Abstract
Objective
The purpose of this systematic review and meta-analysis was to assess the clinical efficacy and safety of ultrasound (US)-guided high intensity focused ultrasound (HIFU) in the treatment of breast fibroadenoma in different studies.
Methods
Studies evaluating the efficacy and safety of US-guided HIFU in the treatment of histologically-proven FA with follow-up outcomes of more than 3 months were searched through MEDLINE/PubMed databases. Volume reduction rate (VRR) and side effects were extracted and compared for further analysis.
Results
Of 29 identified articles, 10 studies involving 385 women and more than 545 FAs met the inclusion criteria. The mean VRR at 6 months and 12 months after HIFU was 52.00% and 72.00%. In terms of intraoperative safety, nine studies reported mild to moderate pain, with an average visual analogue scale (VAS) score ranging from 1.60 to 7.10. The most common postoperative side effect associated with HIFU was subcutaneous ecchymosis and less frequent were pain, erythema, and skin pigmentation, most of which disappeared within weeks. No serious side effects were observed.
Conclusion
S-guided HIFU is an effective and safe noninvasive treatment for breast FA that does not cause serious side effects. Further studies are needed to explore crucial influencing factors of VRR.
1. Introduction
Fibroadenoma (FA) is one of the most common benign breast tumors in adolescent females which accounts for approximately two thirds of all breast tumors and more than half of all biopsied breast lesions. It is most commonly found in women between the age of 14-35 [Citation1]. Since FA is associated with increased sensitivity of breast tissue to estrogen, the incidence decreases as age increases, and it is rarely seen in postmenopausal women [Citation2]. Ultrasound imaging and core-needle biopsy are common methods of diagnosing FA. Although the growth of FA is usually slow and there is an extremely low rate of malignant transformation (0.02-0.125%) [Citation3], it can bring psychological pressure and cause cosmetic concerns in patients due to its superficial location and symptoms caused by multiple FAs [Citation4, Citation5]. Therefore, instead of regular physical examination and ultrasonography for observation only, active clinical intervention is recommended for symptomatic and psychologically burdened patients.
Currently, the treatment of FA includes open surgical resection, vacuum-assisted mammotomy (VAM), and energy ablation (including cryoablation and thermal ablation) [Citation6]. Open surgical resection can completely remove FA, but it may lead to scarring, breast deformity, injury to the mammary ducts, and postoperative complications such as postoperative hematomas, infections, and even abscesses [Citation7]. In addition, multiple incisions or extended incisions are required to remove multiple FAs, which may cause terrible cosmetic issues that would not be accepted by young female patients. VAM with a small skin incision of about 3-5 mm is used as a cosmetic surgery for FA [Citation8]. However, repeated cuts and suction of the lesion may cause some complications, such as inevitable internal injury, residual tumor, loss of surrounding normal breast tissue, postoperative hematoma, and pain. For multiple lesions, a larger number of skin incisions and disposable vacuum probes may also be unacceptable to patients for cosmesis and cost reasons.
Therefore, minimally invasive and noninvasive energy ablation, including radiofrequency ablation (RA), microwave ablation (MWA), laser ablation (LA), cryoablation, high intensity focused ultrasound ablation (HIFU), could become the most suitable choice for patients with FA, among which HIFU is the only noninvasive technique [Citation9]. The concept of HIFU was first proposed by Lynn in the 1940s [Citation10]. Its principle is to focus ultrasound energy from an extracorporeal transducer to the target tissue and rapidly heat the target tissue to more than 60 °C within 1s, resulting in protein degeneration and irreversible coagulation necrosis, while the surrounding normal tissue and skin are not damaged. HIFU has been successfully used to treat various solid tumors such as uterine fibroids, prostate cancer, and bone tumors [Citation11]. In 2015, Cavallo B et al. used ultrasound (US)-guided HIFU to treat FA for the first time [Citation12]. They demonstrated that HIFU can safely and effectively achieve satisfactory ablation of benign breast lesions. In recent years, some studies have also confirmed the efficacy and safety of HIFU in the treatment of FA. Due to its advantages of noninvasiveness, no risk of bleeding, satisfactory cosmetic effect, short hospital stay, and short recovery time, HIFU is increasingly used to treat FA. This systematic review and meta-analysis aimed to investigate the research and clinical application of HIFU in the treatment of FA, mainly to evaluate and analyze the efficacy and safety in different reports, and tried to find the potential factors affecting the efficacy.
