Abstract
Purpose
Intra-arterial conversion therapy (ICT) is a promising option for patients with unresectable hepatocellular carcinoma (uHCC). However, the selection of sequential therapeutic modalities is still controversial. This study compared the efficacy and safety of surgical resection (SR) versus thermal ablation (TA) after patients with uHCC received ICT.
Methods
From May 2008 to November 2021, 3553 consecutive patients were reviewed and 791 patients were downstaged to receive TA or SR. Among them, 340 patients received SR, and 451 received TA after ICTs. The propensity score matching (PSM) method was applied to reduce selection bias between groups. Cumulative overall survival (OS) and progression-free survival (PFS) were compared using the Kaplan–Meier method with the log-rank test. The occurrence of complications and adverse events (AEs) were compared using chi-square test.
Results
After PSM 1:1 (n = 185 in both groups), the 10-year OS and PFS rates for patients who underwent SR were comparable to those of patients who underwent TA (OS: 45.2% vs. 36.1%; p = 0.190; PFS: 19.3% vs. 15.9%; p = 0.533). A total of 237 (29.9%) patients (203 males; mean age:57.1 ± 11.0 years) received downstaging therapy, and long-term OS and PFS remained comparable between the two groups (p = 0.718, 0.636, respectively). However, the cumulative OS and PFS rates in the downstaged cohort were significantly higher than those in the nondownstaged cohort (both ps < 0.001). Additionally, there was no difference in major complications between the two groups (SR: 6.3% vs. TA: 8.6%; p = 0.320).
Conclusions
TA might be an acceptable first-line alternative to SR after patients with uHCC receive ICT, especially patients unsuitable for SR. Better long-term survival was observed among patients in the downstaged cohort compared to those who failed to downstage.
Introduction
Hepatocellular carcinoma (HCC) is the third most common malignant tumor and ranks as the fourth leading cause of death due to cancer, with an annual incidence of 905,000 new cases and 830,000 deaths globally [Citation1,Citation2]. To date, first-line curative therapies, including surgical resection (SR), thermal ablation (TA) and liver transplantation, have been confirmed to effectively improve HCC patient prognosis [Citation3]. Unfortunately, the BRIDGE study reported that more than 64% of patients are ineligible for curative therapies at the time of initial diagnosis [Citation4]. Conversion therapy, which is defined as the conversion of unresectable HCC (uHCC) into resectable HCC, is a promising option for patients with uHCC [Citation5,Citation6]. Moreover, the Chinese Expert Consensus on Conversion Therapy for Hepatocellular Carcinoma (CECCTHC) suggests that conversion therapy is an intermediate goal for the treatment of uHCC, while long-term survival is the ultimate goal in China [Citation7].
Over the past decade, conversion therapies have frequently been attempted, and various therapeutic schemes have emerged [Citation8–10]. For example, multitargeted tyrosine kinase inhibitors (TKIs), including sorafenib and lenvatinib, were recommended as a first-line treatment in advanced HCC by various guidelines [Citation11,Citation12], but their low objective response rate (ORR) has limited further development of conversion therapy. Intra-arterial therapies, mainly including transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC), are new options for patients with uHCC [Citation13–15]. In particular, the FOLFOX regimen (oxaliplatin plus fluorouracil and leucovorin) proposed for HAIC was observed to significantly improve the ORR (46%) compared with sorafenib [Citation16]. Moreover, Shi Ming et al. showed that FOLFOX of HAIC yielded a 48% ORR in a randomized phase III trial of large HCC (largest diameter >7 cm) [Citation15].
Although large HCC is strongly resistant to chemotherapy due to a high tumor burden, intra-arterial therapies or their combination with TKIs and immune checkpoint inhibitors (ICIs) offer new hope for affected patients [Citation17,Citation18]. An increasing number of studies have shown that a triple treatment approach can play an important role in rapidly reducing tumor burden, which can be attributed to the formation of strong synergistic effects among treatments. For example, the CHANCE001 study reported that TACE combined with TKIs and ICIs obtained a 60.1% ORR in patients with advanced HCC [Citation9]. Xu et al. reported that HAIC combined with lenvatinib and ICIs obtained a 64.9% ORR in HCC patients with high-risk features [Citation19]. Therefore, intra-arterial conversion therapies (ICTs) provide a prerequisite for the implementation of sequential curative treatment.
To date, SR and TA after ICT are the most frequently used curative treatments in patients with uHCC; however, a comparison in terms of efficacy, safety and long-term survival is lacking. Herein, the aim of this study was to investigate the long-term survival and safety of these two therapeutic modalities after patients with early-intermediate uHCC receive ICT.
