Abstract
Background
The greater omentum is routinely resected during cytoreductive surgery (CRS), but few studies have analyzed the rationale behind this. This study aimed to assess the prevalence of omental metastases (OM) and the correlation between macroscopically suspected and microscopically confirmed OM, in patients with pseudomyxoma peritonei (PMP) or colorectal peritoneal metastases (PM).
Method
All patients without previous omentectomy, treated with initial CRS and hyperthermic intraperitoneal chemotherapy for PMP or colorectal PM, at Uppsala University Hospital in 2013–2021, were included. Macroscopic OM in surgical reports was compared with histopathological analyses.
Results
In all, 276 patients were included. In those with PMP, 112 (98%) underwent omentectomy and 67 (59%) had macroscopic suspicion of OM. In 5 (4%) patients, the surgeon was uncertain. Histopathology confirmed OM in 81 (72%). In patients with macroscopic suspicion, 96% had confirmed OM (positive predictive value, PPV). In patients with no suspicion, 24% had occult OM (negative predictive value, NPV = 76%). In patients with colorectal PM, 156 (96%) underwent omentectomy and 97 (60%) had macroscopic suspicion. For 5 (3%) patients, the surgeon was uncertain. OM was microscopically confirmed in 90 (58%). PPV was 85% and NPV was 89%. The presence of OM was a univariate risk factor for death in PMP (HR 3.62, 95%CI 1.08–12.1) and colorectal PM (HR 1.67, 95%CI 1.07–2.60), but not in multivariate analyses.
Conclusion
OM was common and there was a high risk of missing occult OM in both PMP and colorectal PM. These results support the practice of routine omentectomy during CRS.
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is considered a curative treatment for pseudomyxoma peritonei (PMP) and colorectal peritoneal metastases (PM) if complete cytoreduction can be achieved [Citation1–3]. PM arise from malignant cells released into the peritoneal cavity from a primary tumor and spread through a redistribution phenomenon [Citation4, Citation5]: The neoplastic cells float freely and circulate toward absorption sites below the right hemi-diaphragm and the greater omentum. This is followed by gravitational distribution to the pelvis and left paracolic gutter, and the ligament of Treitz, while peristaltic movements of the small intestines prevent adhesion until later stages of the disease. For this reason, the greater omentum is considered to be one of the first organs affected by PM and therefore often routinely resected during CRS [Citation6], while other resections are made only if the surgeon suspects tumor growth.
The greater omentum consists of two layers of peritoneum and is an absorption site for peritoneal fluid and solutes [Citation7]. This ‘abdominal policeman’ [Citation8] is also an immunocompetent organ with the ability to contain inflammation and limit the spread of infections. Neoplastic cells are thought to be absorbed into milky spots, a form of lymphoid tissue in the peritoneum that is enriched in the greater omentum [Citation9]. There, the tumor microenvironment affects growth and further spread [Citation7]. Despite this important role of the omentum in peritoneal metastasis, the evidence for routine omentectomy is not clear; nor is the surgeon’s ability to safely judge macroscopic tumor growth in the omentum. Beyond being an important organ in limiting infections, the greater omentum could contain peritoneal metastases, and be resectable in cases of repeated CRS, compared to generalized peritoneal disease.
The primary aim of this study was to investigate the correlation between macroscopically suspected and microscopically confirmed OM in patients with PMP or colorectal PM. A secondary aim was to analyze the prevalence and prognostic impact of OM.
Method
Study population
All patients treated with initial CRS and HIPEC for confirmed PMP or colorectal PM at Uppsala University Hospital, a Swedish center for surgical treatment of peritoneal surface malignancies, between 2013 and 2021, were eligible for inclusion. Patients with other primary tumors (mesothelioma, small bowel adenocarcinoma, or ovarian cancer), repeated CRS, open close/debulking surgery, patients with no macroscopic PM at exploration, and patients not receiving HIPEC were excluded. The following baseline clinical data were retrieved from medical records and radiology and histopathological reports: Age, sex, American Society of Anesthesiologists (ASA) classification [Citation10], primary tumor localization, histopathology, and oncological and surgical treatment information. The study was approved by the regional ethics committee (reference number 2013/203).