2. Materials and methods
2.1. Search strategy
A systematic review of the literature was performed by searching MEDLINE/PubMed databases to identify all studies published up to August 2023 that evaluated the role of HIFU in the treatment of FA. The medical subject heading (MeSH) search terms used were: ‘high-intensity focused ultrasound’, ‘HIFU’, ‘focused ultrasound surgery’, ‘FUS’, ‘focused ultrasound ablation surgery’, or ‘FUAS’, all in combination with ‘breast fibroadenoma’ or ‘fibroadenoma’. The search was restricted to reports in the English language and human subjects; there were no further restrictions. The related articles from the original search were used to broaden the search range, and all abstracts, studies, and citations obtained were reviewed. References to the articles acquired were also manually searched. The last search was conducted on 31 August 2023.
2.2. Study selection
This review was performed in accordance with the Cochrane Handbook and reported following the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) guidelines [Citation13].
The inclusion criteria were papers that reported follow-up outcomes of more than 3 months or safety outcomes on patients with histologically-proven FA, who had undergone US-guided HIFU without other treatment. Studies with fewer than ten patients were not included. Studies of patients with synchronous cancer were excluded from the analysis. Systematic reviews were used to cross-check references, but were excluded from this review. Case reports, conference abstracts, editorials, letters, and animal studies were excluded. The studies with unavailable full text were also excluded. Papers published in English were reviewed by two investigators to meet the inclusion criteria. One author reviewed the titles and abstracts of all the papers according to the eligibility criteria, and the two authors independently reviewed the full text of the papers.
2.3. Data extraction
For the selected studies, we extracted the following data from the articles: first author, publication time, number of patients, age, volume of lesions, ablation mode, acoustic power, treatment energy, treatment time, pain score during treatment, side effects, duration of follow-up, volume reduction rate (VRR), patient satisfaction. The VRR and side effects were also extracted and pooled for further meta-analysis.
2.4. Assessment of risk of bias
The quality of the studies was assessed in accordance with the 2011 Oxford Center for Evidence-Based Medicine (OCEBM) Levels of Evidence [Citation14]. Bias was analyzed using the Methodological Index for Non-Randomized Studies (MINORS) tool for non-randomized studies of interventions [Citation15]. MINORS, which contains 12 items, is commonly used in the assessment of non-randomized controlled trials, particularly of trials exploring surgical interventions. There are 2 points for each item, with 24 points in total. In the scoring system of MINORS, 0-8 points are classified as low-quality literature, 9-16 points as medium-quality literature, and 17-24 points as high-quality literature. If the score of one reported study is less than 12, it should be excluded from the analysis. In this systematic review, two researchers scored the papers independently. If there were discrepancies in the scores, consensus was reached through discussion or consultation with a third researcher.
2.5. Statistical analysis
All extracted data were tabulated and presented as means ± standard deviations (SD), median and range or inter-quartile range, and percentages. To analyze the rate of volume reduction at 6 and 12 months after HIFU, a meta-analysis was conducted using a fixed-effect model. All statistical analyses were carried out in Stata version 17.0 (StataCorp LP, College Station, Texas, USA). P value of <0.05 was defined as statistical significance.