Materials and methods
Study design and patient enrolment
This retrospective, multi-institutional study protocol was approved by the Institutional Review Board (or Ethics Committee) of Sun Yat-sen University Cancer Center (protocol code B2022-694-01) and was conducted following the principles of the 1975 Helsinki Declaration. Due to its retrospective nature, the requirement for written informed consent was waived. The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
From May 2008 to November 2021, a total of 3553 consecutive patients with barcelona clinic liver cancer (BCLC) stage A and B HCC who underwent initial TACE or HAIC at 12 tertiary hospitals in China were reviewed. The distribution of data sources at various hospitals is shown in Supplementary Table 1. These patients were diagnosed based on the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) guidelines [Citation20,Citation21]. The inclusion criteria were as follows: (a) age 18–75 years; (b) Eastern Cooperative Oncology Group (ECOG) performance status <2; (c) Child–Pugh class A or B liver function; (d) absence of macrovascular invasion or extrahepatic metastasis and (e) receiving conversion therapy. The exclusion criteria were as follows: (a) patients who underwent any treatment prior to ICT; (b) HCC combined with other malignancies; (c) lost to follow-up >6 months. shows the study flow of eligible patients. The TACE and HAIC procedures, combination therapy and criteria for discontinuing the treatment protocol are described in Supplementary Information E1.1–1.3.
Figure 1. Enrollment pathway of patients with unresectable hepatocellular carcinoma who underwent intra-arterial conversion therapy.
Successful downstaging therapy was defined as viable intrahepatic tumor burden met the Milan criteria (one lesion up to 5 cm or no more than three lesions ≤3 cm without vascular invasion or extrahepatic metastasis).
HCC: hepatocellular carcinoma; SR: surgical resection; TA: thermal ablation; PSM: propensity score match; IPTW: inverse probability treatment weighting.
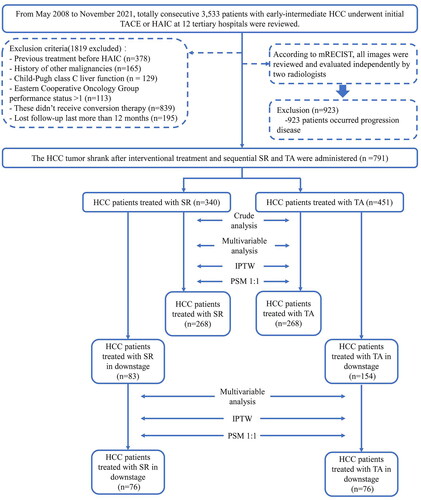
Indication for intra-arterial conversion therapies
In this study, the therapeutic modality selection after ICTs included SR and TA. The procedures of SR and TA after ICTs are described in Supplementary Information E1.4–1.5. SR or microwave ablation (MWA) was indicated based on the following criteria: (1) intrahepatic nodules that received HAIC were evaluated as partial response and stable disease for at least 8 weeks; (2) R0 resection was achieved with sufficient remnant liver volume (≥40% of standard liver volume for patients with liver cirrhosis or ≥30% of standard liver volume for patients without liver cirrhosis); (3) normal coagulation disorders (i.e., prothrombin time <15 s, prothrombin activity >40%, and platelet count >50 cells × 109/L); (4) no severe or persistent adverse events (AEs) occurring due to ICT; (5) patients with serious comorbidities, including heart, lung and renal dysfunction. The final post-ICT treatment strategy for individual patients was determined by a multidisciplinary team that included interventional radiotherapists, hepatobiliary radiologists, surgeons, oncologists, and radiologists and adhered to the patient’s wishes. The selection of ICT modality for treating uHCC is shown in Supplementary Table 2.
Assessments and follow-up
In this study, enrolled patients were censored at the last follow-up date (31 October 2023). After ICT was completed, serum α-fetoprotein (AFP) and dynamic contrast-enhanced images (e.g., computed tomography [CT] or magnetic resonance imaging [MRI]) were examined again at 3- to 6-month intervals during the follow-up period and at approximately 3-month intervals in the first year. If disease progression was not found sequentially, 6-month follow-up intervals were performed. The responses were assessed by dynamic contrast-enhanced CT or MRI based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [Citation22,Citation23]. To maintain balance between the two groups and analyze the risk factors associated with survival outcomes, we collected 16 clinicopathologic variables (Supplementary information E1.6) and two medical examples of ICTs are shown in Supplementary Figures 1 and 2. The description of treatment methods after uHCC recurrence in patients who received ICT is shown in Supplementary Table 3.
