220121. doi: 10.1259/bjr.20220121.
- Qu K, Mao S, Li J, et al. The impact of ultrasound-guided high-intensity focused ultrasound for uterine fibroids on ovarian reserve. Int J Hyperthermia. 2020;37(1):399–403. doi: 10.1080/02656736.2020.1754473.
- Cheung VY, Lam TP, Jenkins CR, et al. Ovarian reserve after ultrasound-guided high-intensity focused ultrasound for uterine fibroids: preliminary experience. J Obstet Gynaecol Can. 2016;38(4):357–361. doi: 10.1016/j.jogc.2016.02.006.
- Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One. 2011;6(12):e26110. doi: 10.1371/journal.pone.0026110.
- Wenhao H, Yingang W, Fan X, et al. Study on a dose prediction model for high-intensity focused ultrasound (HlFU) ablation of uterine fibroids on machine learning. Chin J Ultrasound Med. 2023;39(11):1280–1283. Chinese.
- Yueming H, Jinwen L, Yingying Q, et al. The value of machine learning models based on MRl images in predicting the efficacy of uterine fibroids after HlFU ablation surgery. Mod Oncol Med. 2024;32(06):1093–1100. Chinese.
- Kociuba J, Łoziński T, Zgliczyńska M, et al. Adverse events and complications after magnetic resonance-guided focused ultrasound (MRgFUS) therapy in uterine fibroids - a systematic review and future perspectives. Int J Hyperthermia. 2023;40(1):2174274. doi: 10.1080/02656736.2023.2174274.