3. Results
3.1. Study selection
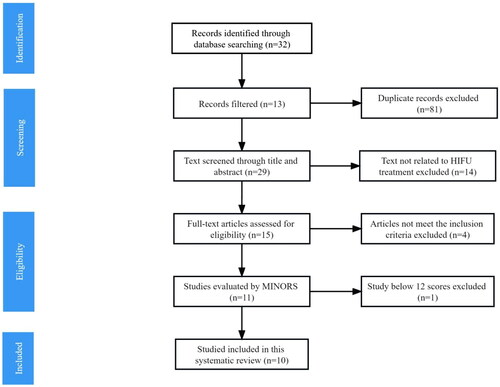
Using the criteria described above, a total of 26 articles published between 1999 and August 2023 were identified from the literature research (). Three additional articles were identified by searching for references of the selected articles. After reviewing the title and abstract, 14 articles were considered irrelevant and excluded, leaving 15 articles for full-text review. After reviewing the full text, another 4 articles were excluded for not meeting the inclusion criteria. In the end, since one article did not reach 12 scores on the MINORS scale after quality evaluation, a total of 10 articles were finally included in this systematic review [Citation16–25].
3.2. Study quality and risk of bias
According to the MINORS scale, the results of scores of 10 included articles were shown in . Data in all studies had been collected according to the objective of this meta-analysis. And follow-up period of each included article met the needs of this study to analyze post-treatment outcomes. However, control groups were set up in only 3 studies [Citation17, Citation19, Citation20], while the other 7 studies were single-arm research [Citation16, Citation18, Citation21–25]. The overall MINORS score of all the articles analyzed in this study was equal to or above 12, ranging between 12 and 16.
Table 1. Quality assessment of included non-randomized trials.
3.3. Inclusion and exclusion criteria
All studies had clear inclusion criteria. Patients more than 18 years old were included in 9 studies [Citation16–23, Citation25], while the other study enrolled patients older than 16 years [Citation24]. Histologically confirmed FA was essential in all studies [Citation16–25], two of which conducted core needle biopsy only in women over the age of 25 [Citation17, Citation20]. In 4 studies, FA was specified to be visible on ultrasound [Citation16–18, Citation20]. In 3 studies, the distance from the shallow margin of FA to the skin and the deep margin of FA to the pectoralis major muscle was required to > 5 mm and > 10 mm, respectively [Citation16, Citation18, Citation22]. Patients over 35 were asked to undergo additional mammograms [Citation16, Citation18, Citation22, Citation25]. Nine studies had clear exclusion criteria, none of which recruited pregnant or lactating women, and patients with ipsilateral breast malignancy or suspected malignancy [Citation16–18, Citation20–25]. Eight studies excluded patients with breast implants [Citation16–18, Citation20–22, Citation24, Citation25]. Previous ipsilateral breast physical ablation or radiation therapy would not meet the enrolled criteria in 5 studies [Citation16–18, Citation20, Citation21]. Other exclusion criteria included microcalcification in the lesion [Citation16, Citation18, Citation22], BI-RADS ≥ 4 [Citation25], and diameter of FA ≤ 1 cm [Citation17, Citation20].
3.4. Baseline
As shown in , a total of 385 patients, aged from 16 to 60 years, with no less than 545 lesions ranging in volume from 0.1 to 44.0 cm3 were ablated with HIFU in 10 included literature [Citation16–25]. In one study [Citation18], a second HIFU ablation was performed in patients with a VRR less than 50% or absolute volume more than 1.5 cm3 at 6 months after the first ablation session, and only data from the first ablation was extracted for comparison and further meta-analysis.
Table 2. Baseline features of studies using HIFU in breast fibroadenomas.