Endpoints and definitions
The primary endpoints were overall survival (OS), progression-free survival (PFS) and extrahepatic progression-free survival (EPFS). OS was calculated from the date of initial treatment to the date of death or follow-up deadline. PFS was calculated from the first ICT to the date of disease progression or end of the follow-up. EPFS was calculated from the first ICT to the date of extrahepatic disease progression or end of the follow-up. Successful downstaging therapy was defined as a viable intrahepatic tumor burden that met the Milan criteria (one lesion up to 5 cm or no more than three lesions ≤3 cm without vascular invasion or extrahepatic metastasis). The secondary endpoints were AEs and complications. Major complications were defined as events that caused substantial morbidity and disability that increased the level of care, led to hospital admission, or substantially prolonged the hospital stay. AEs were evaluated based on Common Terminology Criteria for Adverse Events version 5.0 (National Cancer Institute) [Citation24].
Statistical analysis
Quantitative variables with mean ± standard deviation (SD) or median with interquartile range (IQR) were compared by the Kruskal–Wallis and Mann–Whitney U test. Qualitative data were expressed as frequencies and percentage and were compared using the chi-square test. Cumulative survival was compared using the Kaplan–Meier method using the log-rank test. Univariate and multivariable analyses of independent prognostic factors were evaluated by means of the forward stepwise Cox regression model to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). We used adjusted HRs to compare survival between the two treatment groups, with 95% CIs used to assess the variation around the estimated risk of events. To reduce bias and increase reliability, we applied propensity score matching (PSM) using the nearest-neighbor algorithm to adjust for potentially unbalanced variables in both groups. The calculated propensity scores were then used for case-weight estimation (inverse probability treatment weighting [IPTW]). Weights applied to patients treated with SR were the inverse of the propensity score, and weights applied to patients treated with TA were the inverse of 1 minus the propensity score. All tests of significance were two-sided, and results for which p < 0.05 were interpreted as statistically significant. Statistical analysis was performed using SPSS version 23.0 (IBM Corp., NY, USA) and the ‘RMS package’ using R software version 4.2.0 (http://www.r-project.org/).
Results
Baseline characteristics of enrolled patients
A total of 791 treatment-naïve patients with uHCC who received ICT were enrolled. In total, 676 (85.5%) were male, and the mean age was 57.1 years ± 11.2 (SD). The most common cause of HCC was chronic hepatitis B virus infection (96.9%, 767/791). The mean tumor size was 7.6 cm ± 2.2 (SD). A total of 479 (60.6%) patients were classified as ALBI grade 1. The distribution of BCLC stages was as follows: 342 (43.2%) in stage A and 449 (56.8%) in stage B. Among all enrolled patients, 340 patients were assigned to the SR group, and 451 patients were assigned to the TA group. The baseline characteristics of patients stratified by therapeutic modality are outlined in . Clinical variables, including age, ECOG, ascites, tumor diameter, tumor number, and AFP and aspartate aminotransferase (AST) levels, were different between the TA and SR groups (all ps < 0.05). Patients were PSM 1:1 using the nearest-neighbor method with a caliper of 0.05. After PSM, 536 patients were included in both the TA and SR groups, and no baseline characteristics or ablation parameters were found to differ between groups (, Supplementary Figure 3). Besides, the IPTW was also used for ablation parameters, and standardized mean differences in patients between the two groups were presented in Supplementary Figure 4.
Table 1. Baseline characteristics of the patients with HCC who received intra-arterial conversation therapy in total cohorts.
Survival analysis in total cohorts
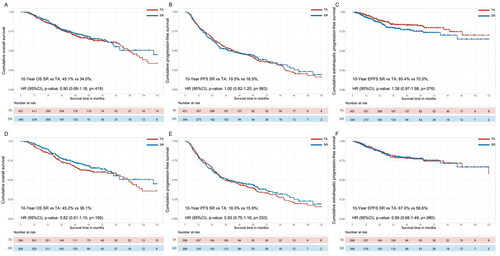
To assess the comparative survival results between SR and TA in patients with uHCC who had first systemic therapy, a long-term follow-up study was conducted. The median follow-up durations for the SR group and TA group were 35.8 months (IQR, 25.7–81.2 months) and 36.0 months (IQR, 27.4–88.0 months), respectively. In the crude Kaplan–Meier analyses, the cumulative 1-, 3-, 5- and 10-year OS rates were 95.5%, 77.6%, 68.1% and 45.1% in the SR group and 94.8%, 76.4%, 65.1% and 34.0% in the TA group, respectively (, HR: 0.90; 95% CI: 0.69–1.16; p = 0.419). The cumulative 1-, 3-, 5- and 10-year PFS rates were 80.5%, 52.2%, 44.3% and 19.5% in the SR group and 80.7%, 53.8%, 41.2% and 16.5% in the TA group (, HR: 1.00; 95% CI: 0.82–1.20; p = 0.963). The cumulative 1-, 3-, 5- and 10-year EPFS rates were 93.0%, 79.6%, 75.5% and 65.4% in the SR group and 95.8%, 84.4%, 82.4% and 70.5% in the TA group, respectively (, HR: 1.38; 95% CI: 0.97–1.98; p = 0.076). After PSM 1:1, the cumulative 1-, 3-, 5- and 10-year OS rates were 96.6%, 78.0%, 68.3% and 45.2% in the SR group and 3.5%, 73.0%, 62.1% and 36.1% in the TA group (, HR: 0.80; 95% CI: 0.61–1.10; p = 0.190). The cumulative 1-, 3-, 5- and 10-year PFS rates were 80.5%, 51.7%, 44.1% and 19.3% in the SR group and 78.5%, 49.5%, 40.1% and 15.9% in the TA group, respectively (, HR: 0.93; 95% CI: 0.75–1.16; p = 0.533). The cumulative 1-, 3-, 5- and 10-year EPFS rates were 92.08%, 81.1%, 77.3% and 67.0% in the SR group and 93.4%, 79.6%, 77.9% and 58.6% in the TA group, respectively (, HR: 0.99; 95% CI: 0.66–1.49; p = 0.980).