Macroscopic assessment
Macroscopic assessment of peritoneal tumor burden is routinely performed at the start of CRS and scored using the peritoneal cancer index (PCI) [Citation11]. The PCI quantifies the tumor load and is calculated by summing lesion size scores (range 0–3) in 13 different abdominal regions. The PCI score ranges between 1 and 39. The extent and distribution of PM are described at the beginning of the surgical report. Macroscopic suspicion of OM was based on these written descriptions in surgical reports, combined with the referral notes sent to the histopathological unit [Citation11].
CRS and HIPEC
After an initial intraoperative assessment of PCI and resectability, CRS is performed by resecting all disease-affected organs and peritoneal surfaces [Citation6]. The omentum is often resected even if there is no suspicion of OM, but the resection is done above or below the gastroepiploic arch depending on the extent of OM. Patients with PM involving the liver hilum, large vessels, extensive small bowel involvement, or with high PCI scores in colorectal PM, are not eligible for CRS and HIPEC, as complete cytoreduction is not possible. A completeness of cytoreduction score (CCS, range 0–3) [Citation11] of 0 is needed in colorectal PM, whereas CCS 0–1 is considered acceptable in PMP. In the present study, an uncertain CCS scoring, ‘0–1′, was classified as CCS 1. HIPEC was performed using the open Coliseum technique [Citation12, Citation13] at the start of the study period and using the closed technique after 2018 [Citation13]. The intraperitoneal chemotherapy used depended on the primary tumor and previous treatments, but mitomycin C was commonly used in PMP and oxaliplatin and/or irinotecan was commonly used in colorectal PM.
Microscopic assessment
Surgical specimens from CRS were fixed in 4% buffered formaldehyde and embedded in paraffin, and macroscopically suspected areas were sliced into 4 µm sections and stained with hematoxylin and eosin. CRS specimens are often voluminous and macroscopically normal tissue were examined by fewer random sections. The primary tumors and the PM were classified based on primary tumor localization, in accordance with the World Health Organization classification system for tumors of the digestive system [Citation14]. Appendiceal tumors were classified as low-grade appendiceal mucinous neoplasm, high-grade appendiceal mucinous neoplasm, mucinous adenocarcinoma, or mucinous signet ring adenocarcinoma. Colorectal primary tumors were classified as adenocarcinoma, mucinous adenocarcinoma, or signet ring cell carcinoma. During the study period, the Ronnett classification of PM [Citation15] was primarily used in Sweden, but the Peritoneal Surface Oncology Group International (PSOGI) classification was also introduced. For this study, the mucinous PM previously classified according to Ronnett were described using the PSOGI classification in mucinous carcinoma peritonei (MCP) G1–G3. Acellular mucin was considered as microscopic disease but reported separately [Citation16]. Patients were divided into those with PMP and those with colorectal PM. PMP included all patients with appendiceal PM except those with non-mucinous adenocarcinoma and non-mucinous PM. The colorectal PM group included all patients with colorectal primary tumors. The PM histopathology was described as adenocarcinoma, mucinous adenocarcinoma, or signet ring cell carcinoma.
Statistical analysis
Continuous data are presented as medians with interquartile ranges (IQRs). To analyze the ability of the surgeon to assess the omentum, positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity were calculated for patients with information on suspicion of OM and histopathological results. Overall survival (OS) was calculated using Kaplan-Meier curves and presented as the cumulative proportion surviving five years after CRS with 95% confidence intervals (CIs). Log-rank test were used to compare differences in OS. Patients were censored at the last follow-up. Follow-up data were retrieved on October 2022. Risk factors for death were analyzed with univariate and multivariate Cox proportional hazard regression analyses and presented as hazard ratios (HRs) with 95% CIs. All variables in the univariate analyses were included in the multivariate analysis, as they were all considered factors relevant for the survival analysis. A p-value of < 0.05 was considered statistically significant. R version 4.2.2 (R foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
Results
Study population
After excluding patients with other primary tumors (n = 35), repeated CRS (n = 18), open close/debulking surgery (n = 74), patients with no macroscopic PM at exploration, and patients not receiving HIPEC (n = 72), 303 patients were eligible for analysis. Of these, 27 patients had previously undergone omentectomy. Thus, 276 patients remained for the final analysis. The included patients were divided into a group with PMP (n = 114) and a group with colorectal PM (n = 162), which were analyzed separately. The baseline clinical characteristics of these patients are shown in .