3.5. Treatment
As shown in , the Echopulse (Theraclion, France) was used in six of the studies [Citation16–18, Citation20–22], and the Haifu system (Chongqing Haifu Medical, China) was used in the latest two studies [Citation24, Citation25], whereas the other two studies did not explicitly describe the devices used for treatment [Citation19, Citation23]. Circumferential ablation was applied in 2 studies [Citation17, Citation20], and entire ablation was used in the remaining 8 studies [Citation16, Citation18, Citation19, Citation21–25]. The ultrasonic output power during treatment was clearly stated in 8 studies [Citation17, Citation18, Citation20–25], and the maximum power was 400 W [Citation25]. Seven studies described treatment energy, which had different measurement units [Citation17, Citation18, Citation20–22, Citation24, Citation25]. Eight studies described the duration of treatment, with average treatment time ranging from 9 min to 118 min [Citation16–18, Citation20–22, Citation24, Citation25].
Table 3. Treatment characteristics of each HIFU study.
3.6. Clinical efficacy and follow-up outcomes
As shown in , the follow-up time of 10 studies was at least 6 months [Citation16–25], and 9 studies were followed-up for more than 12 months [Citation16, Citation18–25]. VRR was used as a clinical efficacy evaluation index and reported in 9 studies [Citation16–18, Citation20–25]. At 12 months, the maximum average VRR of lesions was 84.80% [Citation21]. Three studies reported results related to patient satisfaction, ranging from 88.89% to 95.00% for symptom relief and 88.89% to 100.00% for cosmetic effects [Citation18, Citation21, Citation22].
Table 4. Outcomes for HIFU in each study.
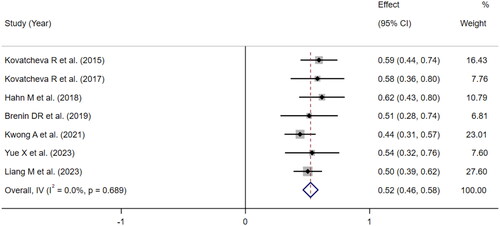
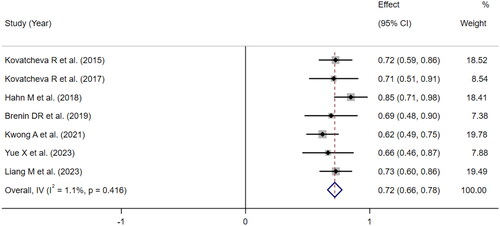
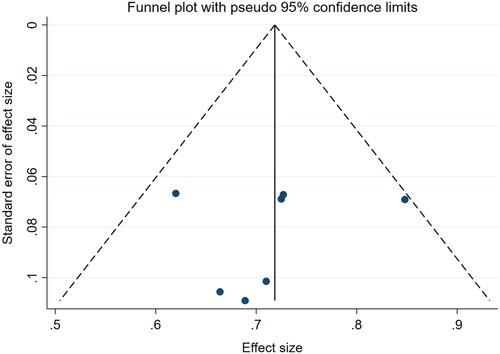
Meta-analysis was performed on the follow-up results of 9 articles that reported VRR to obtain the pooled effect size of tumor VRR at 6 and 12 months. Due to circumferential ablation used in two studies of Peek’s group, the ablation effects in these two articles were quite different from those in the other studies with entire ablation, resulting in significant heterogeneity in the analysis. Thus, these two studies were excluded from the meta-analysis. Finally, the effect size of the 7 articles was combined, and the results were shown in and . Based on the meta-analysis of fixed effects, the effect size of the 7 studies was 52.00% at 6 months and 72.00% at 12 months, and the 95% confidence interval was 46.00%-59.00% and 66.00-78.00%, respectively, which were statistically significant, suggesting that the pooled VRR after 6 months and 12 months of HIFU were 52.00% ( and 72.00% (). The funnel plot showed that there was no publication bias (p > 0.05, ).