Figure 2. Comparing the survival of SR and TA groups for uHCC patients in total cohorts. Kaplan–Meier curves for the (A) overall survival (OS) and (B) progression-free survival (PFS) and (C) extrahepatic progression-free survival (EPFS) of uHCC patients in total cohorts before propensity score matching (PSM). The OS (D), PFS (E) and EPFS (F) were compared with bias reduction after PSM.
The variables matched for PSM included age at diagnosis, gender, ECOG, comorbidity, HBV, BCLC stages, ascites, HCC number, HCC diameter, ALBI grade, AFP, tyrosine kinase inhibitors and immunotherapy.
HCC: hepatocellular carcinoma; SR: surgical resection; TA: thermal ablation; PSM: propensity score match.
shows the different methods of analysis used to compare OS, PFS and EPFS between the two groups. In the adjusted multivariable analysis, OS in the SR group was comparable with the TA group (adjusted HR: 0.88; 95% CI: 0.66–1.18; p = 0.407). This result was also observed after IPTW was applied (HR: 0.88; 95% CI: 0.65–1.19; p = 0.410) and covariate adjustment using the propensity score (HR: 0.82; 95% CI: 0.61–1.10; p = 0.190). For PFS, the adjusted multivariable analysis showed that OS was comparable in the SR and TA groups (adjusted HR: 0.93; 95% CI: 0.75–1.16; p = 0.528), which was also observed after IPTW (HR: 0.99; 95% CI: 0.80–1.22; p = 0.940) and covariate adjustment using the propensity score (HR: 0.93; 95% CI: 0.75–1.16; p = 0.533). For EPFS, the adjusted multivariable analysis showed that OS was comparable in the SR and TA groups (adjusted HR: 1.00; 95% CI: 0.66–1.51; p = 0. 994), after IPTW (HR: 1.03; 95% CI: 0.64–1.66; p = 0.894), and after covariate adjustment using the propensity score (HR: 0.99; 95% CI: 0.66–1.49; p = 0.980).
Table 2. Survival comparison of TA versus SR according to analytic methods.
Survival analysis in downstage cohorts
A total of 237 (29.9%) patients (203 males; mean age: 57.1 ± 11.0 years) received downstaging therapy. The baseline characteristics of the downstage cohorts are shown in . Patients who received downstaging therapy had better OS, PFS and EPFS than patients who did not receive downstaging therapy (p < 0.001) (Supplementary Figure 5). In the downstage cohort, 83 patients received SR, and 154 received TA. Distributions within clinical variables, including age, tumor diameter, and AFP, were different between the two groups (all ps < 0.05). Patients were PSM 1:1 using the nearest-neighbor method with a caliper of 0.05. After PSM 1:1, all baseline variables of patients in the SR group (n = 76) and TA group (n = 76) were balanced (, Supplementary Figure 6). And the standardized mean differences of IPTW in patients between the two groups are presented in Supplementary Figure 7.
Table 3. Baseline characteristics of the patients with HCC who received ICTs in downstage cohorts.