Table 1. Baseline clinical characteristics of patients with pseudomyxoma peritonei and colorectal peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, 2013–2021.
Macroscopic assessment
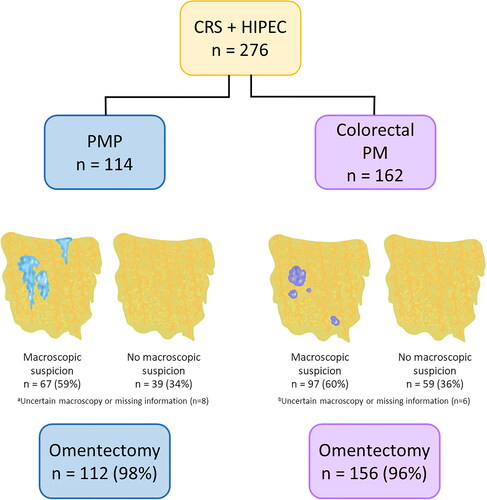
The surgeon registered OM in 67 (59%) PMP patients and 97 (60%) colorectal PM patients, based on macroscopic appearance. In five PMP patients and five colorectal PM patients, the surgeon expressed uncertainty regarding if there was OM or not. For three of the PMP patients and one of the colorectal PM patients there was no information on macroscopic suspicion. Omentectomy was performed in 112 (98%) and 156 (96%) of the PMP and colorectal PM patients, respectively. All patients with macroscopic suspicion, uncertainty, or missing information underwent resection ().
Figure 1. Frequency of macroscopic suspicion of omental metastases and omentectomy in patients with pseudomyxoma peritonei (PMP) or colorectal peritoneal metastases (PM) treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
aFor five patients, the surgeon was uncertain, and for three patients, information was missing.
bFor five patients, the surgeon was uncertain, and for one patient, information was missing.
Histopathology PMP
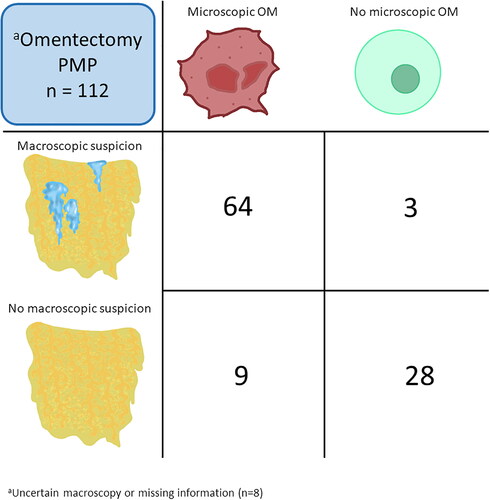
A total of 81 (72%) of the patients with PMP who underwent omentectomy had microscopically confirmed OM. In patients with information on macroscopic suspicion, OM was confirmed in 64 of 67 (96%), yielding the PPV. Occult OM was present in 9 of 37 (24%) patients. Thus, they were incorrectly judged as having no OM. This yielded a NPV of 76%. Of those 9 patients, 7 had acellular mucin only in the omentum, whereas two had neoplastic cells in other specimens, corresponding to a PSOGI MCP 1/2. The sensitivity was calculated to 88% and the specificity to 90% (). Absence of information on macroscopic suspicion was seen in five patients where the surgeon expressed uncertainty and three patients with missing macroscopy information. These patients all had microscopically confirmed OM.
Figure 2. Cross-tabulation Of macroscopic suspicion of omental metastases (OM) versus microscopically confirmed OM in patients with pseudomyxoma peritonei (PMP) who underwent omentectomy.
aFor eight patients, information on macroscopy or microscopy was lacking, and these patients were not included in the cross-tabulation.