3.7. Safety
Postoperative side effects were reported in all studies, nine of which recorded patients’ pain scores over the course of treatment [Citation16–18, Citation20–25]. All the pain reported was mild or moderate except in one study [Citation20]. Side effects occurred in 7 studies [Citation16–18, Citation20–22, Citation25]. As shown in , the most common side effects were subcutaneous ecchymosis (8.57%), pain (7.53%), erythema (5.45%), skin pigmentation (4.16%), subcutaneous edema (3.12%) and skin burn (1.56%). No serious side effects were observed. Most of the above-mentioned side effects disappeared in a short period after ablation, except for subcutaneous induration (n = 1) [Citation16], hyperpigmentation (n = 10) [Citation17, Citation20], and skin numbness (n = 1) [Citation20], which lasted until the end of these studies. All side effects occurred shortly after ablation, except for fat necrosis in one patient, which presented with pain at 9 months after treatment and lasted until surgical excision at 18 months after HIFU [Citation21].
4. Discussion
FA is the most common benign tumor in women of reproductive age. Since the breast is the superficial organ attached to the pectoralis major muscle, the lesion is usually palpable by the patients. Although it is characterized by a benign nature, the presence and growth of FA often bring a lot of psychological pressure to the patients. Thus, the number of patients demanding active treatment is increasing. HIFU ablation provides patients with noninvasive treatment options, which could simultaneously meet the patients’ desire for treating FA and preserving good breast cosmesis. In the past two decades, the clinical application of HIFU in treating FA is increasing. In this meta-analysis, the clinical efficacy and safety of HIFU for FA were evaluated.
All the studies have confirmed that HIFU was effective in the treatment of FA, with gradual tumor volume reduction after treatment. Nine articles reported the results of tumor VRR after 1 year of HIFU treatment, but there was a large heterogeneity, with the range of average reduction rate from 43.20% to 84.80%. After further analysis, it was found that two studies were responsible for the heterogeneity. Peek et al. tried a new ablation mode, called circumferential ablation, that only ablated the margin layer of the FA, leaving the center untreated. This ablation mode was much different from the entire ablation used in the other studies. From the results, it was inferred that circumferential ablation may not give the lesion sufficient energy, leaving the center of FA alive and not shrinking in follow-up, so the VRR of the lesion at 1 year after HIFU (43.20%) was much lower than that of other studies [Citation20]. On the contrary, it was shown in the meta-analysis that the VRR of entire ablation was relatively good, which was 72.00% at 1 year after HIFU.
Unlike surgical procedures that remove tumors from the body, HIFU causes in situ necrosis of tumors. Thus, the VRR of lesions after HIFU is much concerned by the doctors and patients. What’s more, to a certain extent, the VRR also represents the effectiveness of the treatment. According to the results of this meta-analysis, the volume of FA lesions could be effectively reduced after HIFU, but there were discrepancies in the reduction rate in different studies. In Kovatcheva’s study, the average VRR after two sessions of HIFU ablation was 90.47% at 24 months of follow-up [Citation18], which was higher than one session of HIFU ablation (77.32%). This indicated that sufficient ultrasonic energy should be deposited in FA to ensure the effective reduction of tumor volume after treatment. Since it would take a relatively long time for the body to absorb the necrotic tumor tissue, follow-up of more than one year was recommended. Two ablation methods were used in the 10 studies included in this systematic review, circumferential ablation and entire ablation. It was found that tumors with entire ablation had a faster volume reduction during the follow-up period. In addition to the ablation method, factors such as the energy deposition during treatment, the accuracy of imaging monitoring during treatment, the baseline tumor volume, and the number of FA lesions may also affect the absorption rate of lesions after treatment. However, it remains unclear what the critical factors affecting VRR are. Therefore, future studies are needed to explore the influencing factors and to analyze the correlation between each factor of the reduction rate of lesions, to improve the clinical protocol of HIFU, including patient selection, energy deposition, and treatment mode design, and to provide further evidence for treatment effectiveness predicting.