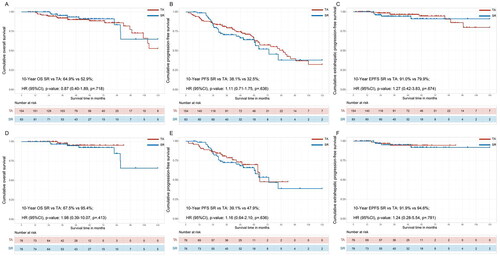
In the crude Kaplan–Meier analyses, the cumulative 1-, 3-, 5- and 10-year OS rates were 100.0%, 95.3%, 91.0% and 64.9% in the SR group and 100%, 92.0%, 88.5% and 52.9% in the TA group (, HR: 0.87; 95% CI: 0.41–1.89; p = 0.718). The cumulative 1-, 3-, 5- and 10-year PFS rates were 98.8%, 70.6%, 60.7% and 38.1% in the SR group and 9.8%, 79.5%, 64.2% and 32.5% in the TA group, respectively (, HR: 1.11; 95% CI: 0.71–1.75; p = 0.636). The cumulative 1-, 3-, 5- and 10-year EPFS rates were 100.0%, 100.0%, 94.2% and 91.0% in the SR group and 100.0%, 100.0%, 96.5% and 79.9% in the TA group, respectively (, HR: 1.27; 95% CI: 0.42–3.83; p = 0.674). After PSM 1:1, the cumulative 1-, 3-, 5- and 10-year OS rates were 100.0%, 100.0%, 96.8% and 67.5% in the SR group and 100.0%, 100.0%, 98.0% and 95.4% in the TA group, respectively (, HR: 1.98; 95% CI: 0.39–10.07; p = 0.413). The cumulative 1-, 3-, 5- and 10-year PFS rates were 98.6%, 72.5%, 66.0%, and 39.1% in the SR group and 93.3%, 78.6%, 69.9%, and 47.9% in the TA group, respectively (, HR: 1.16; 95% CI: 0.64–2.10; p = 0.636). The cumulative 1-, 3-, 5- and 10-year EPFS rates were 100.0%, 100.0%, 95.2% and 91.9% in the SR group and 100.0%, 94.6%, 94.6% and 94.6% in the TA group, respectively (, HR: 1.24; 95% CI: 0.28–5.54; p = 0.781).
Figure 3. Comparing the survival of SR and TA groups for uHCC patients in downstaging cohorts. Kaplan–Meier curves for the (A) overall survival (OS) and (B) progression-free survival (PFS) and (C) extrahepatic progression-free survival (EPFS) of uHCC patients in total cohorts before propensity score matching (PSM). The OS (D), PFS (E) and EPFS (F) were compared with bias reduction after PSM.
The variables matched for PSM included age at diagnosis, gender, ECOG, comorbidity, HBV, ascites, HCC number, HCC diameter, ALBI grade, AFP, tyrosine kinase inhibitors and immunotherapy.
HCC: hepatocellular carcinoma; SR: surgical resection; TA: thermal ablation; PSM: propensity score match.
Risk factors for survival outcomes
The risk factors for OS and PFS in the total cohort were assessed by univariate and multivariate analyses (Supplementary Tables 4 and 5). In the multivariate analysis, tumor size (HR: 1.90; 95% CI: 1.24–2.90, p = 0.003), comorbidity (HR: 1.63; 95% CI: 1.06–2.51; p = 0.025) and ALBI grade (HR: 1.35; 95% CI: 1.04–1.76; p = 0.024) were significant factors for OS. Moreover, comorbidity (HR: 1.54; 95% CI: 1.12–2.11; p = 0.008), tumor number (HR: 1.33; 95% CI: 1.02–1.74; p = 0.037) and targeted immunotherapy (HR: 0.62; 95% CI: 0.51–0.76; p < 0.001) were significant factors for PFS. The risk factors for OS and PFS in the downstage cohort were also assessed by univariate and multivariate analyses (Supplementary Tables 6 and 7). In the multivariate analysis, age (HR: 3.44; 95% CI: 1.53–7.73, p = 0.003) and comorbidities (HR: 4.36; 95% CI: 1.30–14.65; p = 0.017) were significant factors for OS. Moreover, age (HR: 1.89; 95% CI: 1.15–3.11, p = 0.012) and targeted immunotherapy (HR: 1.975; 95% CI: 1.243–3.139; p = 0.004) were significant factors for PFS.
Subgroup analyses
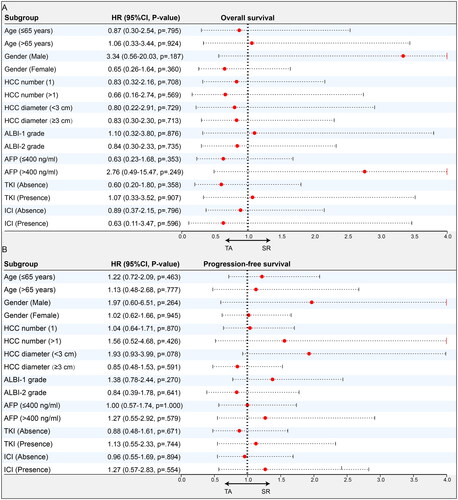
The forest plots of the subgroup analyses for OS and PFS based on the above-mentioned important variables are shown in and . Among all patients, the subgroup and PSM analyses did not change our essential finding that OS was comparable in the SR and TA groups (). However, the findings suggest that TA appeared to be particularly helpful in controlling disease for select patients, including those who received therapy in combination with TKI and those with tumor diameters <10 cm. In the downstaged cohort, neither subgroup nor PSM analyses changed our essential finding that SR provided comparable OS and PFS as TA ().