The histopathology of peritoneal disease in patients with PMP is presented in . Five patients with a benign omentum had no microscopic evidence of peritoneal disease but had a PCI score ≥1. A total of 23 (21%) patients had acellular mucin but no neoplastic epithelium. All patients with PSOGI G3 (signet ring cell differentiation) had OM. Patients with OM more often had CCS 1 and higher PCI scores. In patients with PCI ≥20 (n = 51), all but one patient had OM and in patients with PCI <10 (n = 35), 13 (37%) had OM.
Table 2. Clinical and histopathological characteristics of pseudomyxoma peritonei patients with or without confirmed omental metastases.
Histopathology colorectal PM
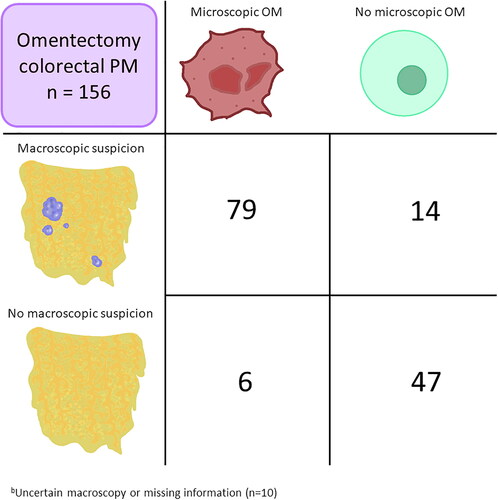
Ninety (58%) patients had microscopically confirmed OM. Patients with information on macroscopic suspicion and histopathological results are shown in . OM was confirmed in 79 of 93 (85%, PPV) patients with macroscopic suspicion, whereas histopathological information was lacking for four patients. Six of 53 (11%) patients were incorrectly judged as having a benign omentum (NPV = 89%). The sensitivity and specificity were 93% and 77%, respectively. Four of five patients with macroscopic uncertainty had confirmed OM, whereas information was missing for one. The patient with missing information on the surgeon’s suspicion had confirmed OM.
Figure 3. Cross-tabulation Of macroscopic suspicion of omental metastases (OM) versus microscopically confirmed OM in patients with colorectal peritoneal metastases (PM) who underwent omentectomy.
aFor ten patients, information on macroscopy or microscopy was lacking, and these patients were not included in the cross-tabulation.
shows the histopathology of the peritoneal disease in patients with colorectal PM. Adenocarcinoma and mucinous adenocarcinoma were most common. No neoplastic epithelium was found for nine patients when specimens from CRS were analyzed. OM tended to be more common in patients with high PCI but 26 (41%) of patients with PCI <10 had OM.
Table 3. Clinical and histopathological characteristics of colorectal peritoneal metastasis patients with or without confirmed omental metastasis.
Survival
Patients with PMP who had confirmed OM had a 5-year OS of 67% (95% CI 56–81), compared with 97% (95% CI 90–100) in those without OM (p = 0.03). Colorectal PM patients generally had a poorer prognosis and OM was associated with an even poorer prognosis, with a 5-year OS of 14% (95% CI 7–28) compared with 41% (29–58, p = 0.02) in patients without OM (). In a Cox proportional hazard regression model, OM was a univariate risk factor for death in both PMP (HR 3.62, 95% CI 1.08–12.1, ) and colorectal PM (HR 1.67, 95% CI 1.07–2.60, ). In the multivariate analyses, OM was not an independent risk factor for death. Peritoneal disease judged as G3 MCP (signet ring cells) was associated with death in PMP, and high PCI scores and higher ASA-classification were associated with an increased risk of death in colorectal PM. Mucinous histopathology was associated with better prognosis in colorectal PM.
Figure 4. a. Overall survival for patients with pseudomyxoma peritonei with or without omental metastases (OM + vs. OM-). b. Overall survival for patients with colorectal peritoneal metastases and OM + or OM.