As stated by the 10 articles included in this review, the most common side effect was subcutaneous ecchymosis, followed by pain and erythema, which did not require clinical intervention and could be recovered in a short time. The overall side effect rate of HIFU treatment for FA was not high, and there were no serious side effects. The occurrence of side effects has a connection with doctor’s experiences. From Xiao et al.’s study, it could be concluded that there was a learning curve when doctors used HIFU to treat FA, and when the learning stage finished, not only the incidence of side effects was greatly reduced, but also the treatment time was significantly shortened [Citation26]. This might be one of the reasons for the relatively high rate of side effects in early published studies. After the first application of ultrasound-guided HIFU for the treatment of FA in 2015, it was found that the treatment time has been reduced with the accumulation of doctor’s experience and the advancement of technology [Citation27]. In the latest study, the average treatment time has been reduced to 9 minutes, only with a few mild side effects [Citation25].
Further analysis revealed that there were differences in side effect rates between the two ablation modes. Compared to entire ablation, circumferential ablation caused more side effects and needed a longer recovery time. In the study of Peek et al. [Citation20], 46 side effects were reported during 1-year follow-up, which was the largest number of side effects in all studies. Meanwhile, the average visual analogue scale (VAS) score of circumferential ablation was as severe as 7.1 [Citation20], significantly higher than that of entire ablation. Among all reported side effects, 32.75% (56/171) were caused by circumferential ablation. Furthermore, for long-term side effects, 91.67% (11/12) were caused by circumferential ablation. This might be related to the different energy input sites. The focus is placed in the center of the lesion in entire ablation, while in circumferential ablation, the focus is put around the lesion, much closer to the skin, with the ablation time treating the margin of FA longer than entire ablation. Hence, for the high VAS score and side effect rate, circumferential ablation might be not recommended for patients who intend to choose HIFU for the treatment of FA.
Furthermore, side effects seem to also be related to treatment equipment. Two kinds of device were used in the studies included in this systematic review, which were EchoPulse and Haifu system. EchoPulse was used in most of the previous studies, leading to different kinds of skin side effects, such as skin burn, ecchymosis, and hyperpigmentation, that lasted from days to months. Haifu system was utilized in the latest two studies, only resulting in mild skin redness and edema, which disappeared within a few days. Compared with EchoPulse, Haifu system has a significant reduction in both the type and incidence of side effects. It might be related to the differences in therapeutic features of these two devices and clinical protocols. In clinical trials using the EchoPulse device, patients were placed in supine or lateral position with the transducer directly contacted with the skin, and a single round of sonication of energy emission was 8 seconds through coupling gel, while in clinical trials using Haifu system device, patients were required to lie prone with the breast immersed in a tank of cold degassed water, and a single sonication of energy emission was 1 second with the transducer not touching the skin () [Citation28]. It is known that, while the ultrasonic energy passes through the body and focuses on the targeted lesion, there might be some energy deposits in the near acoustic pathway, especially in the obvious interface, such as the skin. The energy deposition is usually very low in the skin, but if the sonication is continuously generated in one round with a lack of effective heat dissipation, skin burn may occur. Since the accumulation of energy in one round of sonication with 1 second is lower than with 8 seconds, and the effect of heat dissipation might be better with a large volume of cold water than with a relatively small amount of coupling gel, this might be the reason why both the kinds and the incidence of skin side effects were much lower with Haifu system-related clinical trials than that with EchoPulse-related clinical trials. What’s more, when the transducer of EchoPulse is directly pressed onto the skin, the pressure may slow down the blood flow in capillaries and small vessels of the skin and subcutaneous tissue, or even temporarily close the vessels. This might increase the risk of skin side effects.
Figure 5. Treatment figure of patients with FA using Haifu system. (Zhang C, et al. Int J Hyperthermia. 2022;39(1):743-750.).