Figure 4. Subgroup analyses of SR and TA groups for uHCC patients in total cohorts. Forest plot showing the factors associated with overall survival (OS) and progression-free survival (PFS) in the HCC patients who received SR and TA after intra-arterial conversion therapy. (A) OS in all patients, (B) PFS in all patients.
HCC: hepatocellular carcinoma; SR: surgical resection; TA: thermal ablation; ECOG: Eastern Cooperative Oncology Group; HBV: hepatitis type B viral; AFP: α-fetoprotein; ALBI: albumin–bilirubin; TKI: tyrosine kinase inhibitors; ICI: immune checkpoint inhibitors.
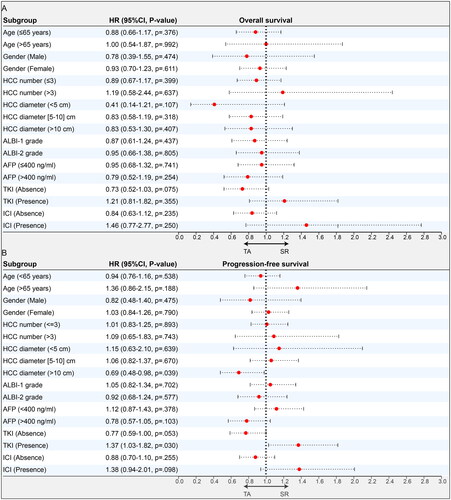
Figure 5. Subgroup analyses of SR and TA groups for uHCC patients in downstaged cohorts. Forest plot showing the factors associated with overall survival (OS) and progression-free survival (PFS) in the HCC patients who received SR and TA after intra-arterial conversion therapy. (A) OS in the downstage cohorts and (B) PFS in the downstage cohorts in the downstage cohorts.
HCC: hepatocellular carcinoma; SR: surgical resection; TA: thermal ablation; ECOG: Eastern Cooperative Oncology Group; HBV: hepatitis type B viral; AFP: α-fetoprotein; ALBI: albumin–bilirubin; TKI: tyrosine kinase inhibitors; ICI: immune checkpoint inhibitors.
Treatment-related AEs and complications
There were no treatment-related deaths in either group. AEs and complications that occurred after SR or TA are shown in . Grade 1–2 and grade 3–4 AEs in both groups were similar (p = 0.364, 0.556). Liver dysfunction was the most common minor complication, which included the presence of mild ascites and increased transaminase levels. Seventeen major complications occurred in the SR group, which comprised two instances of peritoneal effusion, liver abscess (n = 2), biliary fistula (n = 5), hemorrhage (n = 2), massive ascites (n = 10), implantation metastases (n = 2) and others (n = 2). Meanwhile, 23 major complications were found in the TA group, comprising peritoneal effusion (n = 3), liver abscess (n = 4), biliary fistula (n = 7), cholema (n = 5), hemorrhage (n = 2), massive ascites (n = 12), implantation metastases (n = 2) and others (n = 4). There were no significant differences in minor or major complications between the two groups (p = 0.320, 0.521).
Table 4. Adverse events and complications comparison between SR and TA after conversation therapy.
Discussion
The aim of conversion therapy is to achieve tumor downstaging and provide patients with a chance to receive curative therapy. Recently, as combinations involving antiangiogenic therapies and anti-programmed cell death protein 1 (PD-1) antibodies have been observed to induce higher tumor response rates, increasingly, conversion therapy has been used in clinical practice for patients with uHCC. For example, the IMbrave-150 study found encouraging results with the combination of atezolizumab and bevacizumab in terms of ORRs (27.3% by RECIST 1.1 and 33.2% by mRECIST) in patients with advanced HCC [Citation25]. However, their conversion rate remains unsatisfactory, especially among patients with large or giant HCC. TACE and HAIC, standard intra-arterial therapies that are used to treat uHCC, have many advantages including minimal invasiveness, easily replicable operations, high concentration, and low toxicity. Their direct chemical action on tumors can significantly improve ORR, and as a result, they are increasingly being used in conversion or downstaging therapy. Furthermore, Shi et al. Have demonstrated that MWA and radiofrequency ablation following TACE downstaging beyond the Milan criteria obtained comparable survival with TA alone for cases of HCC that met the Milan criteria and had significant survival benefits [Citation26,Citation27].