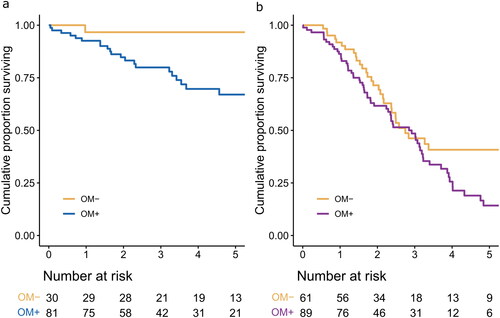
Table 4. Univariate and multivariate cox proportional regression hazard analysis for risk of death in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Table 5. Univariate and multivariate cox proportional regression hazard analysis for risk of death in patients with colorectal peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Of the eight patients who did not undergo omentectomy, all but one had recurrence. The majority had peritoneal recurrence, although one had pleural metastases, one had liver metastases, and one had skeletal metastases. The patient without recurrence had PSOGI MCP G1.
Discussion
This study showed that omentectomy was performed in more than 95% of the patients treated with CRS and HIPEC for PM. Occult OM were not uncommon (24% in PMP, 11% in colorectal PM), which strengthens that omentectomy should be routinely performed as part of CRS.
The prevalence of OM was slightly higher in PMP compared with in colorectal PM (72% vs. 58%), but in PMP, the neoplastic finding was acellular mucin in 19% of cases. Nors et al. routinely resected the omentum, reporting OM in 65% of PMP patients (44% acellular mucin), and 44% of colorectal PM patients [Citation17]. Notably, the reported prevalence of OM in colorectal PM varies widely, between 20% and 80% [Citation5, Citation17–20].
The removal of disease-affected organs and peritoneal surfaces is dependent on the surgeon’s ability to judge the extent and distribution of tumor growth. In our study, when a surgeon suspected OM in PMP patients, they were right in 96% of cases. In colorectal PM, suspicions were correct in 85% of cases. The difference between PMP and colorectal PM can be explained by mucin being easier to recognize than non-mucinous nodules.
Most importantly, the surgeon needs to safely clear the organs and peritoneal surfaces from PM involvement. In PMP patients, occult OM was found in 24%; the majority had acellular mucin. The role of acellular mucin in PMP remains unclear. The prognosis is similar to the in patients without peritoneal involvement [Citation21], and acellular mucin should, therefore, be separated from PSOGI MCP G1 [Citation16]. However, acellular mucin is still considered an indirect sign of peritoneal surface involvement, and neoplastic cells are sometimes found upon repeated examination [Citation22]. In this study, two patients had acellular mucin in the greater omentum, but PSOGI MCP G1/G2 at other sites. Occult OM was also found in some colorectal PM patients (11%). None of them had acellular mucin. This is a slightly lower proportion than has been reported previously [Citation17–20].
The high incidence of occult OM strengthens the argument for routine omentectomy. In addition, OM was not uncommon in patients with limited tumor burden (PCI <10), thus avoiding routine omentectomy in these patients cannot be recommended. Potential disadvantages of omentectomy have not been clearly reported. On the contrary, Khan et al. reported no increase in postoperative morbidity [Citation20], and in a randomized trial, delayed gastric emptying was not worse after resection above the gastroepiploic arch [Citation23]. In addition, animal studies report that the omentum traps neoplastic cells in a pro-tumorigenic environment, promoting disease progression [Citation7]. On the other hand, this first site of entrapment of cancer cells could offer a window of opportunity for limited resection compared with general intraperitoneal dissemination, for example in the case of repeat CRS and HIPEC. However, this potential benefit has not been studied in this context, but could be relevant as repeat CRS and HIPEC is a feasible option for recurring PM.
OM was associated with poorer survival in both PMP and colorectal PM, but was not an independent predictor of survival in a multivariate model. Instead, the well-known risk factors for poor outcome – PCI score and signet ring cells – were predictors. OM has been associated with high PCI scores in several studies [Citation18–20], strengthening the argument that the greater omentum plays an important role in PM.