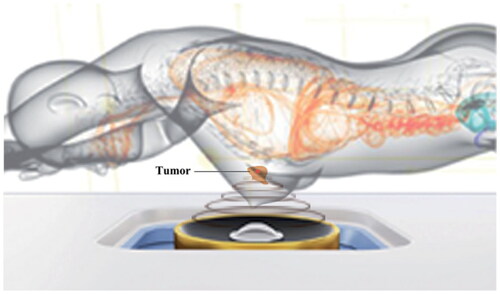
According to Chinese guideline, open surgery is suitable for patients with FA lesions larger than 3.0 cm in diameter, and VAM is recommended for patients with lesions smaller than 2.5 cm in diameter limited to the length of the biopsy needle [Citation29]. For HIFU, FA lesions that can be identified by real-time ultrasound and have a safe distance to the skin could be treated. For patients with multiple FAs, surgical resection would leave multiple skin scars affecting breast cosmesis, while repeated vacuum-assisted excision would be needed by VAM, which might increase the risk of normal breast tissue loss, breast duct injury, and internal trauma. Since HIFU is characterized by noninvasiveness, it could meet FA patients’ desire for cosmesis concern, so it might be a promising choice for patients who have multiple FAs or recurrent FA, and patients who are afraid of undergoing surgery.
This systematic review has some limitations. Firstly, due to the limited number of existing literature, the number of studies included in this analysis was small. With more studies exploring HIFU treatment of FA in the future, more papers can be included and a larger number of data can be pooled together for further analysis. Secondly, the studies included in this review had inconsistent definitions and assessment methods for side effects, so it was not possible to conduct a meta-analysis of side effects. A unification of a standard reporting system was suggested to set up for both efficacy and safety in future studies.
5. Conclusion
HIFU is effective and safe in treating FA. No serious side effects occur during and after treatment, and the lesion volume can gradually decrease in follow-up. The factors affecting the tumor VRR need to be further investigated.
Disclosure statement
The authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Weaver M, Stuckey A. Benign breast disorders. Obstet Gynecol Clin North Am. 2022;49(1):57–72. doi:10.1016/j.ogc.2021.11.003.
- Grouthier V, Chakhtoura Z, Tejedor I, et al. Positive association between progestins and the evolution of multiple fibroadenomas in 72 women. Endocr Connect. 2020;9(6):570–577. doi:10.1530/EC-20-0012.
- El-Wakeel H, Umpleby HC. Systematic review of fibroadenoma as a risk factor for breast cancer. Breast. 2003;12(5):302–307. doi:10.1016/s0960-9776(03)00123-1.
- Brahmachari S, Bhagat V, Patil P, et al. Evaluating the effect of ormeloxifene on multiple fibroadenomas and mastalgia. J Pharm Bioallied Sci. 2021;13(Suppl 2):S1386–S1389. doi:10.4103/jpbs.jpbs_222_21.
- Srivastava V, Meena RK, Ansari MA, et al. A study of anxiety and depression in benign breast disease. J Midlife Health. 2020;11(4):200–209. doi:10.4103/jmh.JMH_85_20.
- Salati SA. Breast fibroadenomas: a review in the light of current liter. ature. Pol Przegl Chir. 2020;93(1):40–48. doi:10.5604/01.3001.0014.5676.
- Javed A, Jenkins SM, Labow B, et al. Intermediate and long-term outcomes of fibroadenoma excision in adolescent and young adult patients. Breast J. 2019;25(1):91–95. doi:10.1111/tbj.13159.
- Huo HP, Wan WB, Wang ZL, et al. Percutaneous removal of benign breast lesions with an ultrasound-guided vacuum-assisted system: influence factors in the hematoma formation. Chin Med Sci J. 2016;31(1):31–36. doi:10.1016/s1001-9294(16)30019-0.
- Wang ZB, Wu F, Wang ZL, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou Province miniswine. Ultrason Sonochem. 1997;4(2):181–182. doi:10.1016/s1350-4177(97)00028-x.
- Lynn JG, Zwemer RL, Chick AJ, et al. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(2):179–193. doi:10.1085/jgp.26.2.179.
- Wu F, Wang ZB, Cao YD, et al. A randomised clinical tril of high-intensity focused ultrasound ablation for the treatment of patients with laocalised breast cancer. Br J Cancer. 2003;89(12):2227–2233. doi:10.1038/sj.bjc.6601411.