In this study, more than 80% of patients had large HCC, while approximately one-third had received ICT. To provide reliable sequential treatment options after conversion therapy, we reported two modalities (SR and TA) that were applied after ICT in patients with uHCC. Several key results were found. First, SR had comparable long-term survival (more than 10 years), including OS, PFS and EPFS, compared with TA. Second, among all patients, 237 (29.9%, mean tumor size = 7.5 cm) obtained downstaging therapy, outperforming the downstaging rate of other conversion therapies in previous studies [Citation25–27], demonstrating the outstanding effectiveness of the ICTs. This satisfactory result may be mainly attributed to over half of the population receiving ICTs combined with targeted immunotherapy. Third, for these patients in the downstage cohort, OS and PFS were further improved, outperforming those who failed to achieve downstaging, and similar long-term survival outcomes were also observed between the SR group and TA group. Given that the tumor burden of HCC was reduced to a low level (one lesion up to 5 cm or no more than three lesions <3 cm), our results showed that tumor burden did not impact further survival benefits. Fourth, ICTs combined with targeted immunotherapy significantly improved survival benefits compared with ICTs alone. This result may be closely associated with the following factors: (1) the patients who underwent successful downstaging therapy obtained more opportunities to eradicate hepatic nodules; and (2) the application of ICTs contributed to eliminating microvascular invasion, which has been shown to reduce the tumor recurrence rate [Citation28].
For the multivariate Cox regression analyses that included all patients in the cohort, tumor burden and hepatic function were crucial factors for the long-term OS of uHCC patients who received ICTs, which is consistent with previously reported studies. Interestingly, targeted immunotherapy plays an important role in PFS. The PFS benefit observed in this study may be due to the synergistic antitumour effects of the chemical agents, antiangiogenic agents, and PD-1 inhibitors. Oxaliplatin can induce immunogenic cell death by releasing tumor antigens, transporting calreticulin to the cell surface and secreting HMGB1 and ATP. These molecules related to cell death bind to their respective receptors and support the evolution of tumor-specific CD8+ T cells. Indeed, combined antiangiogenic and anti-PD-1/PD-L1 therapy has been shown to elicit T-cell function and drive tumor cells to activate immune checkpoints [Citation29–31], thereby generating greater antitumour immunity than HAIC or TACE alone.
For the multivariate Cox regression analyses performed in the downstaged cohort, age and comorbidities were more important than tumor burden and hepatic function for OS. For PFS, the selection of HAIC conversion therapy and targeted immunotherapy were more conducive to tumor control, which is consistent with the findings of Min Deng et al. [Citation10], possibly because (1) HAIC yields a better ORR than TACE because of the stable local high concentrations of chemotherapy agents in the tumor sustainably provided by HAIC for >24 h; (2) the embolization effect of TACE may aggravate liver dysfunction and complications, which results in significant reductions in executable TACE sessions.
In the subgroup analysis, long-term OS was not affected by different subgroup variables and remained similar between the two groups. However, TA, with advantages including minimally invasive and repetitive operations, can effectively control tumor recurrence for treatment in cases of smaller HCC (diameter ≤10 cm). In this study, the number of TA sessions exceeded that of SR. Moreover, TA combined with TKIs may also effectively suppress the progression of tumors. Fukuda et al. found that sorafenib administered before TA increases the ablation-induced coagulation necrosis range due to decreasing blood flow in the tumor and nontumor areas in patients with HCC, which improved the probability of complete ablation and expanding the ablation margin [Citation32].
In regard to safety, there were some differences between the SR group and TA group in terms of the frequencies and severity of AEs. The incidences of both total AEs and grade 3–4 AEs in the TA group were similar to those in the SR group based on the primary data, and any AEs in the two groups were consistent with those in previous phase III trials [Citation15,Citation17,Citation25]. Several gastrointestinal disorders, such as diarrhea and vomiting, in any AE grade were more common in both groups, which may be related to the effects of chemotherapy drugs. Moreover, the symptoms of embolism, including fever, pain, elevated alanine aminotransferase, elevated AST, and hyperbilirubinemia, were found more frequently. There was no significant difference in complications between the two groups, but the incidence of cholema was higher in the ablation group, which is worthy of attention and prompt management when performing TA.
Although our study has many strengths, including a large cohort and multicenter study design, several limitations remain. First, this study enrolled patients from several hospitals across our country, with differences in the application scheme and duration of chemotherapy drugs across hospitals. For example, the infusion time of fluorouracil in some hospitals was 23 h, while in others, it was 46 h. These factors may have affected the final outcomes. Second, this study enrolled most patients in China with large HCC and hepatitis type B infection as a predominant etiology. It remains to be elucidated whether the results could be widely applied in Western countries, where the majority of patients have a low tumor burden or alcoholic liver cirrhosis as the predominant etiology. Third, although we collected data from multiple hospitals, the number of enrolled patients remains limited. In the future, prospective clinical trials should be conducted to further test the reliability and reproducibility of the study results.
In conclusion, our multi-institutional study found that first-line treatment with TA may provide long-term survival outcomes and a safety profile that is comparable to SR after patients with uHCC receive ICT. Thus, TA may be an alternative option for minimally invasive treatment when encountering the potential technical challenges of implementing SR after ICT. Notably, targeted immunotherapy should be recommended as an effective combination therapy strategy that helps reduce the HCC recurrence rate after ICT.