This study has some noteworthy limitations. First, the macroscopic assessments were retrospectively collected from surgical reports and there was a risk of misinterpreting the reports. Sometimes, information was missing. To clarify vague descriptions, histopathological referral notes and PCI protocols were used for confirmation. Second, omentectomy was not routinely performed in all patients, and resections were done both above and below the gastroepiploic arch. However, > 95% of patients underwent resection. Third, histopathological examination is challenging because of large-volume specimens and there is a risk of overlooking neoplastic cells.
In conclusion, OM is common and the risk of missing occult OM in both PMP and colorectal PM argues in favor of routine omentectomy as part of CRS, unless any clear disadvantages of omentectomy are demonstrated.
Disclosure statement
No potential competing interest was reported by the authors.
Data availability statement
The data presented in this study are note publically available according to the ethics committee as the data contains personal information.
Additional information
Funding
References
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):1–8. doi: 10.1245/s10434-008-9966-2.
- Huang C-Q, Min Y, Wang S-Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–55683. doi: 10.18632/oncotarget.17497.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166.
- Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219(2):109–111. doi: 10.1097/00000658-199402000-00001.
- Sugarbaker PH, Chang D. Anatomic sites of disease in colorectal cancer patients recorded at the time of cytoreductive surgery for peritoneal metastases. Eur J Surg Oncol. 2022;48(5):946–955. doi: 10.1016/j.ejso.2022.01.012.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004.
- Koppe MJ, Nagtegaal ID, de Wilt JH, et al. Recent insights into the pathophysiology of omental metastases. J Surg Oncol. 2014;110(6):670–675. doi: 10.1002/jso.23681.
- Morison R. Remarks on some functions of the omentum. Br Med J. 1906;1(2350):76–78. doi: 10.1136/bmj.1.2350.76.
- Hagiwara A, Takahashi T, Sawai K, et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993;53(3):687–692.
- Wolters U, Wolf T, Stützer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217–222. doi: 10.1093/bja/77.2.217.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23.
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6(8):790–796. doi: 10.1007/s10434-999-0790-0.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;2(2):68–75. doi: 10.4251/wjgo.v2.i2.68.
- Bosman F, Carniero F, Hruban R, et al. World Health Organisation classification of tumours of the digestive system. Lyon: IARC Press; 2010.
- Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei. Am J Surg Pathol. 1995;19(12):1390–1408. doi: 10.1097/00000478-199512000-00006.
- Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016;40(1):14–26. doi: 10.1097/PAS.0000000000000535.
- Nors J, Iversen LH, Nielsen K, et al. Peritoneal metastases found in routinely resected specimens after cytoreductive surgery and heated intraperitoneal chemotherapy. Eur J Surg Oncol. 2022;48(4):795–802. doi: 10.1016/j.ejso.2021.12.026.
- Bhatt A, Yonemura Y, Mehta S, et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur J Surg Oncol. 2020;46(4 Pt A):582–589. doi: 10.1016/j.ejso.2019.11.495.
- Bonnefoy I, Mohamed F, Bonnot P-E, et al. Risk of omental metastases in patients undergoing cytoreductive surgery for colorectal peritoneal metastases. Dis Colon Rectum. 2020;63(9):1251–1256. doi: 10.1097/DCR.0000000000001670.
- Khan S, Doan N-H, Hosseini M, et al. Is routine omentectomy a necessary component of cytoreductive surgery and HIPEC? Ann Surg Oncol. 2023;30(2):768–773. doi: 10.1245/s10434-022-12714-7.
- Enblad M, Birgisson H, Wanders A, et al. Importance of absent neoplastic epithelium in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(4):1149–1156. doi: 10.1245/s10434-015-4989-y.
- Al-Azzawi M, Misdraji J, van Velthuysen M-LF, et al. Acellular mucin in pseudomyxoma peritonei of appendiceal origin: what is adequate sampling for histopathology? J Clin Pathol. 2020;73(4):220–222. doi: 10.1136/jclinpath-2019-206213.
- Evers DJ, Smeenk RM, Bottenberg PD, et al. Effect of preservation of the right gastro-epiploic artery on delayed gastric emptying after cytoreductive surgery and HIPEC: a randomized clinical trial. Eur J Surg Oncol. 2011;37(2):162–167. doi: 10.1016/j.ejso.2010.12.005.