- Cavallo Marincola B, Pediconi F, Anzidei M, et al. High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Devices. 2015;12(2):191–199. doi:10.1586/17434440.2015.986096.
- Liberati A, Altman UG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–b2700. doi:10.1136/bmj.b2700.
- Oxford Levels of Evidence Working Group. The Oxford 2011 levels of evidence; 2011. Available from: http://www.cebm.net/index. aspx?o1=45653
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi:10.1046/j.1445-2197.2003.02748.x.
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound. 2015;3(1):1. doi:10.1186/s40349-014-0022-3.
- Peek MC, Ahmed M, Scudder J, et al. High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia. 2016;32(8):881–888. doi:10.1080/02656736.2016.1212278.
- Kovatcheva R, Zaletel K, Vlahov J, et al. Long-term efficacy of ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma. J Ther Ultrasound. 2017;5(1):1. doi:10.1186/s40349-017-0083-1.
- Imankulov S, Tuganbekov T, Razbadauskas A, et al. HIFU treatment for fibroadenoma - a clinical study at National Scientific Research Centre, Astana, Kazakhstan. J Pak Med Assoc. 2018;68(9):1378–1380.
- Peek MCL, Ahmed M, Scudder J, et al. High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial). Int J Hyperthermia. 2018;34(7):1002–1009. doi:10.1080/02656736.2017.1373865.
- Hahn M, Fugunt R, Schoenfisch B, et al. High intensity focused ultrasound (HIFU) for the treatment of symptomatic breast fibroadenoma. Int J Hyperthermia. 2018;35(1):463–470. doi:10.1080/02656736.2018.1508757.
- Brenin DR, Patrie J, Nguyen J, et al. Treatment of breast fibroadenoma with ultrasound-guided high-intensity focused ultrasound ablation: a feasibility study. J Breast Imaging. 2019;1(4):316–323. doi:10.1093/jbi/wbz050.
- Kwong A, Co M, Chen C, et al. Prospective clinical trial on high-intensity focused ultrasound for the treatment of breast fibroadenoma. Breast J. 2021;27(3):294–296. doi:10.1111/tbj.14166.
- Yue X, Li Z, Yin H, et al. Focused ultrasound ablation surgery for multiple breast fibroadenomas: pathological and follow-up results. Int J Hyperthermia. 2023;40(1):2202372. doi:10.1080/02656736.2023.2202372.
- Liang M, Zhang Z, Zhang C, et al. Feasibility and efficacy of ultrasound-guided high-intensity focused ultrasound of breast fibroadenoma. Int J Hyperthermia. 2023;40(1):2240548. doi:10.1080/02656736.2023.2240548.
- Xiao Y, Liang M, Chen M, et al. Evaluating the learning curve of high intensity focus ultrasound for breast fibroadenoma by CUSUM analysis: a multi-center study. Int J Hyperthermia. 2022;39(1):1238–1244. doi:10.1080/02656736.2022.2123566.
- Co M, Chen C, Lee C, et al. Prospective clinical trial on the learning curve of high-intensity-focused ultrasound for the treatment of breast fibroadenoma. Surg Today. 2022;52(7):1048–1053. doi:10.1007/s00595-021-02421-3.
- Zhang C, Liang M, Xia T, et al. Dosimetric analysis of ultrasound-guided high intensity focused ultrasound ablation for breast fibroadenomas: a retrospective study. Int J Hyperthermia. 2022;39(1):743–750. doi:10.1080/02656736.2022.2074151.
- Peng Y, Xie F, Zhao Y, et al. Clinical practice guideline for breast fibroadenoma: Chinese Society of Breast Surgery (CSBrS) practice guideline 2021. Chin Med J (Engl). 2021;134(9):1014–1016. doi:10.1097/CM9.0000000000001462.