Author contributions
Conception and design: Wendao Liu. Collection and assembly of data: Yusen and Chao An. Data analysis and interpretation: Chao An. Manuscript writing: Yusen and Chao An. Final approval of manuscript and accountable for all aspects of the work: all authors.
Supplemental Material
Download MS Word (1.7 MB)Acknowledgments
The authors acknowledge and express their deepest gratitude to the participants of this study.
Disclosure statement
The authors have no conflicts of interest to disclose.
Data availability statement
The in-house developed medical database of this study is publicly accessible at: http://www.yunedc.cn/#/login.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913.
- Park J-W, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818.
- Vitale A, Trevisani F, Farinati F, et al. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020;72(6):2206–2218. doi: 10.1002/hep.31187.
- Zhu X-D, Huang C, Shen Y-H, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10(4):320–329. doi: 10.1159/000514313.
- Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480.
- Zhang W, Tong S, Hu B, et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. 2023;11(9):e007366. doi: 10.1136/jitc-2023-007366.
- Zhu H-D, Li H-L, Huang M-S, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi: 10.1038/s41392-022-01235-0.
- Deng M, Cai H, He B, et al. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization, potential conversion therapies for single huge hepatocellular carcinoma: a retrospective comparison study. Int J Surg. 2023;109(11):3303–3311. doi: 10.1097/JS9.0000000000000654.
- Benson AB, D’Angelica MI, Abrams T, et al. NCCN Guidelines® Insights: biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21(7):694–704. doi: 10.6004/jnccn.2023.0035.
- Kuo Y-H, Lu S-N, Chen Y-Y, et al. Real-world lenvatinib versus sorafenib in patients with advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2021;11:823960. doi: 10.3389/fonc.2021.737767.
- Sidaway P. FOLFOX-HAIC active in large HCC. Nat Rev Clin Oncol. 2022;19(1):5–5. doi: 10.1038/s41571-021-00577-y.
- Salem R. Hepatic arterial infusion chemotherapy for large hepatocellular carcinoma: ready for prime time. J Clin Oncol. 2022;40(2):118–119. doi: 10.1200/JCO.21.02392.
- Li Q-J, He M-K, Chen H-W, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40(2):150–160. doi: 10.1200/JCO.21.00608.
- Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi: 10.1016/j.jhep.2018.02.008.
- Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi: 10.1200/JCO.22.00392.
- Xia D, Bai W, Wang E, et al. Lenvatinib with or without concurrent drug-eluting beads transarterial chemoembolization in patients with unresectable, advanced hepatocellular carcinoma: a real-world, multicenter, retrospective study. Liver Cancer. 2022;11(4):368–382. doi: 10.1159/000523849.
- Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Yu H, Bai Y, Xie X, et al. RECIST 1.1 versus mRECIST for assessment of tumor response to molecular targeted therapies and disease outcomes in patients with hepatocellular carcinoma: a systematic review and meta-analysis. BMJ Open. 2022;12(6):e052294. doi: 10.1136/bmjopen-2021-052294.
- Kuroda H, Oikawa T, Ninomiya M, et al. Objective response by mRECIST to initial lenvatinib therapy is an independent factor contributing to deep response in hepatocellular carcinoma treated with lenvatinib-transcatheter arterial chemoembolization sequential therapy. Liver Cancer. 2022;11(4):383–396. doi: 10.1159/000522424.
- Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology Consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions – part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168–1184.e1. doi: 10.1016/j.jvir.2019.04.017.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745.
- Shi F, Wu M, Lian S-S, et al. Radiofrequency ablation following downstaging of hepatocellular carcinoma by using transarterial chemoembolization: long-term outcomes. Radiology. 2019;293(3):707–715. doi: 10.1148/radiol.2019181991.
- Shi F, Lian S, Mai Q, et al. Microwave ablation after downstaging of hepatocellular carcinoma: outcome was similar to tumor within Milan criteria. Eur Radiol. 2020;30(5):2454–2462. doi: 10.1007/s00330-019-06604-y.
- Li S-H, Mei J, Cheng Y, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III, randomized study. J Clin Oncol. 2023;41(10):1898–1908. doi: 10.1200/JCO.22.01142.
- Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559.
- Liu L, Zhang R, Deng J, et al. Construction of TME and Identification of crosstalk between malignant cells and macrophages by SPP1 in hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71(1):121–136. doi: 10.1007/s00262-021-02967-8.
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x.
- Fukuda H, Numata K, Moriya S, et al. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation–propensity score matching analysis. Radiology. 2014;272(2):598–604. doi: 10.1148/radiol.14131640.
